Abstract
Objective
We wanted to prospectively evaluate the effect of various positions of the patient on gastric distension and lesion conspicuity during performance of CT gastrography (CTG).
Materials and Methods
One hundred thirteen consecutive patients with gastric cancer underwent CTG in the 30° left posterior oblique (LPO), supine, and prone positions. Two radiologists scored (a grade from 1-4) the degree of gastric distension and the lesion conspicuity according to the three scanning positions and the three gastric portions. Two- (2D) and three-dimensional (3D) images were used for analysis. Finally, these data were compared with the endoscopic findings and surgical results.
Results
The mean scores of gastric distension and lesion conspicuity for the LPO and supine positions were higher than those for the prone position (p < 0.001) in the gastric middle and lower portions. However, there was no significant difference between the LPO and supine positions (p ≥ 0.21). As for the gastric upper portion, the mean scores of gastric distension in the prone position were higher than those in the two other positions (p < 0.001). The prone position showed better lesion conspicuity than the two other positions for only one of two cases of gastric cancer in the upper portion of the stomach.
Conclusion
CTG performed in the LPO position or the supine position combined with CTG performed in the prone position is optimal for achieving good gastric distension and evaluating the lesion conspicuity of gastric cancer.
Keywords: Computed tomography (CT), gastrography; Gastric cancer; Distension; Lesion conspicuity
CT gastrography (CTG) is currently being investigated in Asian countries, where gastric cancer is one of the most common causes of cancer-related death (1, 2). Although fiberoptic gastroscopy is used for obtaining biopsies and upper gastrointestinal series is used for showing the site and extent of gastric cancer, CTG is becoming the imaging tool of choice for preoperative gastric cancer staging (3-8). According to the recent advances in multidetector CT technology, CTG can accurately detect and localize both early and advanced gastric cancer with its interactive two- (2D) and three-dimensional (3D) displays that simulate endoscopic viewing and upper gastrointestinal series (9-17). Moreover, the transparent or surface-shaded volume-rendering techniques using CTG have decreased the need for the upper gastrointestinal series.
The gastric antrum is the most common site where cancer develops among all the parts of the stomach, but the gastric antrum tends to be easily collapsed and retain fluid in the dependent portion (18-20), and this can cause masking of gastric cancer during performance of CTG with using air. Especially, minimally invasive surgery such as laparoscopy-assisted gastrectomy or endoscopic mucosal resection requires more accurate preoperative cancer evaluation. As a result, it is becoming important to obtain the optimal CTG with adequate gastric distension and minimal residual fluid. A previous study used the 30° left posterior oblique (LPO) position to achieve better distension of and less residual fluid in the lower part of the stomach (13, 21). However, that study didn't focus on gastric lesions such as cancer for assessing the diagnostic performance and no other position was used, such as the prone position. Therefore, the purpose of this study was to prospectively evaluate the effect of various positions of the patient on the gastric distension and lesion conspicuity during the performance of CTG in gastric cancer patients.
MATERIALS AND METHODS
Patients
This study received institutional review board approval and informed consent was obtained from all the patients. Between July 2005 and March 2006, 123 consecutive patients with endoscopically proven gastric cancer prospectively underwent MDCT gastrography. Ten patients were excluded from our study because the patients didn't undergo surgery due to an advanced stage of inoperable gastric cancer. The remaining 113 patients (73 men and 40 women, mean age: 58 years) were finally enrolled in this study. All the patients underwent laparotomy (n = 90) or endoscopic mucosal resection (n = 23) within a maximum of 63 days after they underwent MDCT gastrography. Among them, 83 patients had early gastric cancers and 30 patients had advanced gastric cancers. The tumors were located in the upper (n = 2), middle (n = 47), both the middle and lower portions (n = 6), and the lower portion (n = 58) of the stomach, respectively.
