Abstract
Background
The analysis and isolation of high numbers of chromosomes smaller than 3 Mb in size (microchromosomes) with good purity is dependent primarily on the detection sensitivity of the flow cytometer and the precision of the sort unit. The aim of this study was to investigate the capability of using a conventional flow cytometer for the detection and sorting at high purity microchromosomes with an estimated size of 2.7Mb.
Methods
Chromosomes were isolated from a human cell line containing a pair of X-derived microchromosomes using a modified polyamine isolation buffer. The chromosome preparation was labelled with Hoechst and Chromomycin and analysed and purified using a MoFlo sorter (DAKO) configured for high speed sorting. The purity of the flow sorted microchromosomes was assessed by reverse chromosome painting.
Results
Improved resolution of the peak of microchromosomes in a bivariate plot of Hoechst versus Chromomycin fluorescence was obtainable after discriminating clumps and debris based on gating data within a FSC versus pulse width plot.
Conclusions
Chromosomes of smaller size, less than 3Mb, can be detected with high resolution and flow sorted with high purity using a conventional flow sorter.
Keywords: microchromosomes; mammalian artificial chromosomes; metaphase; bivariate; univariate, flow karyotype; resolution; sorting; FISH; DOP-PCR
Chromosomes smaller than 20 Mb have been reported previously to occur naturally as chromosome aberrations (1,2) and in animal species such as birds (3) and in certain species of turtles (4). Previously, flow cytometry has been applied successfully to the isolation of microchromosomes greater than 20Mb in size (5-8). To date, we are not aware of any reports on the use of flow cytometry to isolate small (< 3Mb in size), experimentally generated, microchromosomes or mammalian artificial chromosomes, MACs (9-12), which are a useful research tool for the functional characterization of genes as well as potential gene carriers for somatic gene therapy (12-14).
Here we report the first successful application of a conventional flow cytometer for the detection and sorting of microchromosomes smaller than 3Mb using chromosomes prepared from a human cell line using a modified polyamine isolation buffer (15).
MATERIALS AND METHODS
Cell Culture
Chromosomes were prepared from a cell line (B5-3) derived from human fibrosarcoma cell line, HT1080. B5-3 contains a pair of ~2.7Mb X-centromere based microchromosomes generated using an approach involving telomere-associated chromosome fragmentation (10,16,17).
The cell line was cultured in DMEM (Gibco) medium supplemented with 15% fetal bovine serum (FBS, Gibco) and 500μg/ml of Geneticin (Invitrogen). The cell line was treated with demecolcine (0.1μg /ml) for 6hr after subculturing for 24hr.
Chromosome Preparation and Staining
Chromosomes were prepared as described previously (15) and stained overnight with Hoechst 33258 (Sigma) and Chromomycin A3 (Sigma). The stained chromosomes were treated with 25mM of sodium sulphite an hour before flow analysis.
Flow Cytometric Analysis and sorting
Stained chromosome suspensions were analysed on a flow cytometer (MoFlo®, DAKO) as described previously (15). In addition to Hoechst and Chromomycin fluorescence, forward scatter and pulse width parameters were collected. A region (R1, Figure 1D) was created on the plot of linear Forward Scatter (FSC) versus linear Pulse Width to exclude clumps and debris and bivariate plots of Hoechst versus Chromomycin fluorescence were gated on this region. A total of 100,000 events were acquired for the cell line at a data rate of 1000 events per second. Data collected from the experiments were analysed using Summit V3.1 (analysis software from DAKO).
Fig.1.
