Abstract
Extrapulmonary organ involvement in human immunodefiaency virus (HIV)-infected patients with pulmonary tuberculosis (TB) is reported to be 26%, however, the clinical predictors of extrapulmonary involvement in pulmonary TB patients has not been reported yet. We tried to determine the clinical predictors of presence of extrapulmonary involvement in patients with pulmonary TB. Cross-sectional study was performed including all adult patients with culture-proven pulmonary TB diagnosed between January 1, 2004 and July 30, 2006, at a tertiary referral hospital in South Korea. The presence of extra-pulmonary TB involvement was diagnosed based on bacteriological, pathological, or clinical evidence. Among 320 patients with a culture-proven pulmonary TB, 40 had extrapulmonary involvement. Patients with bilateral lung involvement were more likely to have extrapulmonary involvement, with an adjusted odds ratio (OR) of 4.21 (95% confidence interval [CI], 1.82-9.72), while patients older than 60 yr (adjusted OR, 0.27; 95% CI, 0.08-0.89), patients with cavitary lesions (adjusted OR, 0.37; 95% CI, 0.16-0.84), and with higher levels of serum albumin (adjusted OR, 0.45; 95% CI, 0.25-0.78) had less frequent involvement. Clinicians should be aware of the possibility of extrapulmonary involvement in TB patients with bilateral lung involvement without cavity formation or lower levels of serum albumin.
Keywords: Tuberculosis; Tuberculosis, Miliary; Diagnosis
INTRODUCTION
Extrapulmonary tuberculosis (EPTB) comprises 9.7-46% of all cases of tuberculosis (TB) (1-3). Although tuberculous bacilli could spread to any organs, the common organs involved with EPTB include lymph nodes, pleura, bones and joints, brain and meninges, gastrointestinal organs, liver, genitourinary organs, peritoneum, and pericardium. Although TB lymphadenitis or TB pleuritis respond relatively well to anti-TB treatment, some forms of EPTB (e.g., TB meningitis) are notorious for their association with high morbidity and mortality (4, 5). Furthermore, miliary TB, the extreme form of EPTB, presents a great challenge to human health because of its high mortality rate of 18-24%, even in recent reports (6-9).
Extrapulmonary organ involvement (10) in human immunodeficiency virus (HIV)-infected patients with pulmonary TB is reported to be 26%, however, the clinical characteristics of patients with pulmonary TB at risk of simultaneous extrapulmonary organ involvement have not been studied in detail, although the initiation of treatment following early identification of extrapulmonary involvement is crucial. The aim of this study was to determine the prevalence and clinical predictors of the presence of extrapulmonary involvement in patients with pulmonary TB.
MATERIALS AND METHODS
Study settings, subjects, and data collection
All adult patients with culture-proven pulmonary TB diagnosed between January 1, 2004 and July 31, 2006 at Seoul National University Hospital, a tertiary referral hospital were included for this study. We retrospectively reviewed the medical records of these patients, which included demographic data, results of laboratory tests, and so on. We also reviewed the radiographic examinations of the patients. The protocol of this study was approved by the institutional review board of Seoul National University Hospital.
Definition of extra-pulmonary involvement of TB
The presence of extra-pulmonary involvement in patients with pulmonary TB was based on the following criteria: 1) demonstration of acid-fast bacilli or the growth of Mycobacterium tuberculosis from tissue; 2) presence of granulomas with or without caseation necrosis in tissue; 3) positive polymerase chain reaction (PCR) results for the DNA of M. tuberculosis from tissues; or 4) a clinical diagnosis by duty physicians based on symptoms, laboratory, radiographic findings, and treatment response to anti-TB medications. Tuberculous pleuritis was not classified as EPTB because pleura is believed to be involved by direct invasion from frequently accompanying pulmonary parenchymal TB or hypersensitivity reaction by M. tuberculosis rather than blood stream dissemination (11-13).