CT Technique
All the patients, who had fasted at least eight hours prior to the MDCT gastrography, received 10 mg of butyl scopolamine (Buscopan, Boehringer Ingelheim, Seoul, Korea) intravenously to decrease bowel peristalsis and to facilitate hypotonia, and they received 6 g of effervescent granules (Top, Taejoon Pharmaceuticals, Kyungkido, Korea) with 10 mL of water to achieve gastric distension just before CT. CTG was performed using a 16-MDCT scanner (Somatom Sensation 16, Siemens, Erlangan, Germany). The CT parameters included 16×0.75 mm detector configuration, 120 kVp, 120 mAs, 15 mm/sec table feed and 1-mm reconstruction with a 30% overgap. After ensuring adequate gastric distension on the scanogram, the arterial and delayed phase scans were obtained from the diaphragmatic dome to the lower edge of the stomach. The portal phase scans were obtained from the diaphragmatic dome to the symphysis pubis.
Triphasic CT scans were performed during the arterial phase (start of delay: 30 seconds) with the patient in the LPO position, during the portal phase (72 seconds) with the patient in the supine position and during the delayed phase (150 seconds) with the patient in the prone position after injection of 120 mL of nonionic contrast material (Ultravist; Schering, Berlin, Germany) at 4 mL/sec via the antecubital vein by using a 18-gauge needle and an automatic injector. The LPO position was performed by placing a pillow at the patient's back.
Image Analysis
Two board-certified gastrointestinal radiologists, each with thirteen and six years of experience, evaluated the CTG images by working in consensus at a workstation (AW 4.1; GE Healthcare, Milwaukee, WI) before the surgery or endoscopic mucosal resection. They were blinded to the endoscopic results, including the lesion size, location, and characterization, but they knew that CTG was routinely performed in gastric cancer patients. The images from the data sets of the three scanning positions were reviewed. For the purposes of data recording and analysis, the stomach was divided into the three portions: the upper, middle, and lower portion.
Gastric distension was ranked on a four-point scale and this was assessed on the 2D axial and coronal multiplanar reformation images for comparing the degree of distension according to the three scanning positions and the three gastric portions. Each gastric portion was assigned a value by using the following grading system: 1 = less than 25% of the expected maximal luminal distension, 2 = 25-50%, 3 = 51-75%, and 4 = more than 75%.
Next, the two 3D images using virtual gastroscopy and the surface-shaded display were created for lesion detection, lesion localization, and assessing their conspicuity in each of the three scanning positions. The 3D images focused on the gastric lesion after we primarily identified the presence of abnormal gastric wall thickening or a mass on the 2D images. We searched for abnormal gastric lesion by using virtual gastroscopy in the patients who had early gastric cancer, whereas we used the surface-shaded display to accurately evaluate the tumor extent and morphology in the patients who had advanced gastric cancer. Lesion conspicuity was rated according to a four-point scale: 1, no detectable; 2, poor (detectable, but indistinct due to air bubbles, fluid, or a partially collapsed stomach); 3, fair (between 2 and 4), and 4, good (similar to the endoscopic findings or the surgical specimen).
Histopathologic Review
The gross and histopathologic findings were reported by an experienced gastrointestinal pathologist. The location (upper, middle, or lower) and stage (early or advanced gastric cancer) of the gastric cancer were determined.
Data Comparison and Statistical Analysis
All the gastric cancers detected on CTG were correlated with the optical fiberoptic gastroscopic findings and the gross surgical specimens. The gastric cancer that involved two or more gastric portions was incorporated as the one dominant portion for the evaluation of lesion conspicuity, according to the three gastric portions.
The differences of gastric distension and lesion conspicuity, according to the three scanning positions and the three gastric portions, were compared using the Friedman test and the Wilcoxon signed-rank test. The statistical difference between the early and advanced gastric cancers was also analyzed. Statistical analyses were performed using SPSS software (version 12.0, SPSS Inc., Chicago, IL). We applied the Bonferroni correction (p ≤ α / n) to the Wilcoxon singed-rank test in order to avoid a lot of spurious positives because the alpha value needed to be lowered to account for the number of comparisons being performed. Therefore, p values less than 0.05 and 0.017 were considered statistically significant on the Friedman test and the Wilcoxon signed-rank test, respectively.