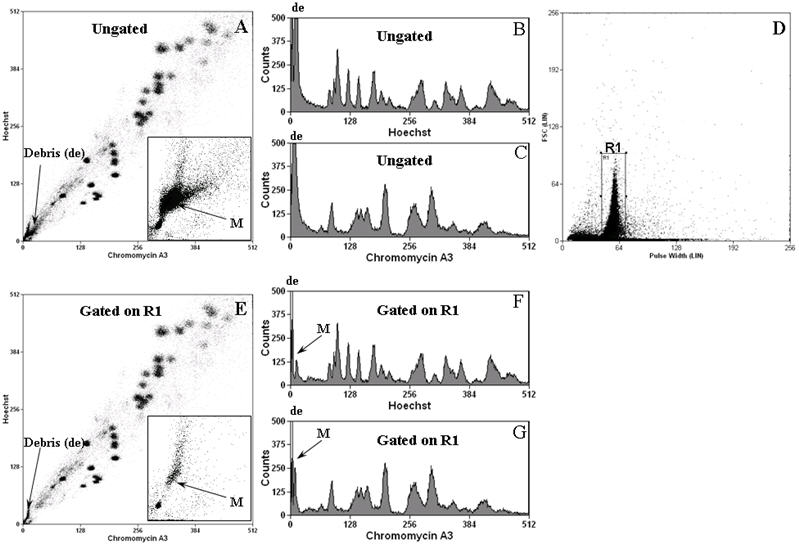
The stained chromosome suspension was flow sorted at a data rate of 10,000-15,000 events per second with an optimal setting of the sheath pressure of ~60 psi and drop drive frequency to ~95 KHz using a 70μm Cytonozzle tip on a high purity sort option of single mode per single drop envelope. The microchromosomes were flow sorted into sterile 500μl Eppendorf tubes containing 33μl of sterile UV treated distilled water.
Verification of microchromosome peak
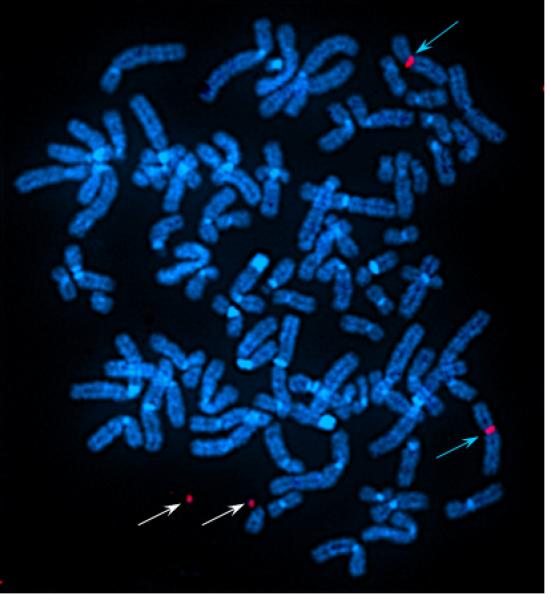
The purity of the flow sorted microchromosome peak was assessed by preparing a chromosome paint as described previously (18,19) from 500 sorted microchromosomes amplified using partially degenerate primers (DOP-PCR). The chromosome paint was directly labelled with Cy3 and reverse painted onto metaphase spreads of the human cell line containing the 2.7 Mb microchromosomes.
RESULTS
Data analysis and gating
Typical bivariate and univariate flow karyograms are shown in Figure 1. The modified polyamine isolation buffer produced ungated flow karyotypes in which all but the microchromosome clusters were well separated and resolved (Figure 1A). The microchromosome cluster (M) was buried in the debris (insert figure 1A and figures 1B and 1C). Back gating of the pulse width plot using regions of the bivariate flow karyogram revealed that chromosomes (and microchromosomes) were contained within the major peak of pulse width measurements with a non-linear correlation between chromosome size and pulse-width length (data not shown). The majority of the debris demonstrated shorter pulse width and aggregates greater pulse width. To discriminate clumps and debris, we applied a gate on the FSC versus pulse width plot (region gate R1, Figure 1D). This additional gating led to an improvement in the resolution of microchromosomes such that, after gating, the microchromosome peak could be identified as a clear and distinct cluster (inset Figure 1E) or as a single individual peak in univariate plots (Figures 1F, 1G).
Peak verification
Verification of the microchromosome peak (M) was carried out by painting back the probes derived from the flow sorted microchromosome onto metaphase spreads of the B5-3 cell line. The painting probe from the sorted microchromosome peak hybridized to the two microchromosomes and centromeres of the X chromosomes as expected with no discernable additional signals.
DISCUSSION AND CONCLUSIONS
The sorting of microchromosomes has been reported previously only for microchromosomes larger than 20 Mb in size (5-8,20). The potential of large scale purification of mammalian artificial chromosomes for gene therapy applications (8,13,14,20-23) has generated the need for a better method for the preparation and isolation of chromosomes of smaller microchromosomes with improved purity.