Statistical analyses
Univariate comparisons between the group with pulmonary TB and extrapulmonary involvement and the group with pulmonary TB without extrapulmonary involvement were performed using Pearson's chi-square test or Fisher's exact test for categorical variables and Student's t-test for continuous variables. Variables analyzed included demographic characteristics, laboratory results, and radiographic findings. Using variables with p values of <0.20 from the univariate comparisons, multiple logistic regression models were constructed to identify predictors of the presence of extrapulmonary involvement. In logistic regression, backward elimination was used to select variables to be maintained in the final model, using a p value of <0.10 as the criterion for statistical significance of associations. The area under the receiver operator characteristic (ROC) curve was used to evaluate the performance of the models. To successfully split patients into more homogeneous subgroups, classification and regression trees (CART) were used to build a binary classification tree through recursive partitioning. All tests of significance were two sided and p<0.05 was considered statistically significant. We used statistical software Stata 9.0 (Stata Corporation, College Station, TX, U.S.A.) to perform the multiple logistic regression and R 2.4.1 (The R foundation for statistical computing) to construct the CART.
RESULTS
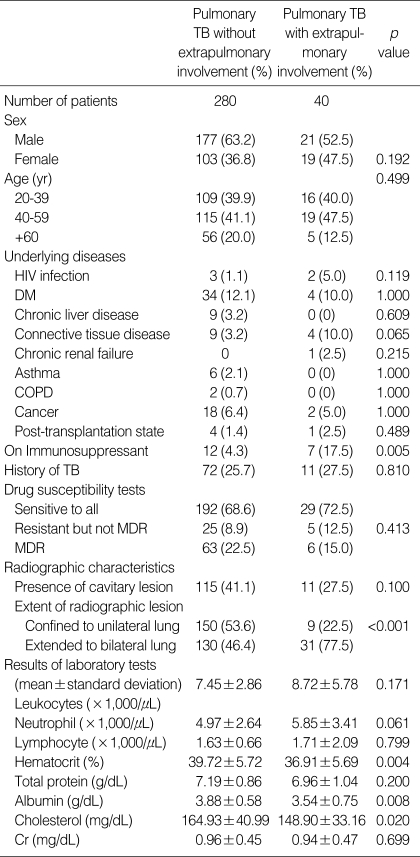
Three hundred and twenty patients were diagnosed with culture-proven pulmonary TB at Seoul National University Hospital between January 1, 2004 and July 31, 2006. Their median age was 45 yr and 198 (62%) were male: 85 patients (26.6%) had underlying diseases including HIV infection, diabetes, chronic liver diseases, and so on; 83 patients (25.9%) had previously diagnosed and treated TB (Table 1).
Table 1.
Demographic and clinical characteristics of enrolled patients
HIV, human immunodefiaency virus; COPD, chronic obstructive pulmmary disease; TB, tuberculosis; AFB, acid-fast bacilli; MDR, multi-drug resistance.
Forty (12.5%) of the 320 patients with pulmonary TB had extrapulmonary involvement. Miliary involvement of the lung was the most common manifestation of EPTB (12 patients, 30%). TB lymphadenitis (8 patients), intestinal TB (8 patients), and TB laryngitis (8 patients) followed. The tuberculous involvement of extrapulmonary organs was confirmed bacteriologically in 11 patients (27.5%) and diagnosed based on positive PCR for M. tuberculosis DNA in 7 patients (Table 2).
Table 2.
Sites and methods of diagnois of extrapulmonary involvement in 40 patients
*, When a patient had more than one organ involved, all of them were counted independently; †, 2 patients with intestinal TB diagnosed based on typical colonosopic findings and the other 2 patients with TB laryngitis without AFB bacilli and caseating granuloma in pathologic examinations.
PCR, polymerase chain reaction; TB, tuberculosis; AFB, acid-fast bacilli.
We compared the clinical characteristics and laboratory results between the 40 pulmonary TB patients with extrapulmonary involvement and the 280 patients without. There was no difference between the two groups in terms of age, underlying diseases, history of previous TB, and drug susceptibility pattern. However, bilateral lung involvement was more common in patients with extrapulmonary involvement (77.5% vs. 46.4%, p<0.001). In addition, the mean hematocrit, albumin, and cholesterol values were lower in the patients with extrapulmonary involvement (Table 3).
Table 3.
Comparison of demographic and clinical characteristics between pulmonary tuberculosis (TB) patients with extrapulmonary involvement and without extrapulmonary involvement (univariate analysis)
DM, diabetes mellitus; COPD, chronic obstructive pulmmary disease; MDR, Multi-drug resistance.
