Abstract
Proliferation of antigen-specific lymphocytes and resulting clonal expansion is essential for adaptive immunity. We report that B cell-specific deletion of CD98hc reduced antibody responses due to total suppression of B cell proliferation and subsequent plasma cell formation. Deletion of CD98hc didn’t impair early B cell activation, but did inhibit later activation of the MAP kinase Erk1/2 and down regulation of the p27 cell cycle inhibitor. Reconstitution of CD98hc-deficient B cells with CD98hc mutants revealed that the integrin-binding domain of CD98hc is required, but the amino acid transport function of CD98hc is dispensable, for B cell proliferation. Thus, CD98hc supports integrin-dependent rapid proliferation of B cells. We propose that the advantage of adaptive immunity favored appearance of CD98hc in vertebrates.
Introduction
Adaptive immunity is a vertebrate specialization that requires selective proliferation of antigen-specific lymphocytes 1, 2. The CD98 heavy chain (CD98hc; also called 4F2hc) (http://www.signaling-gateway.org/molecule/query?afcsid=A000262), encoded by the Slc3a2 gene, is a vertebrate membrane protein whose expression is greatly increased in proliferating lymphocytes 3. Originally described 25 years ago3 as a lymphocyte activation antigen, the role of CD98hc in the immune system remained obscure. CD98 has two distinct functions: facilitating amino acid transport4, 5 and mediating integrin signaling6, 7. The 80kD CD98hc is covalently linked with one of several 40 kD light chains, which function as amino acid transporters 4, 5. Leucine and isoleucine transport is mediated by the CD984, 5 heterodimer, and these amino acids are important regulators of the mTOR pathway that governs nutrient-regulated lymphocyte function8, 9. CD98hc also interacts with certain integrin β-subunits to mediate signaling events that control cell migration, survival, and proliferation 7. CD98hc is first seen in primitive vertebrates, coincident with the appearance of adaptive immunity 2, 10. Consequently, we hypothesized that CD98hc could play a role in the rapid lymphocyte proliferation required for effective adaptive immunity.
Here we report that CD98hc facilitates humoral immunity by supporting the rapid proliferation of B cells that is necessary for clonal expansion and subsequent differentiation into plasma cells. We deleted CD98hc in B cells by crossing mice bearing a floxed Slc3a2 allele (Slc3a2f/f) with those expressing Cre recombinase in B cells (CD19-Cre+). These mice exhibited normal maturation and distribution of peripheral B cells, and normal morphology of secondary lymphoid organs. Slc3a2f/fCD19-Cre+ mice immunized with T cell-dependent or -independent antigens showed markedly reduced antibody responses, compared to control mice, due to complete suppression of B cell proliferation and plasma cell formation in CD98hc-null B cells. Mutant forms of CD98hc that mediate integrin signaling but not amino acid transport supported proliferation of CD98hc-deficient B cells; hence, the region of CD98hc that mediates integrin interaction is required, and the domain facilitating amino acid transport function is dispensable, for B cell proliferation. Furthermore, stimulation of a mixture of CD98hc-deficient and CD98hc-sufficient B cells resulted in strong enrichment of CD98hc-sufficient cells; this finding establishes the capacity of CD98hc to confer a strong selective advantage during rapid B cell proliferation. Thus, the ability of CD98hc to enable clonal expansion, necessary for adaptive immunity, may have favored the appearance and retention of CD98hc in vertebrates10.
