Abstract
Objective
To improve the diagnostic efficacy of transrectal ultrasound (TRUS)-guided targeted prostatic biopsies, we have suggested the use of a new scoring system for the prediction of malignancies regarding the characteristics of focal suspicious lesions as depicted on TRUS.
Materials and Methods
A total of 350 consecutive patients with or without prostate cancer who underwent targeted biopsies for 358 lesions were included in the study. The data obtained from participants were randomized into two groups; the training set (n = 240) and the test set (n = 118). The characteristics of focal suspicious lesions were evaluated for the training set and the correlation between TRUS findings and the presence of a malignancy was analyzed. Multiple logistic regression analysis was used to identify variables capable of predicting prostatic cancer. A scoring system that used a 5-point scale for better malignancy prediction was determined from the training set. Positive predictive values for malignancy prediction and the diagnostic accuracy of the scored components with the use of receiver operating characteristic curve analysis were evaluated by test set analyses.
Results
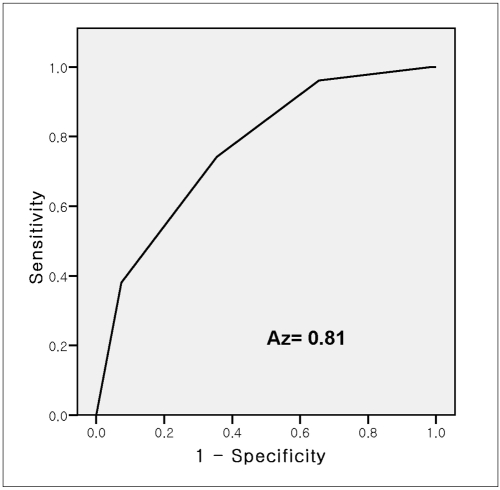
Subsequent multiple logistic regression analysis determined that shape, margin irregularity, and vascularity were factors significantly and independently associated with the presence of a malignancy. Based on the use of the scoring system for malignancy prediction derived from the significant TRUS findings and the interactions of characteristics, a positive predictive value of 80% was achieved for a score of 4 when applied to the test set. The area under the receiver operating characteristic curve (AUC) for the overall lesion score was 0.81.
Conclusion
We have demonstrated that a scoring system for malignancy prediction developed for the characteristics of focal suspicious lesions as depicted on TRUS can help predict the outcome of TRUS-guided biopsies.
Keywords: Prostate biopsy, Transrectal ultrasound, Prostate cancer diagnosis
Transrectal ultrasound (TRUS) is generally recognized as the method of choice for prostatic biopsy guidance, however, only 20% of urologists perform targeted biopsies based on sonographic findings (1, 2). Most contemporary prostate biopsy protocols have concentrated on the use of the systematic prostate biopsy approach and currently require the acquisition of 10 or more cores (3). Ultrasound (US) criteria used to identify and characterize suspicious lesions for diagnosing a malignancy are controversial and have not been well defined (1, 4). Moreover, the low positive predictive value (PPV) for the presence of prostate cancer remains a considerable weakness (5, 6). Although increased cancer detection has been reported for the use of color Doppler US (7, 8), the combined sensitivity of gray-scale and color Doppler imaging is insufficient to preclude the need for systemic biopsies (6, 9). Recently, identification of prostatic lesions with the use of TRUS has been reemphasized in recently published studies. Djavan and Margreiter (10) have suggested that taking the endosonographic morphology of the prostate gland into consideration for prostate biopsy strategies may improve the quality of the prostate biopsy. Furthermore, according to Shim et al. (11), men with suspicious lesions depicted on TRUS had a higher risk of being diagnosed with prostate cancer.
The purpose of this study was to suggest the use of a new scoring system for malignancy prediction for focal suspicious lesions depicted on TRUS and to evaluate the diagnostic efficacy including determination of the PPV for TRUS guided biopsies. In the current study, we developed a new scoring system for malignancy prediction by using TRUS results from a training set of patients. We then evaluated the reliability of the criteria by applying the criteria in a "blinded" fashion to a test set of patients.
