Abstract
Background
The concept of ribosomal constraints on rRNA genes is deduced primarily based on the comparison of consensus rRNA sequences between closely related species, but recent advances in whole-genome sequencing allow evaluation of this concept within organisms with multiple rRNA operons.
Methodology/Principal Findings
Using the 23S rRNA gene as an example, we analyzed the diversity among individual rRNA genes within a genome. Of 184 prokaryotic species containing multiple 23S rRNA genes, diversity was observed in 113 (61.4%) genomes (mean 0.40%, range 0.01%–4.04%). Significant (1.17%–4.04%) intragenomic variation was found in 8 species. In 5 of the 8 species, the diversity in the primary structure had only minimal effect on the secondary structure (stem versus loop transition). In the remaining 3 species, the diversity significantly altered local secondary structure, but the alteration appears minimized through complex rearrangement. Intervening sequences (IVS), ranging between 9 and 1471 nt in size, were found in 7 species. IVS in Deinococcus radiodurans and Nostoc sp. encode transposases. T. tengcongensis was the only species in which intragenomic diversity >3% was observed among 4 paralogous 23S rRNA genes.
Conclusions/Significance
These findings indicate tight ribosomal constraints on individual 23S rRNA genes within a genome. Although classification using primary 23S rRNA sequences could be erroneous, significant diversity among paralogous 23S rRNA genes was observed only once in the 184 species analyzed, indicating little overall impact on the mainstream of 23S rRNA gene-based prokaryotic taxonomy.
Introduction
Ribosomes are the large, ribonucleoprotein machinery in which proteins are synthesized. The structure of ribosomes is largely conserved amongst the three kingdoms of life. In prokaryotes, the ribosome is composed of two subunits: a large 50S subunit, and a small 30S subunit. The 50S subunit contains a 23S, a 5S rRNA, and more than 30 proteins. The 30S subunit contains a 16S rRNA plus 20 proteins. Precise spatial relationships may be essential for assembly of functional ribosomes, constraining ribosomal RNA genes from drastic change [1].
Because of functional constraints on the structure of rRNA gene sequences, certain regions in rRNA genes that are in contact with other components in the ribosome are conserved, while sequences between the conserved regions mutate at faster rates. The conserved regions are useful for determining distant relationships, while the “fast” regions are useful for distinguishing closely-related organisms. Horizontal gene transfer events that could falsify the evolutionary history of an organism are unlikely to occur in the highly constrained rRNA genes[2], [3], [4]. These features make rRNA genes the most suitable molecular chronometer for both phylogenetic analysis and taxonomic classification of cellular organisms [5].
In general, 16S and 23S rRNA based phylogenetic trees are in good agreement[6], [7], while 5S rRNA is considered to not contain sufficiently long sequences for statistically significant comparisons. The detailed and comprehensive phylogenies inferred from 16S as well as 23S rRNA sequence comparisons provide three primary domains [5], [8] the Bacteria, Archaea, and Eukarya. However, 23S rRNA has lost favor to 16S rRNA in phylogenetic analysis and is rarely used in taxonomic classification because of the lack of established broad-range sequencing primers and the difficulty of sequencing larger genes with early sequencing technology.
There has been renewed interest in the use of the 23S rRNA gene, driven by the decrease in sequencing costs with next generation DNA sequencing technology (454 sequencing) and demand from the new Roadmap Initiative in the Human Microbiome Project (http://nihroadmap.nih.gov/hmp/). Compared to 16S rRNA genes, 23S rRNA genes contain more characteristic sequence stretches due to a greater length, unique insertions and/or deletions, and possibly better phylogenetic resolution because of higher sequence variation [9]. A recent study indicated that 23S rRNA genes also contain conserved regions for designing broad-range primers with a similar degree of universality to the broad-range primers for 16S rRNA genes [10].
Gene redundancy is uncommon in prokaryotic genomes, yet the rRNA genes can vary from one to as many as 15 copies in a single genome [11]. Divergent evolution between rRNA genes in the same genome may corrupt the record of evolutionary history and obscure the true identity of an organism. When substantial variation occurs, use of rRNA gene sequences may lead to the artificial classification of an organism into more than one species. In this study, we performed a systematic survey of intragenomic variation of 23S rRNA genes in genomes representing 184 prokaryotic species.
Materials and Methods
Annotation of 23S rRNA genes
Gene sequences were from the Complete Microbial Genomes database at the NCBI website (http://www.ncbi.nlm.nih.gov/genomes/MICROBES/Complete.html). For some species, more than one genome was available. Comparison of homologous 23S rRNA genes between these genomes did not show >3% sequence difference (data not shown). To avoid duplicating any species, only the most completely annotated genome was included for analysis. Lengths of 23S rRNA genes were identified by using experimentally defined 23S rRNA sequences from the closest relatives available and verified by 2° structure analysis based on minimizing free energy, using RNAstructure [12] and Rnaviz [13], with experimentally defined 23S rRNA or the consensus 23S rRNA models [14] used for reference. The number of copies of 23S rRNA genes present in a genome was determined by whole genome BLAST search based on the known 23S rRNA sequence.
Analysis of intragenomic diversity in 23S rRNA genes
Genomes that contained only a single 23S rRNA gene were not further analyzed. Copies of 23S rRNA genes from each remaining genome were aligned with Clustalw [15]. To calculate diversity, the number of revealed substitutions including point mutations and insertions/deletions (indels) was divided by the total number of positions, including gaps in the alignment.