RESULTS
Gastric Distension
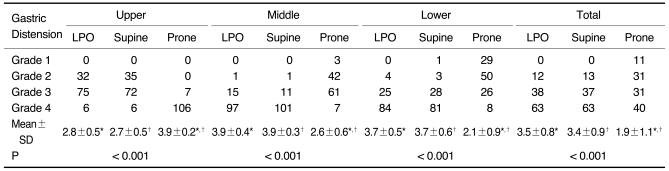
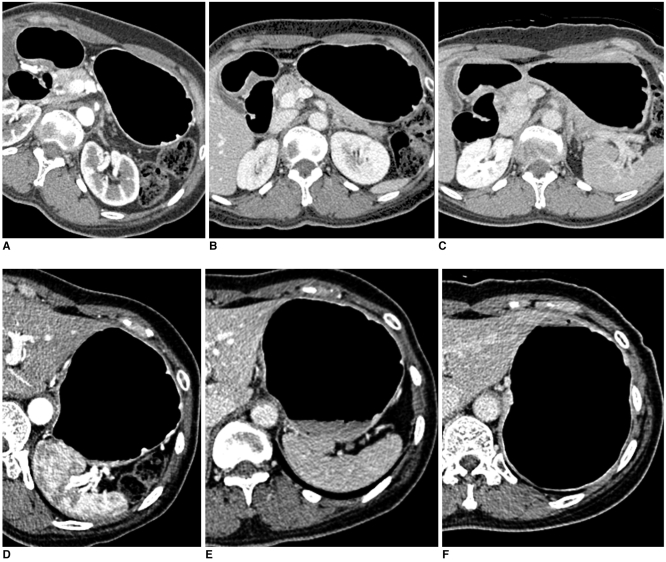
The results for the degree of distension according to the three gastric portions in the LPO, supine, and prone positions to determine the optimal CTG are summarized in Table 1. For the middle and lower gastric portions, the mean scores of distension in both the LPO and supine positions were significantly higher than that in the prone position (p < 0.001) (Fig. 1A-C). The difference between the LPO and supine positions was not statistically significant (p = 0.206 for the middle portion and 0.625 for the lower portion). For the gastric upper portion, the mean score of distension in the prone position was significantly higher than those in both the LPO and supine positions (p < 0.001). However, the difference between the LPO and supine positions was not statistically significant for the gastric upper portion (p = 0.439) (Fig. 1D-F). The total scores of gastric distension in all three gastric portions were significantly higher in both the LPO and supine positions than that in the prone position (p < 0.001).
Table 1.
Degree of Gastric Distension According to Three Scanning Positions and Three Gastric Portions in 113 Gastric Cancer Patients
Note.-Unless otherwise indicated, data is number of patients. Mean±standard deviation (SD) was derived from average score of degree of distension according to three scanning positions and three gastric portions. LPO = 30° left posterior oblique
*,†For mean scores of gastric distension, there was significant difference between LPO and prone positions and between supine and prone positions in upper, middle, and lower gastric portions and total three gastric portions by Wilcoxon signed-rank test (p < 0.017).
Fig. 1.
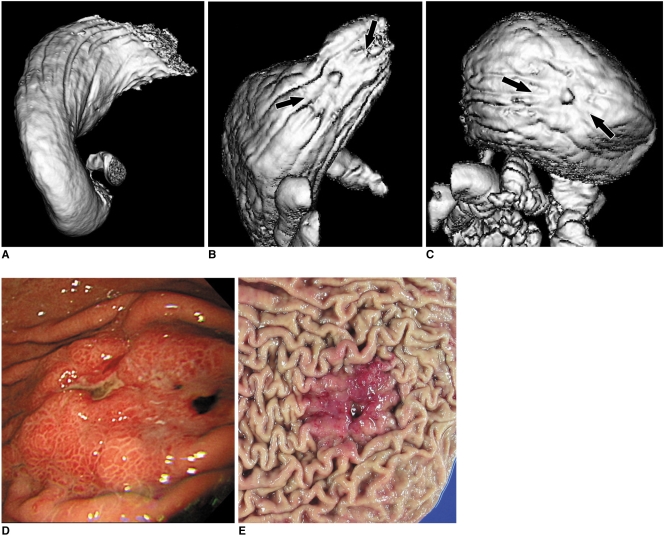
51-year-old woman who underwent CT gastrography according to three scanning positions. On two-dimensional axial images, grade of distension of gastric lower portion was 4 (more than 75%) in 30° left posterior oblique position (A), 3 (between 50% and 75% of expected maximal distension) in supine position (B), and 3 in prone position (C). As for gastric upper portion, grade of distension was 3 in 30° left posterior oblique position (D), 3 in supine position (E), and 4 in prone position (F).