MATERIALS AND METHODS
Our Institutional Review Board approved this retrospective study and waived the requirement for patient informed consent.
Patients
Among patients referred for TRUS with abnormal prostate specific antigen (PSA) levels (> 3.0 ng/mL) or a palpable abnormality detected on a digital rectal examination (DRE) between July 2003 and December 2005, 350 consecutive patients underwent a targeted prostate biopsy for 358 suspicious lesions and were included in this study. Of these patients, 147 patients had prostatic cancer (mean age, 69.7 ± 8.0 years; age range, 49-94 years) and 203 patients had no malignancy (mean age, 64.1 ± 8.6 years; age range, 33-85 years).
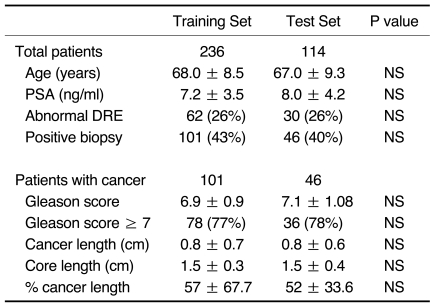
Patients and accompanying data were randomized into two groups; the training set (236 patients with 240 lesions) and the test set (114 patients with 118 lesions). Clinical profiles of the patients showed no statistically significant differences between the two groups (Table 1).
Table 1.
Randomization of Patients by Stratified Random Sampling
Note.-PSA = prostate-specific antigen, DRE = digital rectal examination, NS = not statistically significant
Data presented are median ± standard deviation or number.
US Examination and Interpretation
Transrectal US was performed by a uroradiologist with more than 10 years of uroradiology experience with the use of an iU 22 or HDI 5000 ultrasound scanner (Philips, Bothell, WA) equipped with a 9-4 MHz broadband curved array endocavitary transducer. TRUS included imaging in the transverse and sagittal planes using both gray-scale and color Doppler US. Gray-scale imaging was performed first, followed by color Doppler US imaging. The color window sector width was increased to include the entire transverse width of the gland. To optimize low-velocity flow detection, the pulse repetition frequency was set to 800 Hz with a wall filter of 50 Hz.
The following US characteristics of focal suspicious lesions in the training set (n = 240) were evaluated retrospectively by two radiologists in consensus, blinded to the pathological findings. The characteristics included unilaterality, location, echotexture, outline, definition, shape, vascularity and contour bulging. Lesion location was reported as "outer" when a lesion was located at the outer half of the peripheral zone, as "inner" when a lesion was located at the inner half of the peripheral zone or at the transition zone, or as "both" when a lesion was located in both halves of the peripheral zone. Echotexture was classified as homogeneous or heterogeneous. Lesion margin outline was classified as regular or irregular. The term "regular" was used only if a nodular lesion was round or oval. Lesions were classified as well-defined or ill-defined. In terms of lesion shape, three shapes were identified: nodular, band-like and clusters (i.e., clusters of millimetric foci). During color Doppler US examinations, blood flow patterns and intensities were evaluated for all of the lesions. Doppler amplification was controlled so that normal prostatic tissue did not display any noise. Positive contour-bulging was defined as asymmetric bulging of the contour of the prostate.
After US examinations, biopsies of suspicious lesions both for the training set and test set were performed by a radiologist using an automatic core biopsy device (Pro-Mag 2.2, Manan Medical Products, Northbrook, IL). In addition to twelve randomized biopsies, an additional prostate biopsy was performed targeting focal suspicious lesions. Pathology reports about targeted focal suspicious lesions were evaluated.