Comparison of 2° structures
To compare two related 2° structures, a mismatch was defined as conserved if located in a loop, or located in a stem but causing GC:GU conversions or covariation resulting in no change in base-pairing. In contrast, a non-conserved mismatch altered base-pairing. Substitutions also were classified by the position-specific relative variability rate calculated from the consensus 23S rRNA model based on an alignment of 187 bacterial 23S rRNA genes [14]. Positions were classified as variable or non-variable positions, according to the substitution rate relative to the average substitution rate of all sites [14]. The relative substitution rate for a variable position v>1 indicates a substitution rate higher than that averaged for all sites in the 23S rRNA gene analyzed, while a conserved position had a relative substitution rate v<1; uncommon sites are positions occupied in <25% of organisms due to insertions. The expected variability for certain classes of positions was calculated from the consensus models. Differences between expected and observed variability was analyzed by Chi-Square analysis, considered significant if p<0.05.
Experimental verification of 23S rRNA gene sequences
Representative 23S rRNA gene sequences containing intervening sequences were experimentally verified by sequence-based PCR using allele-specific primers (Table S1). Bacterial genomic DNA was prepared from strains used in the specific bacterial genome projects. The borders of the 23S rRNA gene sequence and inserts were amplified and sequenced. The sequences obtained were compared with sequences of the corresponding 23S rRNA genes from the edited whole genomes to identify discrepancies.
Results
rRNA gene database
In total, 342 complete prokaryotic genomes were available for analysis, 27 from Archaea and 316 from Bacteria. Of the 342 genomes, 73 redundant genomes were removed from the database, as were 85 genomes that contained only a single rrn operon. Remaining were 184 unique (10 Achaea, 174 Bacteria) species whose genomes contained multiple 23S rRNA genes (Table S2). The 184 species represented 11 phyla, of which Proteobacteria was the most abundant phylum (98 species) followed by Firmicutes (43 species), Euryarchaeota (10 species), Actinobacteria (9 species) (Table S2). The remaining 7 phyla were represented by only 24 species.
Diversity of 23S rRNA genes in the primary structure
The 184 genomes had a median of 4.57 23S rRNA genes/genome (range 1 to 15). Diversity was found in 113 genomes, with mean 0.40% and range from 0.01% to 4.04% (median 0.24%, interquartile range (IQR) 0.10%–0.52% sequence variation). The threshold for identification of outliers, calculated as IQR±1.5 IQR, was 0–1.15%. Using this threshold, 8 genomes were identified as having high intragenomic variation amongst their paralogous 23S rRNA genes (Table 1). The eight outliers were concentrated in the four most abundant phyla, four in Proteobateria, two in Firmicutes, one in Actinobacteria, and one in Euryarchaeota. The absence of outliers in the remaining seven phyla is likely because they were least represented in the dataset. In 4 genomes, Carboxydothermus hydrogenoformans, Haloarcula marismortui, Shewanella oneidensis, and Streptococcus pyogenes, there was on average 1.52% diversity due to 44.6 substitutions including 8.8 indels (Table 1). In 3 genomes, Nocardia farcinica (1.18% diversity, 37 point mutations, 1 indel), Clostridium perfringens (1.96% diversity, 16 point mutations, 41 indels), and Salmonella typhimurium (1.27% diversity, 16 point mutations, 41 indels), substitutions tended to concentrate within short segments of 23S rRNA genes causing complex rearrangement of the secondary structure (Figure 1), which will be described in detail separately. Highest diversity was observed in Thermoanaerobacter tengcongensis (4.04% diversity, 107 point mutations, 8 indels) that also contained intervening sequences (IVS) (Figure 2), which will be described below.
Table 1. Genomes with significant intragenomic diversity among paralogous 23S rRNA genes.
| Organism | Accession number | Copy | Alignment (nt) | Diversity (%) | Substitution* | Covariation | G:U = G:C | In-loop | Stem-loop transition | Indel |
| Carboxydothermus hydrogenoformans | CP000141 | 4 | 2921 | 1.30 | 38 | 4 | 2 | 25 | 7 | 2 |
| Haloarcula marismortui | AY596297 | 3 | 2922 | 1.54 | 45 | 24 | 2 | 11 | 8 | 0 |
| Shewanella oneidensis | AE014299 | 9 | 2894 | 1.17 | 34 | 22 | 4 | 5 | 3 | 1 |
| Streptococcus pyogenes | AE004092 | 6 | 2912 | 1.65 | 48 | 22 | 8 | 11 | 7 | 8 |
| Average | 6.4 | 2912 | 1.52 | 44.6 | 14.4 | 4 | 14.4 | 8.2 | 8.8 | |
| Nocardia farcinica | AP006618 | 3 | 3132 | 1.18 | 37 | Complex rearrangement** | 1 | |||
| Clostridium perfringens | BA000016 | 10 | 2915 | 1.96 | 57 | 41 | ||||
| Salmonella typhimurium | AE006468 | 7 | 3093 | 1.27 | 39 | 3 | ||||
| Thermoanaerobacter tengcongensis | AE008691 | 4 | 2965 | 4.04 | 115 | 24 | 19 | 62 | 10 | 8 |
Substitution includes both point mutations and indels. **See Figure 1.