CT Gastrography and Histopathologic Correlation
One hundred eleven of the 113 gastric cancers on CTG were detected and these were well correlated with both the fiberoptic gastroscopic findings and the surgical specimens. Two early gastric cancers located in the middle and lower portions, respectively, were missed on CTG by the two radiologists. For the two early gastric cancers that were missed, one early gastric cancer located in the lower portion was not detected due to retained fluid in all three scanning positions, although there was adequate gastric distension. The other early gastric cancer that was missed was located in the middle portion. A pseudo-lesion created by an irregular mucosal fold on virtual gastroscopy was interpreted as a true lesion in all three scanning positions.
Lesion Conspicuity
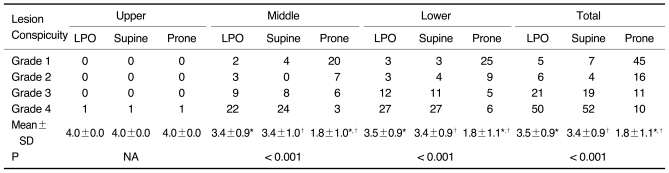
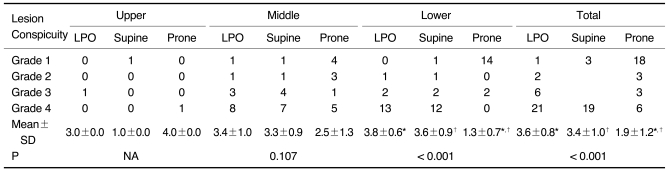
Tables 2 and 3 show the results of the lesion conspicuity, according to the location of the gastric cancer and the three scanning positions, for the 112 gastric cancer patients, except for one patient. As for the 81 early gastric cancers involving the middle and lower portion, respectively, the mean scores of lesion conspicuity in the LPO and supine positions were significantly higher than those in the prone position (p < 0.001) (Fig. 2). There was no significant difference between the LPO and supine positions (p = 0.671 for the middle portion and 0.938 for the lower portion). For only one early gastric cancer involving the upper portion, the score of lesion conspicuity was the same among the three different positions. As for the 16 advanced gastric cancers involving the lower portion, the mean scores of lesion conspicuity in the LPO and supine positions were significantly higher than that in the prone position (p < 0.001). There was no significant difference between the LPO and supine positions (p = 0.461). However, for the 13 advanced gastric cancers involving the middle portion, there was no significant difference among the three different positions. For only one advanced gastric cancer located in the upper portion, the score of lesion conspicuity in the prone position was higher than that in the other two positions (Fig. 3). For the total results, the lesion conspicuity in the middle and lower gastric portions was significantly higher in both the LPO and supine positions than that in the prone position, regardless of the stage (p < 0.001).
Table 2.
Degree of Lesion Conspicuity According to Three Scanning Positions and Three Gastric Portions in 82 Early Gastric Cancer Patients
Note.-Unless otherwise indicated, data is number of patients. Mean±standard deviation (SD) was derived from average score of degree of lesion conspicuity according to three scanning positions and three gastric portions. LPO = 30° left posterior oblique, NA = not applicable
*,†For mean scores of lesion conspicuity, there was significant difference between LPO and prone positions and between supine and prone positions in middle and lower gastric portions and total three gastric portions by Wilcoxon signed-rank test (p < 0.017).
Table 3.