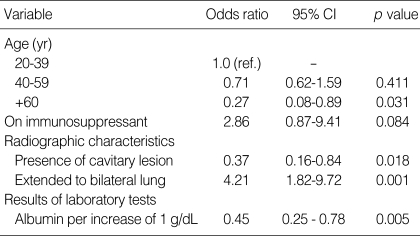
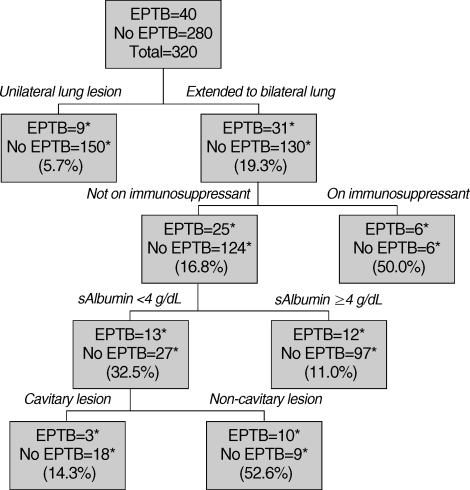
The final multiple logistic regression model showed that after adjustment only the presence of cavitary lesions, absence of bilateral lung involvement, and lower albumin levels were associated with extrapulmonary involvement in patients with pulmonary TB. Patients with bilateral lung involvement were more likely to have extrapulmonary involvement, with an adjusted odds ratio (OR) of 4.21 (95% confidence interval [CI], 1.82-9.72), while patients older than 60 yr (adjusted OR, 0.27; 95% CI, 0.08-0.89) and patients with cavitary lesions were less likely to have extrapulmonary involvement (adjusted OR, 0.37; 95% CI, 0.16-0.84). In addition, patients with higher levels of albumin had less frequent extrapulmonary involvement (adjusted OR, 0.45; 95% CI, 0.25-0.78) (Table 4). The fitness of the final model was good in terms of multiple logistic regression (area under the ROC curve, 0.76; 95% CI, 0.68-0.84) as well as CART analysis (area under the ROC curve, 0.73; 95% CI, 0.65-0.82) (Fig. 1).
Table 4.
Risk factors for combined extra-pulmonary involvement in patients with pulmonary TB (multiple logistic regression-final model)
TB, tuberculosis; CI, confidence interval.
Fig. 1.
Classification and regression trees (CART) analysis for predicting combined extra-pulmonary involvement in patients with pulmonary TB
EPTB, pulmonary TB with extra-pulmonary involvement; sAlbumin, serum level of albumin.
DISCUSSION
The presence of cavities in patients with pulmonary TB is regarded as a marker for high bacillary burden and is reported to be associated with relapse after completion of treatment (14). Our observation that the extrapulmonary involvement was less frequently observed in cavitary pulmonary TB patients suggests that the higher bacillary burden per se does not make the host prone to extrapulmonary involvement. On the contrary, the presence of cavities was associated with a lower possibility of the spread of tuberculous bacilli to extrapulmonary organs in this study. Given that pulmonary cavities have been reported to be rare in TB patients with immune compromise (15, 16), the presence of cavities could be a hallmark of a certain level of intact immunity against tuberculous bacilli, guaranteeing protection from further dissemination to other organs. This hypothesis could be tested through future study comparing systemic as well as local immunity against M. tuberculosis between TB patients with or without pulmonary cavity should be performed through future studies. In fact, differences were already reported in expression of various genes between pulmonary TB patients and extrapulmonary TB patients (17).
In contrast to the presence of pulmonary cavities, bilateral lung involvement might better reflect attenuated host immunity than bacillary burden (18). Considering that various types of impaired cell-mediated immunity have been considered to play an important role in the development of EPTB (10, 19-22), the decreased host immunity suggested by the presence of bilateral lung involvement could be crucial in the dissemination of tuberculous bacilli to extrapulmonary organs. In fact, pulmonary TB patients on immunosuppressants were prone to have extrapulmonary involvement (p=0.08) in this study, although we failed to get statistical significance because of the small numbers of patients on immunosuppressants.
Hypoalbuminemia is generally regarded as a marker of poor nutritional status in patients with TB (23, 24). In addition, hypoalbuminemia/protein malnutrition itself could impair host immunity against M. tuberculosis through decreased production of cytokines including interferon-γ (25) or the reduction of CD4 and CD8 T cell numbers observed in animal models (26). Hypoalbuminemia as a predictor for the presence of extrapulmonary organ involvement as observed in this study could be explained by probable immune dysfunction against tuberculous bacilli and matches previous reports showing lower albumin levels in patients with disseminated TB (27).