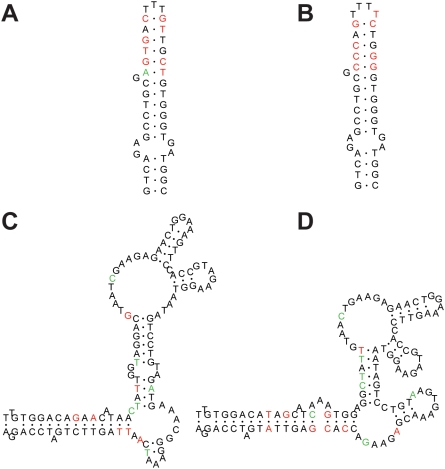
Figure 1. Conservation of 2° structure by complex rearrangement of base pairing and substitutions in rrn23S of Nocardia farcinica (A and B) and Clostridium perfringens (C and D).
Nucleotides related to substitutions are highlighted in red and indels in green. Segments of rrn23S of Nocardia farcinica shown correspond to positions 620 through 659 of rrnC23S. The segments of rrnC23S (A) and rrnB23S (B) differ by 9 positions, including one indel and eight substitutions. Segments of rrn23S of Clostridium perfringens shown correspond to positions 292 through 406 of rrnH23S. The segments of rrnH23S (C) and rrnD23S (D) differ by 22 positions, including 13 indels and 8 substitutions.
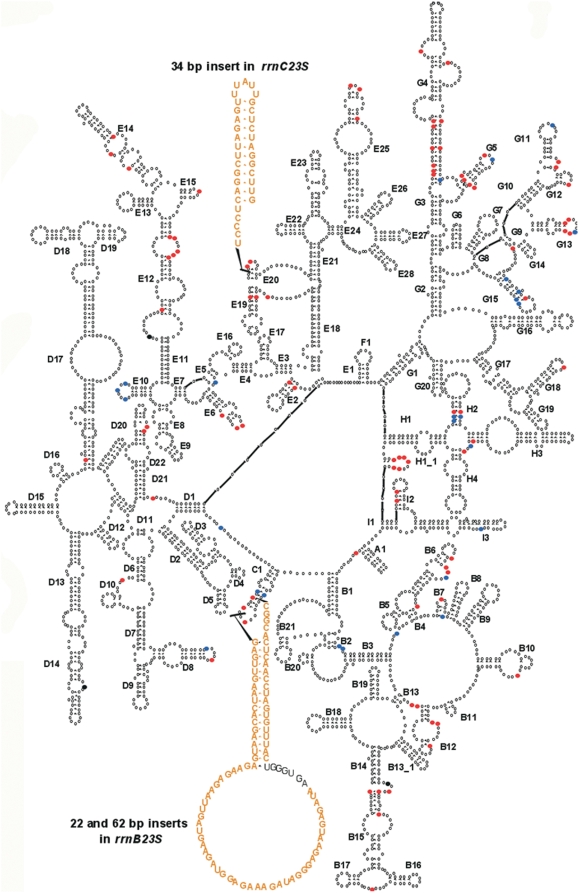
Figure 2. Distribution of substituted positions and IVS in 23S rRNA genes of T. tengcongensis.
Secondary structure of rrnB16S and rrnB23S was based on the 2° structure models for Thermus thermophilus [14]. Substituted positions between rrnB23S and rrnC23S are highlighted in colors according to the position-specific relative variability rate calculated from the consensus rrn23S model based on an alignment of 184 bacterial 23S rRNA genes [14]. A position with a relative substitution rate v>1 (red) implies that it has a substitution rate higher than the average substitution rate of all its sites in the rRNA gene analyzed, while v<1 (blue) indicates that the rate is lower than the average rate. Uncommon sites are positions that are occupied in <25% of organisms because of insertions, which are shown by black dots.
Effect on secondary structure
In T. tengcongensis, compared with the consensus 2° structure model of 23S rRNA [14] (Figure 2), 86 (74.8%) of the 115 mismatch positions occurred at highly variable positions - significantly higher than expected (40.4%, p<0.0001) (Table 2). Comparing the 2° structures of rrnB23S and rrnC23S, 105 of the 115 substitutions were conservative (62 in loops [including 4 insertions], 24 covariations, and 19 GU:GC conversions). Only 10 (9 point mutations and 1 single nucleotide insertion) of the 115 substitutions altered the 2° structure by causing stem-loop transition. These features indicate strong structural constraints, suggesting that rrnC23S may be functional.
Table 2. Observed and expected variability in 23S rRNA genes in T. tengcongensis.