Degree of Lesion Conspicuity According to Three Scanning Positions and Three Gastric Portions in 30 Advanced Gastric Cancer Patients
Note.-Unless otherwise indicated, data is number of patients. Mean±standard deviation (SD) was derived from average score of degree of lesion conspicuity according to three scanning positions and three gastric portions. LPO = 30° left posterior oblique, NA = not applicable
*,†For mean scores of lesion conspicuity, there was significant difference between LPO and prone positions and between supine and prone positions in lower gastric portion and total three gastric portions by Wilcoxon signed-rank test (p < 0.017).
Fig. 2.
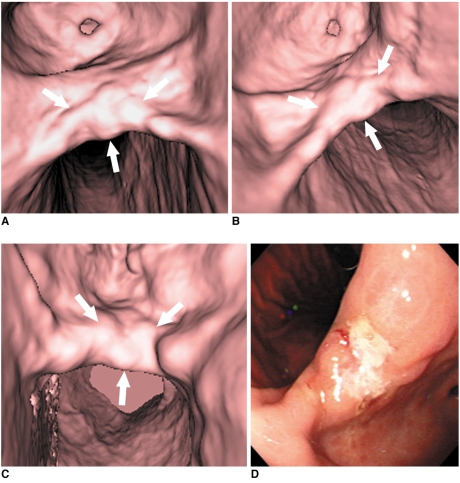
65-year-old man with early gastric cancer in middle portion. This lesion shows irregular mucosal nodularity with depressed lesion in gastric angle (arrows in A-C). Grade of lesion conspicuity in both 30° left posterior oblique (A) and supine positions (B) was 4 (good). However, lesion conspicuity in prone position (C) was 2 (poor) due to partially collapsed stomach with exaggerated rugal folds. This fiberoptic gastroscopic finding well corresponds to virtual gastroscopic images (D). This lesion was histopathologically diagnosed as early gastric cancer after surgery.
Fig. 3.
53-year-old man with advanced gastric cancer in upper portion. This lesion shows ulcerofungating mass in upper portion (arrows in B, C). Lesion conspicuity in 30° left posterior oblique position (A) was 1 (not detectable) due to retained fluid, whereas grade of lesion conspicuity in both supine (B) and prone positions (C) was 4 (good). Fiberoptic gastroscopic findings (D) and surgical specimen (E) well corresponds to surface shaded display images. This lesion was histopathologically diagnosed as advanced gastric cancer.
DISCUSSION
Early gastric cancer confined to the mucosa or submucosa can be cured with surgery or endoscopic mucosal resection, but advanced gastric cancer has a poor prognosis (2, 22). For the patients with early gastric cancer, accurate tumor detection is the first task. Although the MDCT techniques have been refined, the 2D axial or multiplanar reformation images may not detect early gastric cancer that has only subtle mucosal change because this type of early gastric cancer doesn't have adequate wall thickening or enhancement that is visible on the 2D images. Virtual gastroscopy is a 3D endoluminal image, the same as conventional fiberoptic gastroscopy, and it can even detect subtle mucosal abnormality that is suggestive of early gastric cancer compared with 2D images (4-6, 8-12, 14-17, 23). When evaluating advanced gastric cancer, accurately determining the extent of tumor is one of the most important tasks for the presurgical planning. Volume rendering techniques such as transparent or surface-shaded imaging can display the extent of advanced gastric cancer (6, 13). Therefore, in our study, the lesion conspicuity of early gastric cancer according to the scanning positions and the gastric portions was graded on the virtual gastroscopic images and that of advanced gastric cancer was graded on the surface-shaded display images.
These interactive 2D and 3D techniques, which are essential for the accurate detection, localization, and characterization of gastric cancer, require optimal CTG. Performing optimal CTG requires adequate gastric distension and minimal residual fluid, the same as CT colonography. If the stomach is not well distended or it is collapsed, virtual gastroscopy cannot see the endoluminal image and the surface shaded display also cannot accurately depict the tumor. Moreover, exaggerated gastric rugal folds in the partially collapsed stomach can also mimic gastric cancer. Especially, the gastric antrum is the most common site where gastric cancer occurs and the gastric antrum tends to easily collapse compared with the other portions such as the body or fundus. Residual fluid with inadequately dissolved gas bubbles in its dependent portion may also prevent the detection of gastric cancer by virtual gastroscopy or the other 3D volume rendering techniques.