Data Analysis
For each of the US criteria (i.e., unilaterality, location, echotexture, outline, lesion definition, shape, vascularity and prostate contour), PPV, sensitivity and specificity for the detection of malignant lesions were calculated using standard procedures for the training set. Statistical significance was calculated using the chi-squared test and p values of < 0.05 were considered to indicate statistical significance. To determine which US finding best depicted prostate cancer, multiple logistic regression analysis was used to examine each US finding. The backward stepwise elimination technique was used to remove non-significant interactions. SPSS for Windows version 11.0 (SPSS, Chicago, IL) was used to perform all statistical computations.
Scoring System for Focal Suspicious Lesions Seen on Transrectal US
Two of the investigators developed a new diagnostic scoring system using US findings that were suggestive for the presence of a malignancy from the training set and scored 118 focal suspicious lesions seen on TRUS for the test set based on the use of the proposed scoring system. PPVs for the use of a five-point scale were calculated for malignancy and benignity. PPVs for malignancy and benignity of focal suspicious lesions in patients with a PSA of < 10 ng/mL were assessed. In addition, the diagnostic accuracy of the scored components was determined with the use of receiver operating characteristic curve (ROC) analysis. PPV differences between malignancy and benignity for each of the scores of the five-point scale were statistically evaluated using the chi-squared test. The PPV of a systemic biopsy only and systemic biopsy plus targeted biopsy were calculated on a per-patient basis, and were statistically compared with the use of McNemar's test.
RESULTS
Evaluation of the US Findings of Lesions Determined as Suspicious by the Use of Transrectal US in the Training Set
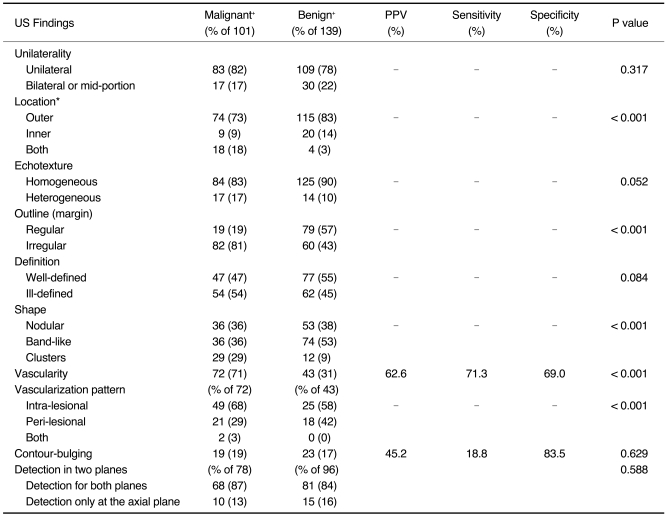
Of the US characteristics evaluated for all suspicious lesions, location, shape, outline, vascularity and vascular pattern were found to be statistically significant indicators for the presence of prostate cancer (Table 2). An inner location was found in 14% of benign lesions and in 9% of malignant lesions (χ2 test, p < 0.001). A statistically significant difference was also found for the number of lesions with a band-like shape, which was more often present in benign lesions (53.2%) than in malignant lesions (35.6%; χ2 test, p < 0.001). Furthermore, an irregular outline was observed for 81% of malignant lesions and for 43% of benign lesions (χ2 test, p < 0.001). In addition, it was determined that vascularity was associated with prostate cancer. Increased vascularity was found in 71% of malignant lesions but in only 31% of benign lesions (χ2 test, p < 0.001).
Table 2.
Evaluation of US Characteristics of Lesions Determined as Suspicious on Transrectal US
Note.-PPV = positive predictive value
*Location was described as "outer" when lesion was limited to outer peripheral zone and was reported as "inner" if lesion was limited to inner peripheral zone or to transition zone. When lesion involved all sides of peripheral zone, it was described as "both".
†Data are presented as counts, with corresponding percentages in parentheses.
Multivariate Logistic Regression Analysis
Multivariate logistic regression analysis was performed for all of the US characteristics. When all of the lesions were evaluated, three criteria, including lesion shape (nodular or cluster), an irregular margin and increased vascularity were found to be significantly and independently associated with the presence of prostate cancer (p < 0.001).