| Relative variability(i) | Observed substitution rate (%) | Expected substitution rate(%) | P value |
| i>1 | 78.4 (86/115) | 40.4 (1175/2912) | <0.001 |
| I<1 | 22.6 (26/115) | 58.6 (1707/2912) | <0.001 |
| Uncommon sites | 2.6 (3/115) | 1.0 (30/2912) | 0.110 |
Besides T tengcongensis, conservation of the 2° structure also is apparent despite changes in the primary structure (Table 1) in other species. In C. hydrogenoformans, H. marismortui, S. oneidensis, and S. pyogenes, there are on average 44.6 substitutions per genome, of which only ∼20% (8.2 substitutions) may potentially cause stem-loop transition. The effect of other substitutions are minimized by either covariations (14.4 substitutions), G:U to G:C transitions (4 substitutions) or in-loop substitutions (14.4). Complex rearrangements of the 2° structure likely occurred in N. farcinica, C. perfringens, and S. typhimurium as a result of changes in the primary structure (Figure 1). The complex rearrangement appears to be associated with indels. One example is between rrnC23S and rrnB23S of Nocardia farcinica in which a single deletion accompanied 8 point mutations in a segment of 40 nt. The topographic structure can be conserved by repositioning the bases rrnB23S although the resultant stem is one base pair shorter and the loop is one base larger (Figure 1A and 1B). Another example is the rrnH23S and rrnD23S of C. perfringens in which there are 13 indels and 8 point mutations in a segment of 115 nt. Although there was an apparent change in the 2° structure, rearrangement of the positions of the loops and the length of the stems appear to have minimized the effect on the topographic structure (Figure 1C and 1D). Variation of rrn23S in S. typhimurium has been previously reported [16].
Intervening sequences
Another type of alteration of 23S rRNA genes is insertion of an intervening sequence (Table 3). IVS was found in 7 genomes (Table 3). If the IVS are not included in calculations, the remaining sequences of paralogous 23S rRNA genes have <1% diversity in 6 of the 7 genomes except for T. tengcongensis whose diversity is due to both IVS-like insertions and a high level of substitutions.
Table 3. Genomes with intervening sequences in 23S rRNA genes.
| Species | Accession # | No. of copies | Length Range (nt) | IVS (nt) | Diversity % | Type of IVS |
| Agrobacterium tumefaciens | AE007869.2 | 4 | 2233–2252 | 9,13 | 0.67 | Short IVS |
| Deinococcus radiodurans | AE000513.1 | 3 | 2877–3944 | 1067 | 0.07 | IVS encoding transposon |
| Nostoc sp. | BA000019.2 | 4 | 2828–4299 | 1471 | 0 | IVS encoding transposon |
| Photobacterium profundum | CR354531.1 | 15 | 2894–2926 | 14,17 | 0.83 | Short IVS |
| Photorhabdus luminescens | BX470251.1 | 7 | 2909–3021 | 112 | 0.96 | IVS |
| Salmonella typhimurium | AE006468.1 | 7 | 2994–3093 | 83, 99 | 1.27 | IVS |
| Thermoanaerobacter tengcongensis | AE008691.1 | 4 | 2881–2965 | 22, 62, 34 | 4.04 | IVS and mutations |
Transposon-mediated insertion of IVS
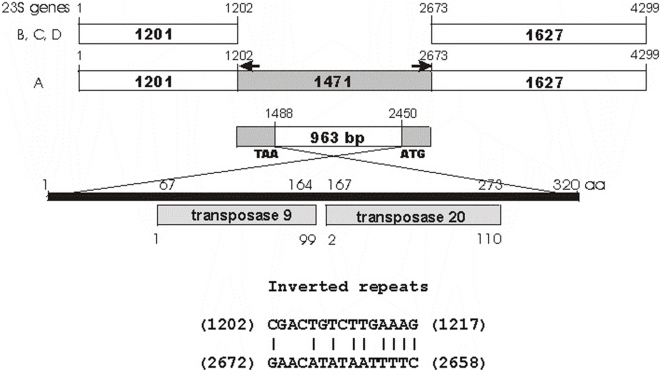
Nostoc sp PCC7120 contained four 23S rRNA genes; rrnA23S is 4299-nt, while the other three each are 2828-nt [17]. The variation was due to the presence of a 1471-nt insert in rrnA23S (Figure 3), including 15-nt imperfect inverted repeats at both ends of the insertion and a 963-nt ORF. The deduced protein is predicted to be a transposase in Clusters of Orthologous Group (COG) COG3547 (Expected value = 7e−27). The 303-amino acid of COG3547 consensus consists of both transposases 9 and 20. Blast of the 1471-nt insert against the genome of Nostoc sp PCC7122 revealed 11 other homologs, each with the terminal inverted repeats. Without the insertion, all four rrn23S copies were identical.
Figure 3. IVS in Nostoc sp (strain PCC 7120) 23S rRNA genes.
A 1471-bp IVS flanked by partial 16-bp inverted repeats (arrows) was found in rrnA23S, which contains a 963-bp open reading frame that encodes proteins with strong homology (Expected value = 7e−27) to transposases 9 and 20 in COG3547.
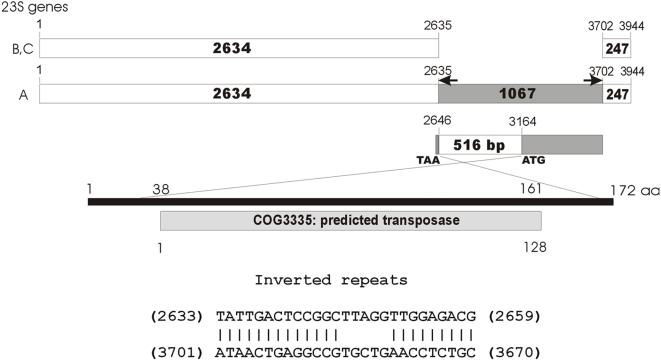
The 23S rRNA genes of Deinococcus radiodurans were only partially annotated (GenBank accession: AE000513) [18]. The full-length 23S rRNA genes were identified by search of the D. radiodurans genome using the unassigned RNA (GenBank accession:30749903) associated with the 50S ribosomal subunit [19]. Three putative 23S rRNA genes were identified. The 2° structures of the putative 23S rRNA genes were compared with the consensus prokaryotic 23S rRNA model to confirm identity and to define length [14]. rrnC23S was 3944-nt while rrnA23S and rrnB23S were only 2877-nt (Figure 4). rrnC23S contains a 1067-nt insertion including a 519-nt ORF encoding a putative172-amino acid protein. The protein was predicted to be a transposase in COG3335 (Expected value = 8e−14) and 27-nt imperfect inverted repeats were present at both ends of the insert. BLAST of the 1067-nt insert against the D. radiodurans genome revealed two other nearly identical (>98% homology) copies, including the inserted repeats, located on plasmids MP1 and CP1. rrnA23S and rrnB23S were nearly identical. Excluding the insertion, only six single-nucleotide substitutions were observed between the three 23S rRNA genes.