Results
B cell CD98hc is needed for antibody responses
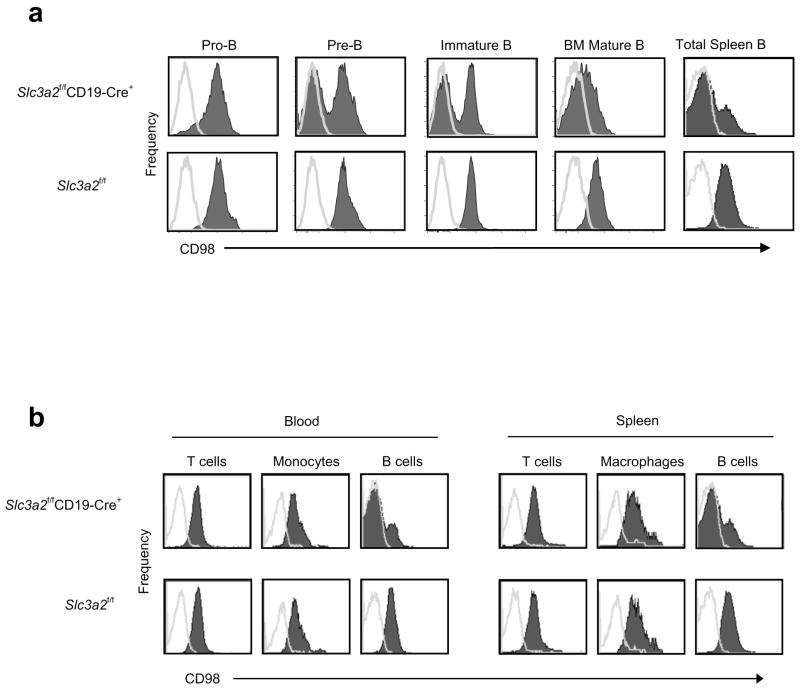
Because germline loss of CD98hc is embryonic lethal 11, we targeted CD98hc by flanking exons one and two of Slc3a2 with loxP sites that specified Cre-recombinase-mediated deletion of the cytoplasmic and transmembrane region of CD98hc (Supplementary Fig. 1a), online) thus leading to complete loss of CD98hc expression12. We crossed Slc3a2f/f mice with mice bearing Cre recombinase under the control of the endogenous B cell-specific Cd19 locus 13. The resulting Slc3a2f/fCD19-Cre+ mice showed specific deletion of CD98hc in B lymphocytes beginning at the pro-B to pre-B cell transition, when CD19 expression is first induced 14; Within the splenic B cell compartment, 70–90% of cells lacked CD98hc (Fig. 1a), and we did not detect any deletion in T cells or monocyte/macrophages (Fig.1b), consistent with the efficiency and specificity of CD19-Cre-mediated recombination 13. B cells in Slc3a2flf littermates were uniformly positive for CD98hc (Fig 1a).
Figure 1.
CD98hc deletion in Slc3a2f/fCD19-Cre+ mice. (a) CD98hc deletion in B cells. BM cells from adult Slc3a2f/fCD19-Cre+ and control mice were isolated and CD98hc expression (filled peaks) measured at different stages of B cell maturation compared to isotype control staining (lines); experiment was repeated once. (b) Specificity of CD98 deletion. Peripheral blood and spleen cells from Slc3a2f/fCD19-Cre+ and control mice were prepared by lysing erythrocytes. CD98hc expression was measured on T cells (CD3+), macrophages (CD11b+B220−), and B cells (B220+) by flow cytometry. Data from one representative mouse of each genotype are shown (n=4 per group for each panel); experiment was repeated once.
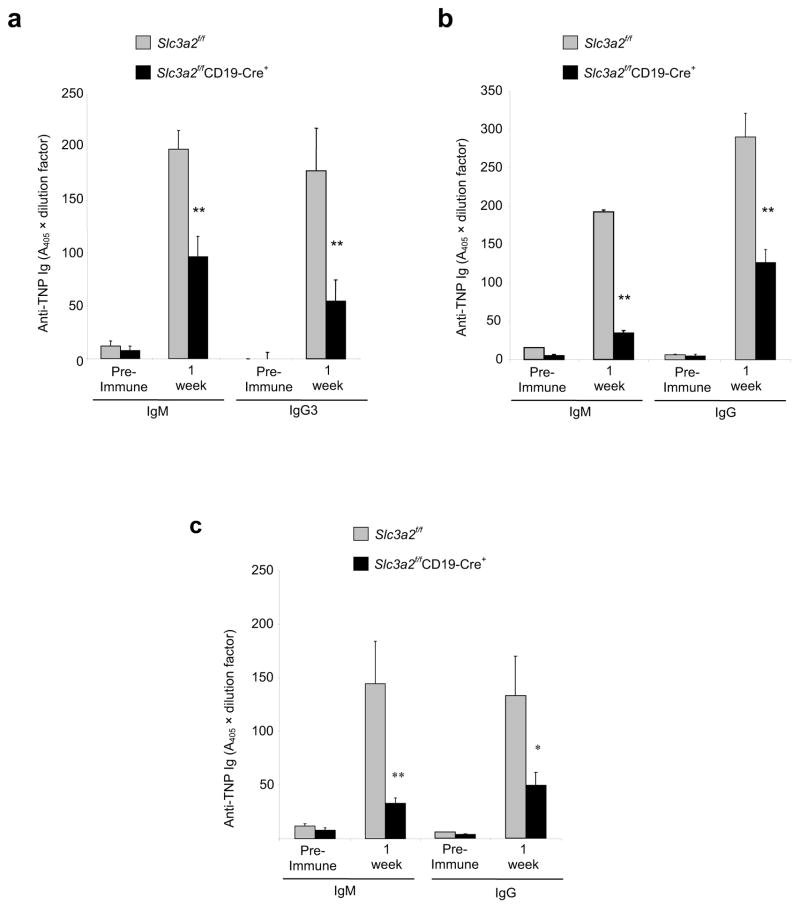