Results from our study that older patients with pulmonary TB have a lower risk of having a extrapulmonary involvement (adjusted OR, 0.27; 95% CI, 0.08-0.89) disagrees with previous reports that show that EPTB was higher in the elderly (28). In addition, the lower risk of EPTB in the elderly does not support immunity as a determinant of the spread of tuberculous bacilli to other organs because of the higher incidence of TB in the aged group (29, 30) and decreased immunity to tuberculous bacilli in older mice (31). This observation could be interpreted in two ways. First, the decreased risk for extrapulmonary involvement in the elderly could result from the small number of patients older than 60 yr (61 patients, 19.1%) in this study. In this setting, a small change in the number of patients with extrapulmonary involvement could make significant changes in the OR. Second, extrapulmonary dissemination with bilateral lung involvement but without cavity formation could be understood as a characteristic of TB bacilli rather than host immune status. The clinical manifestations might differ among TB patients infected with different strains of M. tuberculosis. For example, the 'Beijing strain' was reported to cause more severe pathology in mice (32) as well as more advanced radiographic lesions in humans (33). In this context, infection by specific strains of M. tuberculosis might cause intra- and extrapulmonary dissemination rather than cavity formation.
In conclusion, the extrapulmonary organ involvement in patients with pulmonary TB was more common in patients with bilateral lung involvement but without cavity formation or low levels of serum albumin. Clinicians should keep in mind the possibility of extrapulmonary involvement in these patients.
References
- 1.Rieder HL, Snider DE, Jr, Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141:347–351. doi: 10.1164/ajrccm/141.2.347. [DOI] [PubMed] [Google Scholar]
- 2.Cowie RL, Sharpe JW. Extra-pulmonary tuberculosis: a high frequency in the absence of HIV infection. Int J Tuberc Lung Dis. 1997;1:159–162. [PubMed] [Google Scholar]
- 3.Huang J, Shen M, Sun Y. Epidemiological analysis of extrapulmonary tuberculosis in Shanghai. Zhonghua Jie He He Hu Xi Za Zhi. 2000;23:606–608. [PubMed] [Google Scholar]
- 4.Kent SJ, Crowe SM, Yung A, Lucas CR, Mijch AM. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993;17:987–994. doi: 10.1093/clinids/17.6.987. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran P, Duraipandian M, Reetha AM, Mahalakshmi SM, Prabhakar R. Long-term status of children treated for tuberculous meningitis in south India. Tubercle. 1989;70:235–239. doi: 10.1016/0041-3879(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Langston AA, Gallis HA. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis. 1990;12:583–590. doi: 10.1093/clinids/12.4.583. [DOI] [PubMed] [Google Scholar]
- 7.Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med. 1990;89:291–296. doi: 10.1016/0002-9343(90)90340-j. [DOI] [PubMed] [Google Scholar]
- 8.Mert A, Bilir M, Tabak F, Ozaras R, Ozturk R, Senturk H, Aki H, Seyhan N, Karayel T, Aktuglu Y. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology. 2001;6:217–224. doi: 10.1046/j.1440-1843.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Park YB, Kim YS, Kang SB, Shin JW, Park IW, Choi BW. Miliary tuberculosis and acute respiratory distress syndrome. Int J Tuberc Lung Dis. 2003;7:359–364. [PubMed] [Google Scholar]
- 10.Lado Lado FL, Barrio Gomez E, Carballo Arceo E, Cabarcos Ortiz de Barron A. Clinical presentation of tuberculosis and the degree of immunodeficiency in patients with HIV infection. Scand J Infect Dis. 1999;31:387–391. doi: 10.1080/00365549950163842. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Lee HJ, Kwon SY, Yoon HI, Lee CT, Han SK, Shim YS, Yim JJ. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest. 2006;129:1253–1258. doi: 10.1378/chest.129.5.1253. [DOI] [PubMed] [Google Scholar]
- 12.Moudgil H, Sridhar G, Leitch AG. Reactivation disease: the commonest form of tuberculous pleural effusion in Edinburgh, 1980-1991. Respir Med. 1994;88:301–304. doi: 10.1016/0954-6111(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 13.Antoniskis D, Amin K, Barnes PF. Pleuritis as a manifestation of reactivation tuberculosis. Am J Med. 1990;89:447–450. doi: 10.1016/0002-9343(90)90374-m. [DOI] [PubMed] [Google Scholar]