Kim et al. (21) reported that the LPO position for CTG guaranteed the distensibility of and minimal residual fluid in the lower part of the stomach, as compared with the supine position. This stand in contrast with our study results, as we obtained similar results for gastric distension between the LPO position and the supine position. In our study, the LPO and supine positions were consecutively obtained in the same patient group within a short time interval during performance of CTG. This method might have an effect to prolong distension of the gastric lower portion because the air that shifted to the gastric lower portion was preserved in the supine position immediately after it was obtained in the LPO position. However, in the study by Kim et al., the LPO position and the supine position were used in two different patient groups. The left lateral decubitus position, before the LPO position, was also used. This different positioning protocol between the two studies is thought to be a principal cause for the different results of gastric distension.
Our study is the first trial to evaluate the degree of lesion conspicuity of gastric cancer according to the three scanning positions and the three gastric portions. We prospectively detected 111 of 113 gastric cancers on CTG. Early gastric cancer was accurately detected and localized on virtual gastroscopy in 81 of 83 patients, and this result showed higher sensitivity than the previous studies (5, 14, 15, 23). This higher sensitivity may be due to the fact that most radiologists are aware that CTG is routinely performed in patients with gastric cancer and so they make extra effort to detect early gastric cancer. As a result, we were able to obtain high sensitivity because there was a tendency for the radiologists to over-diagnose even subtle abnormal gastric lesions as early gastric cancer on CTG.
The degree of lesion conspicuity, when the cancer is located in the middle or lower portion of the stomach, showed adequate results for both the LPO and supine positions. However, there was no significant difference between the LPO and supine positions. This result didn't affect the stage of gastric cancer. On the contrary, when the gastric cancer was located in the upper portion, the degree of lesion conspicuity in the prone position was higher than that in both the LPO and supine positions. However, this result didn't show a statistically significant difference because only two of 113 gastric cancers were located in the upper portion. As for advanced gastric cancer located in the middle portion, there was no significant difference for lesion conspicuity among the three different positions. It is thought that advanced gastric cancer in the middle portion is less affected by gastric distension or residual fluid than that in the upper or lower portion. In practice, we can't predict the area where the gastric cancer is located if the patient doesn't undergo endoscopy before CTG. Therefore, we recommended that CTG performed with the patient in the LPO or supine position should be combined with CTG performed with the patient in the prone position for the optimal evaluation of gastric cancer.
Our study had some limitations. First, the triple-phase CTG to obtain images in the three scanning positions in one patient would increase the radiation dose and scanning time. To minimize the radiation dose, we reduced the scan range by only covering the air-distended stomach in the LPO and prone positions. Second, two radiologists evaluated the lesion conspicuity of gastric cancer by working in consensus. Although these radiologists were well trained to analyze CTG, substantial interobserver variability in data collection might occur and this could affect the study results. Finally, we didn't subdivide the gastric middle and lower portions. Although our results were not affected by the degree of lesion conspicuity of the middle or lower gastric cancer between the LPO and supine positions, further studies with subdivisions will be needed.
In conclusion, CTG performed with the patient in the LPO position or the supine position combined with CTG performed with the patient in the prone position is optimal for achieving good gastric distension and evaluating the lesion conspicuity of gastric cancer.
Footnotes
This work was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (A060247). The authors declare no competing financial interests.