Figure 4. IVS in Deinococcus radiodurans 23S rRNA genes.
rrnA23S contains a 1067-bp IVS flanked by 27-bp inverted repeats. The arrows indicate the location of the inverted repeats. The 1067-bp insert contains a 516-bp open reading frame encoding a 172-amino acid protein with strong homology (Expected value = 8e−14) to predicted transposase COG3335.
Small IVS
Salmonella typhimurium has seven 23S rRNA genes with sizes ranging from 2994 to 3093 bp [20]. Two insertions were found, 83-nt and 99-nt, consistent with previously described IVS in S. typhimurium [16] (Table 3). The largest 23S rRNA gene contains both inserts. The second largest 23S rRNA gene contains the 98-nt insert. The remaining five 23S rRNA genes only contain the 83-nt insert. The largest difference (1.27%, 37/2903) was found between rrnB23S and rrnF23S with 37 substitutions (Table 1).
Photorhabdus luminescens contains seven 23S rRNA genes [21]. rrnC23S and rrnE23S contain a shared 112-nt insert (Table 3). The other five 23S rRNA genes are 2909 nt in length. The insert is similar to those from S. typhimurium in size and in that it does not contain an ORF. No repetitive DNA was present in or near the insertion. Excluding the insert, the largest difference was observed between rrnD23S and rrnE23S, differing by 28 nt (0.96%). No sequences homologous to the insert were found in P. luminescens or GenBank.
Photobacterium profundum contains 15 paralogous 23S rRNA genes ranging from 2893–2926 nt in size (GeneBank accession: CR354531.1, unpublished). The size variation was caused by two IVS of 14 and 17 nt, respectively. Maximal diversity was 0.83% (24/2893) between rrnC23S and rrnJ23S (Table 3). No additional copy of the IVS was found outside of the 23S rRNA genes within the P. profundum genome.
Agrobacterium tumefaciens contains four copies of 23S rRNA genes, two on each of its linear and circular chromosomes, respectively [22]. The sizes of the 23S rRNA genes vary between 2233 and 2252 nt because the second 23S rRNA gene is shorter, caused by two small IVS of 9 and 13 nt, respectively (Table 3). Besides the IVS, 15 mutations were observed (0.67% diversity).
IVS and point mutations
T. tengcongensis had four 23S rRNA genes with sizes ranging from 2881 to 2965-nt, reflecting 3 major alleles [23]. The largest difference was between rrnB23S and rrnC23S, due to inserts of 22, 62, and 34-nt (Figure 2, Table 1) and 115 substitutions. The 22 and 62-nt inserts were present in rrnA23S and rrnB23S, respectively, the 34-nt insert in rrnC23S, and none in rrnD23S. No sequences homologous to the three inserts were found in GenBank. Excluding the 3 inserts, there remained differences of 4.0% (115/2884) between rrnB23S and rrnC23S.
Verification of 23S rRNA gene sequences by PCR-based sequencing
To examine the reliability of the whole genome sequences, we experimentally verified the IVS of T. tengcongensis, D. radiodurans, and Nostoc sp. The predicted junctions between the rRNA sequences and the IVS were present in all three strains tested. The sequences of cloned PCR products are identical to those published in the genome studies, providing validation of the presence of the diversity observed in the whole genome sequences. Because we did not experimentally verify the IVS of all species, our results are limited, although in total, the data suggest that the whole genome sequences are highly reliable.
Discussion
This is the first large scale survey of intragenomic variation of paralogous 23S rRNA genes. We analyzed 23S rRNA genes from genomes representing 184 prokaryotic species to examine for evidence of ribosomal constraint of rRNA structures at the intragenomic level. Our findings support the hypothesis that individual 23S rRNA genes within a genome are conserved due to such structural constraints.