- 14.Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, Gordin F, Horsburgh CR, Horton J, Khan A, Lahart C, Metchock B, Pachucki C, Stanton L, Vernon A, Villarino ME, Wang YC, Weiner M, Weis S. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360:528–534. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 15.Aderaye G, Bruchfeld J, Assefa G, Feleke D, Kallenius G, Baat M, Lindquist L. The relationship between disease pattern and disease burden by chest radiography, M. tuberculosis Load, and HIV status in patients with pulmonary tuberculosis in Addis Ababa. Infection. 2004;32:333–338. doi: 10.1007/s15010-004-3089-x. [DOI] [PubMed] [Google Scholar]
- 16.Batungwanayo J, Taelman H, Dhote R, Bogaerts J, Allen S, Van de Perre P. Pulmonary tuberculosis in Kigali, Rwanda. Impact of human immunodeficiency virus infection on clinical and radiographic presentation. Am Rev Respir Dis. 1992;146:53–56. doi: 10.1164/ajrccm/146.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Kim DK, Park GM, Hwang YI, Kim HJ, Han SK, Shim YS, Yim JJ. Microarray analysis of gene expression associated with extrapulmonary dissemination of tuberculosis. Respirology. 2006;11:557–565. doi: 10.1111/j.1440-1843.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 18.Mabiala Babela JR, Makosso E, Senga P. Radiological specifities of pulmonary tuberculosis in Congolese children: effect of HIV infection. Med Trop (Mars) 2006;66:255–259. [PubMed] [Google Scholar]
- 19.Ergun I, Ekmekci Y, Sengul S, Kutlay S, Dede F, Canbakan B, Erbay B. Mycobacterium tuberculosis infection in renal transplant recipients. Transplant Proc. 2006;38:1344–1345. doi: 10.1016/j.transproceed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Hussein MM, Mooij JM, Roujouleh H. Tuberculosis and chronic renal disease. Semin Dial. 2003;16:38–44. doi: 10.1046/j.1525-139x.2003.03010.x. [DOI] [PubMed] [Google Scholar]
- 21.Keiper MD, Beumont M, Elshami A, Langlotz CP, Miller WT., Jr CD4 T lymphocyte count and the radiographic presentation of pulmonary tuberculosis. A study of the relationship between these factors in patients with human immunodeficiency virus infection. Chest. 1995;107:74–80. doi: 10.1378/chest.107.1.74. [DOI] [PubMed] [Google Scholar]
- 22.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 23.Karyadi E, Schultink W, Nelwan RH, Gross R, Amin Z, Dolmans WM, van der Meer JW, Hautvast JG, West CE. Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. J Nutr. 2000;130:2953–2958. doi: 10.1093/jn/130.12.2953. [DOI] [PubMed] [Google Scholar]
- 24.Onwubalili JK. Malnutrition among tuberculosis patients in Harrow, England. Eur J Clin Nutr. 1988;42:363–366. [PubMed] [Google Scholar]
- 25.Dai G, McMurray DN. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect Immun. 1998;66:3562–3568. doi: 10.1128/iai.66.8.3562-3568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mainali ES, McMurray DN. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect Immun. 1998;66:927–931. doi: 10.1128/iai.66.3.927-931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crump JA, Reller LB. Two decades of disseminated tuberculosis at a university medical center: the expanding role of mycobacterial blood culture. Clin Infect Dis. 2003;37:1037–1043. doi: 10.1086/378273. [DOI] [PubMed] [Google Scholar]
- 28.Cailhol J, Decludt B, Che D. Sociodemographic factors that contribute to the development of extrapulmonary tuberculosis were identified. J Clin Epidemiol. 2005;58:1066–1071. doi: 10.1016/j.jclinepi.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2:27–36. [PubMed] [Google Scholar]
- 30.Stead WW, Dutt AK. Tuberculosis in elderly persons. Annu Rev Med. 1991;42:267–276. doi: 10.1146/annurev.me.42.020191.001411. [DOI] [PubMed] [Google Scholar]
- 31.Vesosky B, Turner J. The influence of age on immunity to infection with Mycobacterium tuberculosis. Immunol Rev. 2005;205:229–243. doi: 10.1111/j.0105-2896.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 32.Lopez B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, van Soolingen D. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. 2003;133:30–37. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drobniewski F, Balabanova Y, Nikolayevsky V, Ruddy M, Kuznetzov S, Zakharova S, Melentyev A, Fedorin I. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA. 2005;293:2726–2731. doi: 10.1001/jama.293.22.2726. [DOI] [PubMed] [Google Scholar]