The use of a modified PAB buffer with exclusion of NaCl as previously described (15) improves the resolution of flow karyotypes and facilitates the separation of microchromosomes. The amount of debris produced in chromosome preparations was reduced in the absence of NaCl in the PAB buffer and a further improvement in resolution was achieved by removal of sodium citrate in the staining step (data not shown). More importantly, we found that the separation of the microchromosomes from the debris region was improved significantly upon application of a logic gate based on FSC versus Pulse Width (see figure 1E, 1F and 1G) rather than our usual gating based on low forward scatter and high Hoechst fluorescence (data not shown). Using this region gating, we were able to flow sort microchromosomes smaller than 3 Mb with high purity and yield. The purity of the sorted microchromosomes was confirmed by hybridisation onto B5-3 metaphases using a chromosome paint prepared from the flow sorted microchromosomes.
Using this chromosome preparation and gating strategy, we were able to flow sort approximately 100,000 microchromosomes with high purity in an hour at a data flow rate of more than 10,000 events/sec from a population of microchromosomes which made up on average just 0.6% of the total events.
Fig.2.
Acknowledgements
The authors would like to thank Dr. Christine Farr (University of Cambridge, Dept. of Genetics) who kindly provided the B5-3 cell line employed in this study. This work was supported by the Wellcome Trust.
LITERATURE CITED
- 1.Steinbach P, Djalali M, Hansmann I, Kattner E, Meisel-Stosiek M, Probeck HD, Schmidt A, Wolf M. The genetic significance of accessory bisatellited marker chromosomes. Hum Genet. 1983;65(2):155–64. doi: 10.1007/BF00286654. [DOI] [PubMed] [Google Scholar]
- 2.Blennow E, Nielsen KB, Telenius H, Carter NP, Kristoffersson U, Holmberg E, Gillberg C, Nordenskjold M. Fifty probands with extra structurally abnormal chromosomes characterized by fluorescence in situ hybridization. Am J Med Genet. 1995;55(1):85–94. doi: 10.1002/ajmg.1320550122. [DOI] [PubMed] [Google Scholar]
- 3.Griffin DK, Haberman F, Masabanda J, O’Brien P, Bagga M, Sazanov A, Smith J, Burt DW, Ferguson-Smith M, Wienberg J. Micro- and macrochromosome paints generated by flow cytometry and microdissection: tools for mapping the chicken genome. Cytogenet Cell Genet. 1999;87(3-4):278–81. doi: 10.1159/000015449. [DOI] [PubMed] [Google Scholar]
- 4.Ezaz T, Valenzuela N, Grutzner F, Miura I, Georges A, Burke RL, Graves JA. An XX/XY sex microchromosome system in a freshwater turtle, Chelodina longicollis (Testudines: Chelidae) with genetic sex determination. Chromosome Res. 2006;14(2):139–50. doi: 10.1007/s10577-006-1029-6. [DOI] [PubMed] [Google Scholar]
- 5.Ferretti LRE, Davis L, et al. Cytological analysis and sorting of a human supernumerary minichromosome. Cytotechnology. 1987;1:7–12. doi: 10.1007/BF00351115. [DOI] [PubMed] [Google Scholar]
- 6.Raimondi E, Ferretti L, Young BD, Sgaramella V, De Carli L. The origin of a morphologically unidentifiable human supernumerary minichromosome traced through sorting, molecular cloning, and in situ hybridisation. J Med Genet. 1991;28(2):92–6. doi: 10.1136/jmg.28.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferretti L, Raimondi E, Gamberi C, Young BD, De Carli L, Sgaramella V. Molecular cloning of DNA from a sorted human minichromosome. Gene. 1991;99(2):229–34. doi: 10.1016/0378-1119(91)90131-t. [DOI] [PubMed] [Google Scholar]
- 8.deJong G, Telenius AH, Telenius H, Perez CF, Drayer JI, Hadlaczky G. Mammalian artificial chromosome pilot production facility: large-scale isolation of functional satellite DNA-based artificial chromosomes. Cytometry. 1999;35(2):129–33. [PubMed] [Google Scholar]