One level of ribosomal constraint on 23S rRNA genes was observed at the level of primary structure. In the large majority of the genomes (183/184) analyzed, variation of primary structure among paralogous 23S rRNA genes was small (<3%, which is the 16S rrn-based species boundary) [24]. In our dataset, 38% of the 184 genomes containing multiple paralogous 23S rRNA genes were invariable, similar to the finding in 16S rRNA genes [25]. Our findings of 0.4% average diversity for intragenomic variation of rRNA sequences among the 113 diversified genomes is higher than the reported 0.17% diversity among 16S rRNA genes [26], but will be more similar to the 16S rRNA genes diversity (0.25%) if all 184 genomes are included in the calculation (Table S2). Excluding T. tengcongensis, which has unusually high diversity among 23S rRNA genes, the maximal diversity among paralogous 23S rRNA genes is 1.96%, higher than the maximal variation of 1.26% among 16S rRNA genes [27]. In organisms containing multiple rRNA genes, the homogeneity of primary sequences is believed to be maintained through gene conversion by homologous recombination [2], [28]. The evolution of these paralogous rRNA genes is concerted in such a fashion that within a species the rRNA genes are nearly identical, whereas orthologous rRNA genes between species can vary considerably [2], [28]. However, our study suggests that homologous recombination is not always efficient in keeping the primary structure in check. In T. tengcongensis, the 4% difference (without considering the IVS) in primary structure between rrnB23S and rrnC23S is in agreement with the high % divergence reported between 16S rRNA paralogues [25]. It is tempting to attribute the unusual variation to the presence of IVS in the T. tengcongensis 23S rRNA genes, but variation amongst 23S rRNA genes in six other genomes containing IVS is much lower (Table 3). It appears that gene conversion or concerted evolution is one, but not the only, way to meet the requirement of ribosomal constraint.
Another level of ribosomal constraint was observed at the level of 2° structures. For example, the detailed analysis of the 23S rRNA genes of T. tengcongensis showed only in uncommon instance (10 of 115 point substitutions) a point substitution altered the 2° structure. This observation is in line with the current concept derived from comparing consensus rRNA sequences from closely related species [29], [30], [31], [32], [33] in that selection pressure from ribosomal constraints is to preserve 2° structures that are essential for the assembly of functional ribosomes [34]. In organisms with a single rRNA operon, ribosomal constraints could follow a simple birth-and-death model by linking the fate of a mutation to the viability of the organisms. However, in an organism with multiple rRNA operons, the nature of the selection pressure is unclear. In Escherichia coli, which has seven rRNA operons, after deletion of the other 6 operons only a single operon is sufficient to produce >50% of wild-type rRNA levels and support >50% of the wild-type growth rate [35]. This experiment suggests that in an organism with multiple rRNA operons, it may not be necessary for survival to keep all rRNA genes under ribosomal constraint. The observed tight constraints on individual 23S rRNA in T. tengcongensis needs a new explanation. T. tengcongensis, isolated from a fresh water hot spring in Tengchong, China, is a rod-shaped, gram-negative bacterium that grows anaerobically in extreme environments. It propagates at wide ranges of temperatures from 50° to 80°C and pH values between 5.5 and 9 [23]. It is possible that all four 23S rRNA genes are essential for survival of T. tengcongensis in its natural inhabit, though one might be sufficient under laboratory conditions. It also is possible that the divergence of 23S rRNA genes offers competitive advantage in a changing environment in nature and thus are selectively maintained. Ribosomal constraints could be met by maintaining conserved 2° structures through conserved point mutations under sufficient selective pressure to resist the tide of homologous recombination.
Our finding is consistent with the finding by Acinas et al. in rrnC16S of T. tengcongensis [25]. Both studies indicate that the 2° structures are conserved despite a high level of change in the primary structures and suggest that the rrnC operon is functional. Although Acinas et al. suggested that the rrnC operon could be brought into T. tengcongensis by horizontal gene transfer, the hypothetic donor has yet to be identified. The ribosomal constraint observed at the level of 2° structures is also evident in 23S rRNA genes in C. hydrogenoformans, H. marismortui, S. oneidensis, and S. pyogenes.
Complex rearrangement of the position of nucleotides, the position and size of loops, and the length of stems might be another mechanism to conserve the functional structure of an rRNA gene. The examples shown in N. farcinica and C. perfringens are beyond simple alteration or conservation of the 2° structures since there is no straightforward way to quantify the changes and their effects. Rather, this type of rearrangement seems to aim at conserving the topographic structure in the presence of drastic changes in the primary and secondary structures. However, an experimental approach will be required to address the relationship between the conservation of the topographic structure and function of an rRNA gene.
The concept of rRNA conservation has revolutionized exploration of microbial ecosystems and identifying the causes of diseases of unknown etiologies [36], [37], [38], [39]. The most common molecular sequences used for phylogenetic analysis are ribosomal RNAs. The 16S rRNA genes have become the most widely used sequences for phylogenetic analysis as seen by the vast and growing database of 16S rRNA genes [40], [41]. The choice of 16S rRNA as the most widely used sequences for phylogenetic analysis has been based on the simple assumption that 16S rRNA is optimal because 5S rRNA is too short while 23S rRNA sequencing requires extra effort without a commensurate increase in phylogenetic signal. However, misidentification has been reported. Strains from different species may have identical 16S rRNA gene sequences, and strains of one species may have 16S rRNA that differ by >4% while 3% is considered as the threshold for within-species dissimilarity [42], [43], [44]. On the other hand, the main obstacle limiting 23S rRNA from being used in classification of microorganisms has significantly diminished, as the cost for DNA sequencing is becoming less expensive. Evaluation of intragenomic variation of the rRNA genes has become possible since more and more sequences of whole microbial genomes have recently become available. It would be a logical expectation that the intragenomic diversity of a marker for taxonomic classification of microorganisms is below the level of interspecies diversity. We evaluated the suitability of 23S rRNA genes in this regard. Our study indicates that high-level variation among paralogous 23S rRNA genes might exceed the level of variation among 23S rRNA genes from different strains within a species but will not have a significant effect on the integrity of a 23S rRNA-based phylogenetic system, because its occurrence was rare. The insertions encountered when using 23S rRNA genes in phylogenetic analysis can be easily recognized and eliminated in comparisons of homologous sequences.