Scoring System for Focal Suspicious Lesions and Application to the Test Set
The three criteria identified above by the use of multiple logistic regression analysis were selected as new criteria and location (identified by the chi-squared test) was added as a fourth criterion. Figure 1 shows the scoring system using a 5-point scale for focal suspicious lesions identified by TRUS.
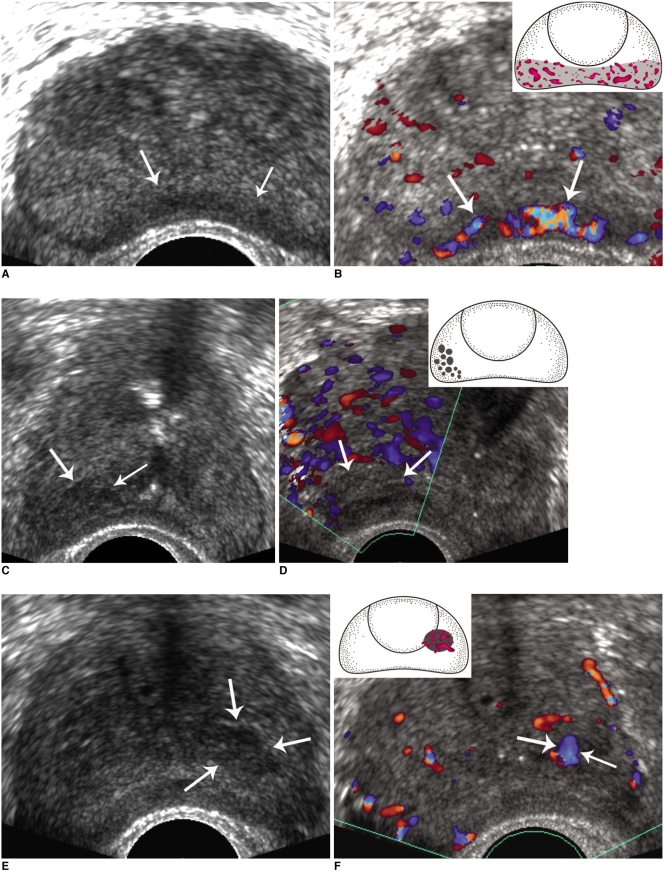
Fig. 1.
Scoring system for focal suspicious lesions.
A, B. Findings for 45-year-old male are presented. Transrectal US image shows band-like low echoic lesion (arrows) with regular outline at outer peripheral zone. Color Doppler US shows prominent hypervascularity (arrows) within lesion, indicating likelihood of malignancy. However, based on use of algorithm for scoring, lesion was given score 2, and positive predictive value for benignity was higher than that for malignancy (64.7% versus 35.3%). Pathology revealed no evidence of malignancy in prostate biopsy samples.
C, D. Findings for 70-year-old male are presented. Transrectal US image shows cluster-like hypoechogenic lesion (arrows) at right outer peripheral zone. Color Doppler US image shows no definite vascularity (arrows) within lesion, indicating that lesion is probably benign. However, devised algorithm classified lesion with score 3, which has considerable positive predictive value for malignancy (48.6%). Pathology confirmed lesion as prostatic cancer.
E, F. Findings for 75-year-old male are presented. Transrectal US image shows nodular low echogenic lesion (arrows) in left prostate. Portion of this lesion was located in transition zone, although most of lesion was located at inner peripheral zone. Color Doppler US image shows focal prominent vascularity (arrows) within lesion. This lesion corresponded to score 2, and pathology revealed lesion as due to prostatic cancer.