References
- 1.Forman D, Goodman KJ. The epidemiology of stomach cancer: correlating the past with the present. Socioeconomic influences in early life can influence mortality in adult life. BMJ. 2000;320:1682–1683. doi: 10.1136/bmj.320.7251.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houghton J, Wang TC. Tumors of the stomach. In: Feldman, editor. Sleisenger and Fordtran's gastrointestinal and liver disease. 8th eds. Philadelphia: Saunders; 2006. pp. 1139–1156. [Google Scholar]
- 3.Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230:465–471. doi: 10.1148/radiol.2302020828. [DOI] [PubMed] [Google Scholar]
- 4.Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465–472. doi: 10.1007/s00261-004-0273-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236:879–885. doi: 10.1148/radiol.2363041101. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Park SH, Hong HS, Auh YH. CT gastrography. Abdom Imaging. 2005;30:509–517. doi: 10.1007/s00261-004-0282-4. [DOI] [PubMed] [Google Scholar]
- 7.Kumano S, Murakami T, Kim T, Hori M, Iannaccone R, Nakata S, et al. T staging of gastric cancer: role of multi-detector row CT. Radiology. 2005;237:961–966. doi: 10.1148/radiol.2373041380. [DOI] [PubMed] [Google Scholar]
- 8.Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, et al. Gastric cancer: preoperative local staging with 3D multidetector row CT-correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. [DOI] [PubMed] [Google Scholar]
- 9.Springer P, Dessl A, Giacomuzzi SM, Buchberger W, Stöger A, Oberwalder M, et al. Virtual computed tomography gastroscopy: a new technique. Endoscopy. 1997;29:632–634. doi: 10.1055/s-2007-1004269. [DOI] [PubMed] [Google Scholar]
- 10.Ogata I, Komohara Y, Yamashita Y, Mitsuzaki K, Takahashi M, Ogawa M. CT evaluation of gastric lesions with three-dimensional display and interactive virtual endoscopy: comparison with conventional barium study and endoscopy. AJR Am J Roentgenol. 1999;172:1263–1270. doi: 10.2214/ajr.172.5.10227500. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Takashima S, Kaminou T, Hayashi S, Nishida N, Matsuoka T, et al. Clinical studies on the visualization of gastric lesions using virtual CT endoscopy. Osaka City Med J. 2001;47:115–126. [PubMed] [Google Scholar]
- 12.Oto A. Virtual endoscopy. Eur J Radiol. 2002;42:231–239. doi: 10.1016/s0720-048x(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Han JK, Lee KH, Chung JW, Yang HK, Choi BI. Computed tomography gastrography with volume-rendering technique: correlation with double-contrast barium study and conventional gastroscopy. J Comput Assist Tomogr. 2003;27:140–149. doi: 10.1097/00004728-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, et al. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004;59:619–626. doi: 10.1016/s0016-5107(04)00169-5. [DOI] [PubMed] [Google Scholar]
- 15.Inamoto K, Kouzai K, Ueeda T, Marukawa T. CT virtual endoscopy of the stomach: comparison study with gastric fiberscopy. Abdom Imaging. 2005;30:473–479. doi: 10.1007/s00261-004-0278-0. [DOI] [PubMed] [Google Scholar]
- 16.Carrascosa P, Capuńay C, Ulla M, López EM, Corti R, Carrascosa J. Elevated gastric lesions: virtual gastroscopy. Abdom Imaging. 2006;31:261–267. doi: 10.1007/s00261-005-0373-x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Eun HW, Hong SS, Auh YH. Early gastric cancer: virtual gastroscopy. Abdom Imaging. 2006;31:507–513. doi: 10.1007/s00261-005-0183-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Ko YT. Gastric lesions: evaluation with three-dimensional images using helical CT. AJR Am J Roentgenol. 1997;169:787–789. doi: 10.2214/ajr.169.3.9275897. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Ko YT. The role of 3D spiral CT in early gastric carcinoma. J Comput Assist Tomogr. 1998;22:709–713. doi: 10.1097/00004728-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lee DH, Ko YT. Advanced gastric carcinoma: the role of three-dimensional and axial imaging by spiral CT. Abdom Imaging. 1999;24:111–116. doi: 10.1007/s002619900456. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Lee JM, Han JK, Lee JY, Yang HK, Lee HJ, et al. Effect of adjusted positioning on gastric distention and fluid distribution during CT gastrography. AJR Am J Roentgenol. 2005;185:1180–1184. doi: 10.2214/AJR.04.1812. [DOI] [PubMed] [Google Scholar]
- 22.Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Eun HW, Choi JH, Hong SS, Kang W, Auh YH. Diagnostic performance of virtual gastroscopy using MDCT in early gastric cancer compared with 2D axial CT: focusing on interobserver variation. AJR Am J Roentgenol. 2007;189:299–305. doi: 10.2214/AJR.07.2201. [DOI] [PubMed] [Google Scholar]