Intervening sequences (IVS) were frequently observed in 23S rRNA genes. In the seven species in which IVS were identified, five species contain small IVS that did not contain any ORFs but in D. radiodurans and Nostoc sp, IVS were large and contained ORFs encoding putative transposases. Experimental studies have demonstrated that IVS in 23S rRNA does not interfere with its functions[45]. The 23S rRNA of Salmonella typhimurium LT2 are known to carry IVS at two sites, helix-25 and helix-45, which are excised by RNase III during rRNA maturation, resulting in rRNA which is fragmented, but nevertheless functional [45]. Fragmentation of the 23S rRNA also has been observed in diverse Eubacteria [46]. 23S rRNAs with insertions have been shown to rescue an E. coli strain in which all chromosomal rRNA operons were deleted [47].
Supporting Information
Bacterial strains and primers used in verification of IVS in 23S rRNA genes.
(0.04 MB DOC)
Intragenomic diversity of 23S rRNA genes in Bacteria and Archaea.
(0.26 MB DOC)
Acknowledgments
We thank Mr. Meisheng Zhou for assistance in verification of sequence diversity.
http://nihroadmap.nih.gov/hmp/; Roadmap Initiative in Human Microbiome Project.
http://www.ncbi.nlm.nih.gov/genomes/MICROBES/Complete.html; Completed Microbial Genomes database.
http://rtfm.arb-home.de; The ARB project (latin, “arbor” = tree) home page.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study is supported in part by NIH grants UH2CA140233, R01CA97946, and R01AI063477 and by the Medical Research Service of the Department of Veterans Affairs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Doolittle WF. Phylogenetic classification and the universal tree. Science. 1999;284:2124–2129. doi: 10.1126/science.284.5423.2124. [DOI] [PubMed] [Google Scholar]
- 2.Eickbush TH, Eickbush DG. Finely orchestrated movements: evolution of the ribosomal RNA genes. Genetics. 2007;175:477–485. doi: 10.1534/genetics.107.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santoyo G, Romero D. Gene conversion and concerted evolution in bacterial genomes. FEMS Microbiol Rev. 2005;29:169–183. doi: 10.1016/j.femsre.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Gurtler V. The role of recombination and mutation in 16S–23S rDNA spacer rearrangements. Gene. 1999;238:241–252. doi: 10.1016/s0378-1119(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 5.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Rijk P, Van de Peer Y, Van den Broeck I, De Wachter R. Evolution according to large ribosomal subunit RNA. J Mol Evol. 1995;41:366–375. doi: 10.1007/BF01215184. [DOI] [PubMed] [Google Scholar]
- 7.Cedergren R, Gray MW, Abel Y, Sankoff D. The evolutionary relationships among known life forms. J Mol Evol. 1988;28:98–112. doi: 10.1007/BF02143501. [DOI] [PubMed] [Google Scholar]
- 8.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwig W, Schleifer KH. Bacterial phylogeny based on 16S and 23S rRNA sequence analysis. FEMS Microbiol Rev. 1994;15:155–173. doi: 10.1111/j.1574-6976.1994.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 10.Hunt DE, Klepac-Ceraj V, Acinas SG, Gautier C, Bertilsson S, et al. Evaluation of 23S rRNA PCR primers for use in phylogenetic studies of bacterial diversity. Appl Environ Microbiol. 2006;72:2221–2225. doi: 10.1128/AEM.72.3.2221-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klappenbach JA, Saxman PR, Cole JR, Schmidt TM. rrndb: the Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res. 2001;29:181–184. doi: 10.1093/nar/29.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews DH, Disney MD, Childs JL, Schroeder SJ, Zuker M, et al. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc Natl Acad Sci U S A. 2004;101:7287–7292. doi: 10.1073/pnas.0401799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rijk P, De Wachter R. RnaViz, a program for the visualisation of RNA secondary structure. Nucleic Acids Res. 1997;25:4679–4684. doi: 10.1093/nar/25.22.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wuyts J, Van de Peer Y, De Wachter R. Distribution of substitution rates and location of insertion sites in the tertiary structure of ribosomal RNA. Nucleic Acids Res. 2001;29:5017–5028. doi: 10.1093/nar/29.24.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattatall NR, Sanderson KE. Salmonella typhimurium LT2 possesses three distinct 23S rRNA intervening sequences. J Bacteriol. 1996;178:2272–2278. doi: 10.1128/jb.178.8.2272-2278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko T, Nakamura Y, Wolk CP, Kuritz T, Sasamoto S, et al. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2001;8:227–253. doi: 10.1093/dnares/8.5.205. [DOI] [PubMed] [Google Scholar]
- 18.White O, Eisen JA, Heidelberg JF, Hickey EK, Peterson JD, et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlunzen F, Zarivach R, Harms J, Bashan A, Tocilj A, et al. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001;413:814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- 20.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 21.Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat Biotechnol. 2003;21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- 22.Wood DW, Setubal JC, Kaul R, Monks DE, Kitajima JP, et al. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science. 2001;294:2317–2323. doi: 10.1126/science.1066804. [DOI] [PubMed] [Google Scholar]
- 23.Bao Q, Tian Y, Li W, Xu Z, Xuan Z, et al. A complete sequence of the T. tengcongensis genome. Genome Res. 2002;12:689–700. doi: 10.1101/gr.219302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stackebrandt E, Goebel BM. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. 1994. pp. 846–849.