- 9.Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF. Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet. 1997;15(4):345–55. doi: 10.1038/ng0497-345. [DOI] [PubMed] [Google Scholar]
- 10.Farr CJ, Bayne RA, Kipling D, Mills W, Critcher R, Cooke HJ. Generation of a human X-derived minichromosome using telomere-associated chromosome fragmentation. Embo J. 1995;14(21):5444–54. doi: 10.1002/j.1460-2075.1995.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiducci C, Ascenzioni F, Auriche C, Piccolella E, Guerrini AM, Donini P. Use of a human minichromosome as a cloning and expression vector for mammalian cells. Hum Mol Genet. 1999;8(8):1417–24. doi: 10.1093/hmg/8.8.1417. [DOI] [PubMed] [Google Scholar]
- 12.Huxley C, Farr C, Gennaro ML, Haaf T. Ordering up big MACs. Biotechnology (N Y) 1994;12(6):586–90. doi: 10.1038/nbt0694-586. [DOI] [PubMed] [Google Scholar]
- 13.Huxley C. Mammalian artificial chromosomes: a new tool for gene therapy. Gene Ther. 1994;1(1):7–12. [PubMed] [Google Scholar]
- 14.Hadlaczky G. Satellite DNA-based artificial chromosomes for use in gene therapy. Curr Opin Mol Ther. 2001;3(2):125–32. [PubMed] [Google Scholar]
- 15.Ng BL, Carter NP. Factors affecting flow karyotype resolution. Cytometry A. 2006;69(9):1028–36. doi: 10.1002/cyto.a.20330. [DOI] [PubMed] [Google Scholar]
- 16.Mills W, Critcher R, Lee C, Farr CJ. Generation of an approximately 2.4 Mb human X centromere-based minichromosome by targeted telomere-associated chromosome fragmentation in DT40. Hum Mol Genet. 1999;8(5):751–61. doi: 10.1093/hmg/8.5.751. [DOI] [PubMed] [Google Scholar]
- 17.Farr CJ, Stevanovic M, Thomson EJ, Goodfellow PN, Cooke HJ. Telomere-associated chromosome fragmentation: applications in genome manipulation and analysis. Nat Genet. 1992;2(4):275–82. doi: 10.1038/ng1292-275. [DOI] [PubMed] [Google Scholar]
- 18.Rabbitts P, Impey H, Heppell-Parton A, Langford C, Tease C, Lowe N, Bailey D, Ferguson-Smith M, Carter N. Chromosome specific paints from a high resolution flow karyotype of the mouse. Nat Genet. 1995;9(4):369–75. doi: 10.1038/ng0495-369. [DOI] [PubMed] [Google Scholar]
- 19.Carter NP, Ferguson-Smith MA, Perryman MT, Telenius H, Pelmear AH, Leversha MA, Glancy MT, Wood SL, Cook K, Dyson HM. Reverse chromosome painting: a method for the rapid analysis of aberrant chromosomes in clinical cytogenetics. J Med Genet. 1992;29(5):299–307. doi: 10.1136/jmg.29.5.299. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderbyl S, MacDonald N, de Jong G. A flow cytometry technique for measuring chromosome-mediated gene transfer. Cytometry. 2001;44(2):100–5. doi: 10.1002/1097-0320(20010601)44:2<100::aid-cyto1087>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Vanderbyl S, MacDonald GN, Sidhu S, Gung L, Telenius A, Perez C, Perkins E. Transfer and stable transgene expression of a mammalian artificial chromosome into bone marrow-derived human mesenchymal stem cells. Stem Cells. 2004;22(3):324–33. doi: 10.1634/stemcells.22-3-324. [DOI] [PubMed] [Google Scholar]
- 22.Co DO, Borowski AH, Leung JD, van der Kaa J, Hengst S, Platenburg GJ, Pieper FR, Perez CF, Jirik FR, Drayer JI. Generation of transgenic mice and germline transmission of a mammalian artificial chromosome introduced into embryos by pronuclear microinjection. Chromosome Res. 2000;8(3):183–91. doi: 10.1023/a:1009206926548. [DOI] [PubMed] [Google Scholar]
- 23.Oberle V, de Jong G, Drayer JI, Hoekstra D. Efficient transfer of chromosome-based DNA constructs into mammalian cells. Biochim Biophys Acta. 2004;1676(3):223–30. doi: 10.1016/j.bbaexp.2003.12.003. [DOI] [PubMed] [Google Scholar]