To test whether CD98hc is important for B cell antibody secretion, we measured concentrations of circulating IgG in Slc3a2f/fCD19-Cre+ and Slc3a2f/fCD19-Cre− mice. Whereas basal circulating IgM concentrations were not significantly lower in naive Slc3a2f/fCD19-Cre+ animals, we noted a significant reduction in class-switched IgG in naive Slc3a2f/fCD19-Cre+ mice (Supplementary Fig. 1b). Furthermore, after challenge with T cell-independent antigen (Fig. 2a), or T cell-dependent antigen in Complete Freund’s Adjuvant (Fig. 2b), we noted a profound reduction in antigen-specific IgM and IgG concentrations in Slc3a2f/fCD19-Cre+ compared with Slc3a2f/fCD19-Cre− mice. This reduction was not specific to Toll-like receptor signals, because similar reductions were observed after immunization with T cell-dependent antigens in adjuvant lacking microbial components (Incomplete Freund’s Adjuvant) (Fig. 2c). In addition, this reduction correlated with Slc3a2 gene dosage; compared to Slc3a2f/+CD19-Cre+ mice, Slc3a2f/− CD19-Cre+ animals showed a more severe defect (Supplementary Fig. 2). Thus, B cell CD98hc is important for mounting specific antibody responses to antigenic challenge.
Figure 2.
Impaired antibody responses in Slc3a2f/fCD19-Cre+ mice. (a) T cell-independent antibody response. Adult (8–12 wk old) Slc3a2f/fCD19-Cre+ or control mice were immunized with 50 μg of a T cell-independent antigen, TNP-LPS, in PBS. Mice were bled before immunization (pre-immune) and at one wk after immunization to obtain serum. Concentrations of anti-TNP IgM or anti-TNP IgG3 were measured by direct ELISA. Error bars represent s.e.m. from 5 mice in each group. ** P < 0.025 (b, c) T cell-dependent antibody responses. Slc3a2f/fCD19-Cre+ or control mice were immunized with 100 μg of the T cell-dependent antigen TNP-KLH in Complete Freund’s Adjuvant (CFA) (b) or Incomplete Freund’s Adjuvant (IFA) (c). Anti-TNP IgM and anti-TNP IgG in serum were measured by direct ELISA. Error bars represent s.e.m. 5 mice for each group. (**P <0.025, *P = 0.057). Experiments in (a–c) were repeated once.