- 25.Acinas SG, Marcelino LA, Klepac-Ceraj V, Polz MF. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J Bacteriol. 2004;186:2629–2635. doi: 10.1128/JB.186.9.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart FJ, Cavanaugh CM. Intragenomic variation and evolution of the internal transcribed spacer of the rRNA operon in bacteria. J Mol Evol. 2007;65:44–67. doi: 10.1007/s00239-006-0235-3. [DOI] [PubMed] [Google Scholar]
- 27.Coenye T, Vandamme P. Intragenomic heterogeneity between multiple 16S ribosomal RNA operons in sequenced bacterial genomes. FEMS Microbiol Lett. 2003;228:45–49. doi: 10.1016/S0378-1097(03)00717-1. [DOI] [PubMed] [Google Scholar]
- 28.Liao D. Gene conversion drives within genic sequences: concerted evolution of ribosomal RNA genes in bacteria and archaea. J Mol Evol. 2000;51:305–317. doi: 10.1007/s002390010093. [DOI] [PubMed] [Google Scholar]
- 29.Zwieb C, Glotz C, Brimacombe R. Secondary structure comparisons between small subunit ribosomal RNA molecules from six different species. Nucleic Acids Res. 1981;9:3621–3640. doi: 10.1093/nar/9.15.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiegler P, Carbon P, Ebel JP, Ehresmann C. A general secondary-structure model for procaryotic and eucaryotic RNAs from the small ribosomal subunits. Eur J Biochem. 1981;120:487–495. doi: 10.1111/j.1432-1033.1981.tb05727.x. [DOI] [PubMed] [Google Scholar]
- 31.Curtiss WC, Vournakis JN. Quantitation of base substitutions in eukaryotic 5S rRNA: selection for the maintenance of RNA secondary structure. J Mol Evol. 1984;20:351–361. doi: 10.1007/BF02104741. [DOI] [PubMed] [Google Scholar]
- 32.Hori H, Osawa S. Evolutionary change in 5S rRNA secondary structure and a phylogenic tree of 352 5S rRNA species. Biosystems. 1986;19:163–172. doi: 10.1016/0303-2647(86)90037-7. [DOI] [PubMed] [Google Scholar]
- 33.Gutell RR, Larsen N, Woese CR. Lessons from an evolving rRNA: 16S and 23S rRNA structures from a comparative perspective. Microbiol Rev. 1994;58:10–26. doi: 10.1128/mr.58.1.10-26.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgs PG. Compensatory neutral mutations and the evolution of RNA. Genetica. 1998;102–103:91–101. [PubMed] [Google Scholar]
- 35.Asai T, Zaporojets D, Squires C, Squires CL. An Escherichia coli strain with all chromosomal rRNA operons inactivated: complete exchange of rRNA genes between bacteria. Proc Natl Acad Sci U S A. 1999;96:1971–1976. doi: 10.1073/pnas.96.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, et al. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci U S A. 2004;101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Relman DA. The search for unrecognized pathogens. Science. 1999;284:1308–1310. doi: 10.1126/science.284.5418.1308. [DOI] [PubMed] [Google Scholar]
- 40.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandamme P, Harrington CS, Jalava K, On SL. Misidentifying helicobacters: the Helicobacter cinaedi example. J Clin Microbiol. 2000;38:2261–2266. doi: 10.1128/jcm.38.6.2261-2266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberreuter H, Charzinski J, Scherer S. Intraspecific diversity of Brevibacterium linens, Corynebacterium glutamicum and Rhodococcus erythropolis based on partial 16S rDNA sequence analysis and Fourier-transform infrared (FT-IR) spectroscopy. Microbiology. 2002;148:1523–1532. doi: 10.1099/00221287-148-5-1523. [DOI] [PubMed] [Google Scholar]
- 44.Harrington CS, On SL. Extensive 16S rRNA gene sequence diversity in Campylobacter hyointestinalis strains: taxonomic and applied implications. Int J Syst Bacteriol. 1999;49 Pt 3:1171–1175. doi: 10.1099/00207713-49-3-1171. [DOI] [PubMed] [Google Scholar]
- 45.Burgin AB, Parodos K, Lane DJ, Pace NR. The excision of intervening sequences from Salmonella 23S ribosomal RNA. Cell. 1990;60:405–414. doi: 10.1016/0092-8674(90)90592-3. [DOI] [PubMed] [Google Scholar]
- 46.Evguenieva-Hackenberg E. Bacterial ribosomal RNA in pieces. Mol Microbiol. 2005;57:318–325. doi: 10.1111/j.1365-2958.2005.04662.x. [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama T, Suzuki T. Ribosomal RNAs are tolerant toward genetic insertions: evolutionary origin of the expansion segments. Nucleic Acids Res. 2008;36:3539–3551. doi: 10.1093/nar/gkn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial strains and primers used in verification of IVS in 23S rRNA genes.
(0.04 MB DOC)
Intragenomic diversity of 23S rRNA genes in Bacteria and Archaea.
(0.26 MB DOC)