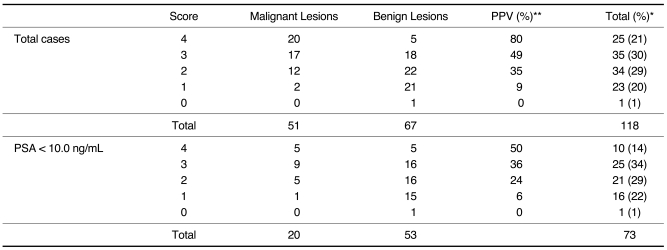
The algorithm determines how many features are present among the following four criteria: a nodular or cluster shape, an irregular outline, increased vascularity and extension to the outer side of peripheral zone; a score of one is added for each trait. Sensitivity, specificity, positive and negative predictive values for the prediction of malignancy were evaluated by applying the scoring system to the test set (n = 118). Each of the scores for the five-point scale showed statistically significant differences between malignant and benign lesions (Table 3). In addition, for the use of the above algorithm, cases with PSA values of < 10 ng/mL were also shown to be significantly associated with the presence of a malignancy, especially for a score of 4 (Table 3). The area under the receiver operating characteristic curve (AUC) for the overall lesion score was 0.81 (Fig. 2).
Table 3.
Evaluation of Scoring System for Focal Suspicious Lesions Depicted on Transrectal US
Note.-PPV = positive predictive value, PSA = prostate specific antigen
*Data are presented as counts, and corresponding percentages in parentheses. **P value < 0.001 by chi-square test
Fig. 2.
Receiver operating characteristic curves of scoring system.
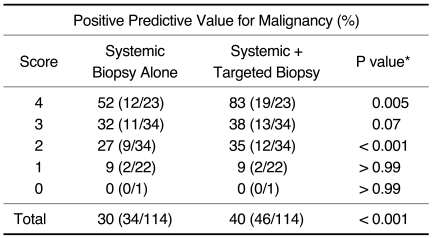
The PPVs for a systemic biopsy alone and a systemic biopsy plus a targeted biopsy are summarized in Table 4. By adding the targeted biopsy to the systemic biopsy, prostate cancer was diagnosed in an additional 15 of 114 patients (13.2%), including seven of 23 (30.4%) patients with a score of 4, five of 34 (14.7%) patients with a score of 3 and three of 34 (8.8%) patients with a score of 2. In terms of the PPV for a malignancy, a significant difference was found between a systemic biopsy alone and systemic biopsy plus targeted biopsy (all p < 0.05 except for a score of 0).
Table 4.
Comparison of Positive Predictive Values for Malignancy by Use of Systemic Biopsy and Targeted Biopsy
Note.-This comparison included test set of 114 patients.
Data in parentheses are per patient and are values used to calculate percentage.
*P values were calculated by use of McNemar's test.
DISCUSSION
Even though hypoechoic lesions of the prostate are frequently detected by the use of TRUS, these lesions have been shown to correspond to a wide-spectrum of pathologies such as prostatic carcinoma, prostate dysplasia, inflammatory changes, granulomatous prostatitis, benign prostatic hyperplastic nodules, smooth muscle bundles, fibrosis or dilated prostatic ducts or cysts (12). Moreover, while the sensitivity of TRUS for lesion detection is high, the specificity of TRUS is disappointingly low and therefore the modality has limits to differentiate benign and malignant lesions.
Recently, several investigators have suggested a reemphasis of TRUS identification of prostatic lesions. According to Toi et al. (13), an increase in the cancer detection rate in patients referred for all indications has been shown for intraprostatic lesions identified by TRUS after a targeted prostate biopsy. Biopsy specimens from these lesions have a greater volume of cancer detected for each positive core and a higher grade of cancer, which concurs with our finding that a positive core tissue had a 56% cancer length. Shim et al. (11) and Lee et al. (14) reported that patients with suspicious lesions depicted on TRUS had more aggressive features of cancer in a comparison to patients without a suspicious lesion. Therefore, identification and a prostate biopsy of US suspicious lesions continue to be useful and important.