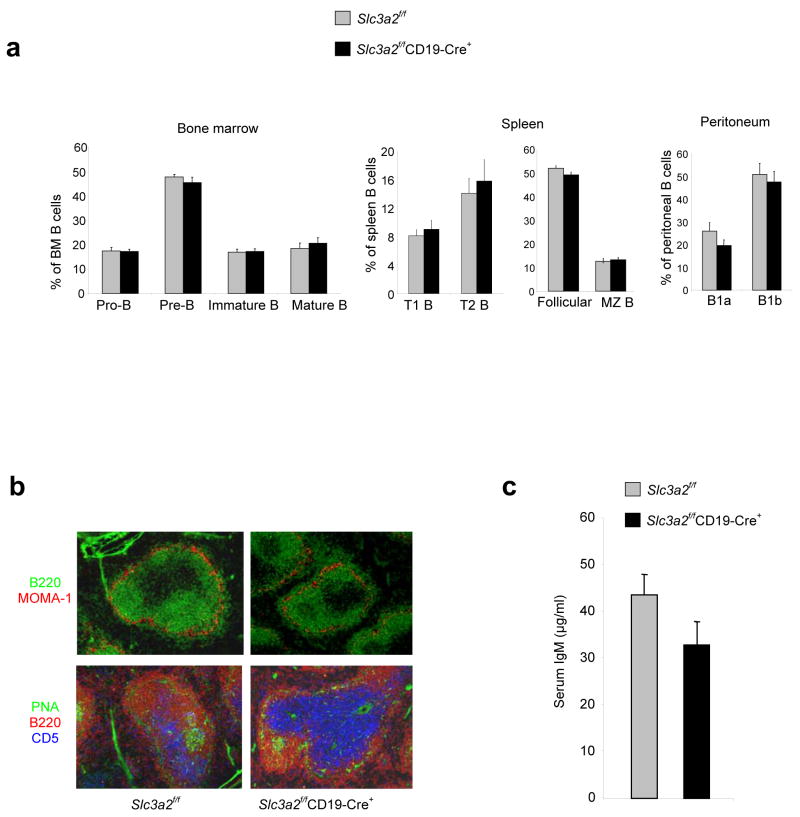
CD98hc is involved in integrin signaling, and integrins are involved in localization and distribution of B cells subsets 15, 16. In addition, CD98hc is important for integrin-mediated mesenchymal cell migration7. To test whether loss or misdistribution of B cell subsets could explain the impaired antibody production in Slc3a2f/fCD19-Cre+ mice, we examined lymphoid tissue for the presence and localization of B cells subsets. Despite the role of CD98hc in adhesive signaling and amino acid transport 6, 7, 17, CD98hc-deficient follicular B cells, marginal zone B cells, transitional B cells, and peritoneal B1 cells were present in normal percentages in the periphery; in addition, pro-B, pre-B, immature B, and mature B cells were present in normal proportions in the bone marrow (BM) (Fig. 3a). Similarly, we detected no significant differences in the absolute numbers of these subsets between Slc3a2f/fCD19-Cre+ and Slc3a2f/fCD19-Cre− mice (Supplementary Fig. 3). Slc3a2f/fCD19-Cre+ animals also exhibited normal splenic architecture, as follicular B cells segregated with intact marginal zones, and Slc3a2f/fCD19-Cre− and Slc3a2f/fCD19-Cre+ mice contained similar numbers of germinal centers (Fig. 3b). Finally, we detected no differences between naïve Slc3a2f/fCD19-Cre− and Slc3a2f/fCD19-Cre+ mice with regard to formed elements of the blood (Supplementary Fig. 4) or concentrations of circulating natural IgM (Fig. 3c). Thus, CD98hc is not required for the formation of mature B cells or their capacity to populate secondary lymphoid organs.
Figure 3.
Normal B cell distribution and natural antibody concentrations in Slc3a2f/fCD19-Cre+ mice. (a) Enumeration of B cell subsets. Indicated B cell subsets in spleen, BM, and peritoneal cells from adult (8–12 wk old) Slc3a2f/fCD19-Cre+ and control mice were analyzed flow cytometry. Error bars show s.e.m. from 4 mice in each group; experiment was repeated with similar results (b) Analysis of secondary lymphoid architecture. Frozen spleen sections from Slc3a2f/fCD19-Cre+ and littermate control mice were stained with the antibodies specific for the indicated proteins to detect B220+ B cells, metalophillic macrophages that outline the marginal zone (MOMA-1+), T cells (CD5+), and germinal center B cells (PNA+). (c) Natural antibody concentrations. Naïve adult (8–12 wk old) Slc3a2f/fCD19-Cre+ and littermate control mice were bled and serum analyzed by sandwich ELISA for total IgM. Error bars show s.e.m. from 30 mice per group (P = 0.11).