This study has refocused on the importance of the TRUS examination to identify and characterize suspicious lesions. Based on our findings, focal suspicious lesions allocated to different TRUS findings had different associated diagnostic accuracies, and these diagnostic accuracies were statistically significant. In particular, individuals with a relatively low PSA level (PSA < 10 ng/mL) were also found to have a significantly different diagnostic accuracy among different scores. Therefore, the proposed scoring system we have described could be used to recruit prostate biopsy candidates, especially when PSA levels are relatively low or equivocal. In addition, the proposed scoring system may be useful to select sites for a targeted prostate biopsy and to predict malignancies.
Our findings suggest that not only prominent vascularity but also abnormal gray scale TRUS findings should be considered together to differentiate chronic inflammation from a malignancy. Prostatic intraepithelial neoplasia (PIN), especially high-grade PIN, is accepted as a premalignant lesion and as a precursor of prostate cancer. Moreover, even though PIN and prostate cancer foci have reportedly been shown to have similar appearances as depicted on TRUS, clusters of millimetric hypoechoic foci may indicate high-grade PIN foci (15). Our results support findings of previous reports in terms of the significance of clusters; clusters were found in 29% of malignant lesions and in 9% of benign lesions (p < 0.001).
Our study has several limitations. The study was limited inherently by its retrospective design, and there may be a selection bias. This study also has a potential limitation with regard to the use of contrast-enhanced color Doppler imaging. Recent clinical trials have demonstrated improved prostate carcinoma detection with the use of targeted biopsies or grading of prostate cancers by the use of contrast-enhanced color Doppler imaging (16). However, other studies have demonstrated the importance of training personnel to interpret properly contrast-enhanced images due to a high level of false-positive results, which substantially reduce specificity (17). Moreover, cost-effectiveness must also be considered. Although TRUS findings alone are limited in terms of prostate cancer detection, we propose a diagnostic scheme with the use of the scoring system that can aid decision-making as to whether or not a patient should undergo a prostate biopsy when focal suspicious lesions are detected by the use of TRUS.
In conclusion, the use of a scoring system for focal suspicious lesions as defined in the present study is found to be associated with different PPVs. The devised scoring system for focal suspicious lesions depicted on TRUS is found to improve the PPV for the use of TRUS and to be helpful to predict the outcomes of TRUS guided biopsies. It is hoped that the use of our suggested scoring system will improve diagnostic decision making for focal suspicious lesions depicted on TRUS. A further controlled prospective study in a larger population would provide a more detailed overview of the ability of US examinations to diagnose prostate cancer and to help decision-making concerning the need for targeted biopsies.
Footnotes
This study was supported by the Seoul National University Bundang Hospital Research Fund (Grant No. 02-2006-031).
References
- 1.Rifkin MM, Choi H. Implications of small, peripheral hypoechoic lesions in endorectal US of the prostate. Radiology. 1988;166:619–622. doi: 10.1148/radiology.166.3.3277237. [DOI] [PubMed] [Google Scholar]
- 2.Plawker MW, Fleisher JM, Vapnek EM, Macchia RJ. Current trends in prostate cancer diagnosis and staging among United States urologists. J Urol. 1997;158:1853–1858. doi: 10.1016/s0022-5347(01)64145-4. [DOI] [PubMed] [Google Scholar]