B cell CD98hc is necessary for plasma cell formation
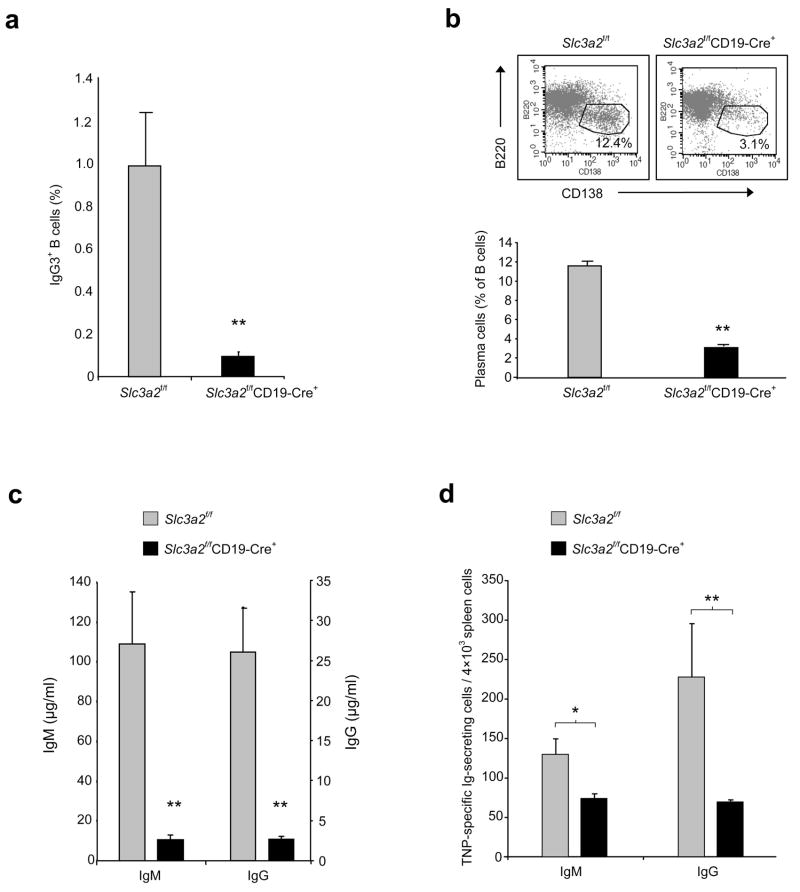
B cells differentiate into antibody-secreting plasma cells following antigenic challenge, suggesting that a CD98hc requirement in plasma cell formation might explain the reduced humoral immune responses of Slc3a2f/fCD19-Cre+ mice. To test this idea, we purified resting splenic B cells from Slc3a2f/fCD19-Cre+ mice, depleted the population of any B cells that expressed CD98hc, and stimulated the remaining population with lipopolysaccharide (LPS) to induce plasma cell formation. B cells from Slc3a2f/fCD19-Cre+ mice were defective both in class-switching (Fig. 4a) and in development into CD138+ plasma cells (Fig. 4b). This phenotype bears similarities to that of β1-integrin-deficient B cells16. The few plasma cells formed by Slc3a2f/fCD19-Cre+ B cells expressed CD98hc, indicating that they were the progeny of a few remaining CD98hc-expressing cells that escaped Cre-mediated recombination and in vitro depletion (Supplementary Fig. 5). Indeed, when we omitted the step of in vitro depletion of CD98hc-expressing cells, the 10–20% of B cells that expressed CD98hc in Slc3a2f/fCD19-Cre+ mice generated near normal percentages of plasma cells in vitro (Supplementary Fig. 6, 7).
Figure 4.
Defective plasma cell formation in Slc3a2f/fCD19-Cre+ mice. (a,b) In vitro plasma cell formation. Resting splenic B cells (CD43−CD98hc-deficient) were purified from Slc3a2f/fCD19-Cre+ or littermate control mice, cultured with LPS for 5 days, and stained for surface IgG3 (a) and CD138 (b) (Syndecan-1, a plasma cell marker). Cells were analyzed by flow cytometry. Dot plot is representative data from one mouse; bar graphs summarize IgG3 or CD138 staining on B220lo cells. Error bars show s.e.m. from 3 mice per group. **P < 0.025 Experiment was repeated with similar results. (c) In vitro antibody secretion. Supernatants from resting B cells stimulated for 4 days with LPS were assayed for total IgM and IgG by sandwich ELISA. Error bars show s.e.m. from 4 mice per group. *P <0.05 Experiment was repeated with similar results. (d) In vivo plasma cell formation. Slc3a2f/fCD19-Cre+ and control mice were immunized with TNP-KLH in CFA. One wk later, splenocytes were cultured for 2–3 h on TNP-coated PVDF membrane 96-well plates. After washing to remove cells, and incubation with anti-IgG HRP-conjugated secondary antibody, AEC substrate was used to develop red spots indicating TNP-specific IgG-secreting plasma cells. Error bars indicate s.e.m. from 4 mice for each group (*P = 0.05, **P = 0.08). Experiment was repeated once.
Consistent with defective formation of antibody-secreting plasma cells, B cells from Slc3a2f/fCD19-Cre+ mice also showed impaired antibody secretion after LPS stimulation in vitro (Fig. 4c). As shown by ELISPOT, Slc3a2f/fCD19-Cre+ mice immunized with a T-dependent antigen also contained fewer cells secreting antigen-specific antibody directly ex vivo, compared to immunized Slc3a2f/fCD19-Cre− mice (Fig. 4d). Taken together, these data show that without CD98hc, B cells are markedly impaired in their capacity to form plasma cells.
CD98hc is required for rapid B cell proliferation
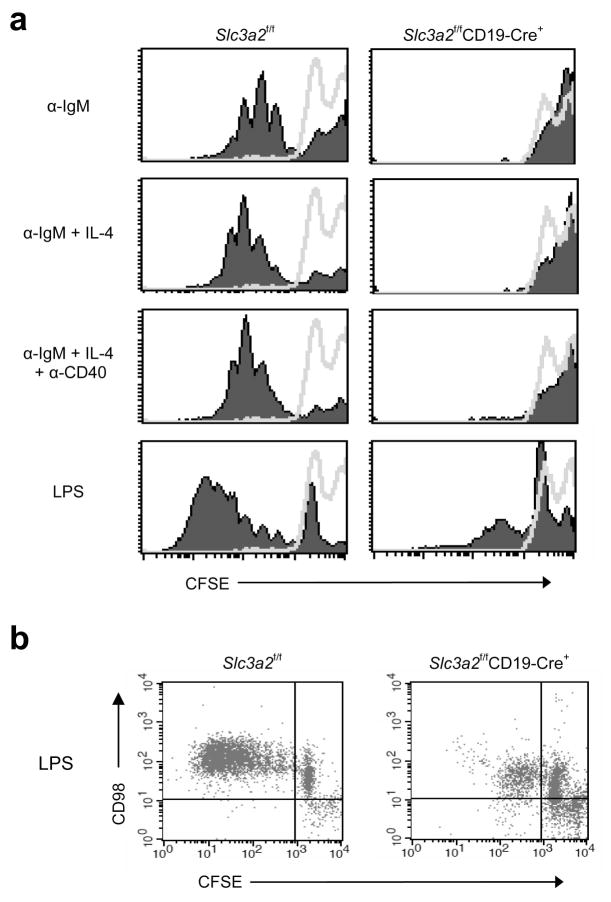
Differentiation into plasma cells is preceded by multiple rounds of proliferation, leading to increased numbers of antigen-specific B cells 18; class switching is also independently regulated by cell division19. Previous reports utilizing blocking or cross-linking antibody suggested CD98hc was involved in T cell 20 21 and keratinocyte proliferation22. To test the role of CD98hc in B cell proliferation, we purified CD98hc-deficient or CD98hc-sufficient resting splenic B cells from Slc3a2f/fCD19-Cre+ or Slc3a2f/fCD19-Cre− mice, respectively, labeled them with the intracellular dye carboxyfluorescein succinimidyl ester (CFSE), stimulated them with B cell mitogens, and measured their proliferation by dye dilution via flow cytometry. Five days after stimulation, B cells from Slc3a2f/fCD19-Cre− mice divided 5–8 times, as seen by the discrete populations of daughter cells exhibiting exponential dilution of fluorescence (Fig. 5a). In sharp contrast, B cells from Slc3a2f/fCD19-Cre+ littermates showed minimal division in response to the B cell receptor crosslinking agent, anti-IgM, provided alone or together with interleukin 4 (IL-4) or anti-CD40; similar data were obtained by direct enumeration of viable cells (Supplementary Fig. 8). A few cells from Slc3a2f/fCD19-Cre+ mice did undergo 1–2 divisions in response to high-dose LPS stimulation (Fig. 5a). However, these divided cells expressed CD98hc, suggesting that they escaped Cre-mediated deletion of Slc3a2 (Fig. 5b). Thus, CD98hc is necessary for rapid proliferation of mature B cells in response to antigen or other mitogenic signals. In the absence of stimulation, the lack of CD98hc did not appreciably alter the viability of cultured B cells (Supplementary Fig. 8), suggesting the low expression of CD98 expression in the resting state is not required for B cell survival. Thus, CD98hc is crucial for the rapid B cell expansion and plasma cell formation in response to external stimuli that drive adaptive humoral immunity.