- 3.Elabbady AA, Khedr MM. Extended 12-core prostate biopsy increases both the detection of prostate cancer and the accuracy of Gleason score. Eur Urol. 2006;49:49–53. doi: 10.1016/j.eururo.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Lee F, Siders DB, Torp-Pedersen ST, Kirscht JL, McHugh TA, Mitchell AE. Prostate cancer: transrectal ultrasound and pathology comparison. A preliminary study of outer gland (peripheral and central zones) and inner gland (transition zone) cancer. Cancer. 1991;67:1132–1142. doi: 10.1002/1097-0142(19910215)67:4+<1132::aid-cncr2820671506>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Littrup PJ, Lee F, Mettlin C. Prostate cancer screening: current trends and future implications. CA Cancer J Clin. 1992;42:198–211. doi: 10.3322/canjclin.42.4.198. [DOI] [PubMed] [Google Scholar]
- 6.Kuligowska E, Barish MA, Fenlon HM, Blake M. Predictors of prostate carcinoma: accuracy of gray-scale and color Doppler US and serum markers. Radiology. 2001;220:757–764. doi: 10.1148/radiol.2203001179. [DOI] [PubMed] [Google Scholar]
- 7.Newman JS, Bree RL, Rubin JM. Prostate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology. 1995;195:86–90. doi: 10.1148/radiology.195.1.7534429. [DOI] [PubMed] [Google Scholar]
- 8.Lavoipierre AM, Snow RM, Frydenberg M, Gunter D, Reisner G, Royce PL, et al. Prostatic cancer: role of color Doppler imaging in transrectal sonography. AJR Am J Roentgenol. 1998;171:205–210. doi: 10.2214/ajr.171.1.9648790. [DOI] [PubMed] [Google Scholar]
- 9.Cornud F, Belin X, Piron D, Chrétien Y, Flam T, Casanova JM, et al. Color Doppler-guided prostate biopsies in 591 patients with an elevated serum PSA level: impact on Gleason score for nonpalpable lesions. Urology. 1997;49:709–715. doi: 10.1016/S0090-4295(96)00632-2. [DOI] [PubMed] [Google Scholar]
- 10.Djavan B, Margreiter M. Biopsy standards for detection of prostate cancer. World J Urol. 2007;25:11–17. doi: 10.1007/s00345-007-0151-1. [DOI] [PubMed] [Google Scholar]
- 11.Shim HB, Lee SE, Park HK, Ku JH. Significance of suspicious lesions at transrectal ultrasonography in men with serum prostate-specific antigen levels of < 20 ng/ml. Tumori. 2007;93:178–181. doi: 10.1177/030089160709300211. [DOI] [PubMed] [Google Scholar]
- 12.Hamper UM, Sheth S, Walsh PC, Holtz PM, Epstein JI. Stage B adenocarcinoma of the prostate: transrectal US and pathologic correlation of nonmalignant hypoechoic peripheral zone lesions. Radiology. 1991;180:101–104. doi: 10.1148/radiology.180.1.2052673. [DOI] [PubMed] [Google Scholar]
- 13.Toi A, Neill MG, Lockwood GA, Sweet JM, Tammsalu LA, Fleshner NE. The continuing importance of transrectal ultrasound identification of prostatic lesions. J Urol. 2007;177:516–520. doi: 10.1016/j.juro.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Kim KG, Lee SE, Byun SS, Hwang SI, Jung SI, et al. Role of transrectal ultrasonography in the prediction of prostate cancer: artificial neural network analysis. J Ultrasound Med. 2006;25:815–821. doi: 10.7863/jum.2006.25.7.815. [DOI] [PubMed] [Google Scholar]
- 15.Ozden E, Göğüs C, Karamürsel T, Baltaci S, Küpeli S, Göğüs O. Transrectal sonographic features of prostatic intraepithelial neoplasia: correlation with pathologic findings. J Clin Ultrasound. 2005;33:5–9. doi: 10.1002/jcu.20080. [DOI] [PubMed] [Google Scholar]
- 16.Mitterberger M, Pinggera GM, Horninger W, Bartsch G, Strasser H, Schäfer G, et al. Comparison of contrast enhanced color Doppler targeted biopsy to conventional systematic biopsy: impact on Gleason score. J Urol. 2007;178:464–468. doi: 10.1016/j.juro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 17.Halpern EJ, Ramey JR, Strup SE, Frauscher F, McCue P, Gomella LG. Detection of prostate carcinoma with contrast-enhanced sonography using intermittent harmonic imaging. Cancer. 2005;104:2373–2383. doi: 10.1002/cncr.21440. [DOI] [PubMed] [Google Scholar]