Figure 5.
Proliferation of splenic B cells from Slc3a2f/fCD19-Cre+ mice. (a) In vitro proliferation analysis. Resting B cells (CD43−) were purified from splenocytes of 8–12 wk-old Slc3a2f/fCD19-Cre+ and littermate control mice and labeled with CFSE. 400,000 CFSE-labeled B cells were cultured per well in a 48-well plate with the indicated stimuli (filled peaks) for 5 days, and proliferation was measured by the dilution of CFSE fluorescence by flow cytometry, as compared with unstimulated resting cells (lines). Each histogram is data from one mouse that represents data from 3 mice of each genotype in one experiment. This experiment was repeated twice with additional mice. (b) CD98hc expression on proliferating cells. CD98 expression was measured on CFSElo (dividing) vs. CFSEhi (non-dividing) cells by antibody staining and 2-color flow cytometry; experiment was repeated once.
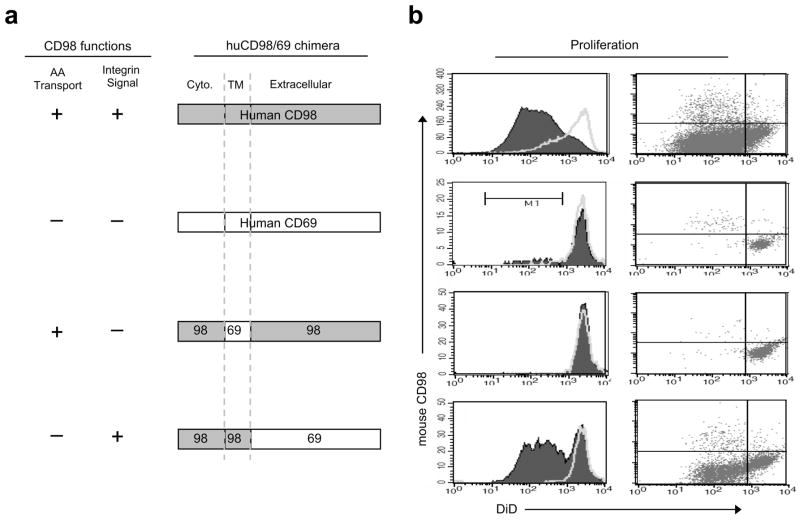
CD98hc has two well-documented biochemical functions: it interacts with certain integrin β subunits to mediate integrin signaling 6, 23, thus influencing adhesion-induced signaling events such as activation of pp125FAK, p130CAS, PI3 kinase, and Akt, that control cell proliferation 7. In addition, by associating with light chains such as LAT-1, CD98hc facilitates transport of amino acids such as leucine and isoleucine 4, 5. These amino acids are important regulators of mTOR 24, a critical node in a signaling pathway that controls immune responses 8, 9 Chimeras in which portions of CD98hc are replaced with portions of CD69, another Type II transmembrane protein, allow decisive separation of these two CD98hc functions17 (Fig. 6a). We used these chimeras to identify the mechanism whereby CD98hc enables B cell proliferation.
Figure 6.
Mechanism by which CD98hc enables B cell proliferation. (a) CD98-CD69 chimeric constructs encoded by retroviruses. (b) Rescue of B cell proliferation with CD98hc integrin signaling function. Six to eight wk after transplant of BM cells infected with indicated retroviruses, resting B cells (CD43− mCD98hc−) were purified from splenocytes of recipient mice. Cells were labeled with DiD dye. 350,000 DiD-labeled B cells were cultured per well in a 48-well plate with LPS (filled peaks) and proliferation was measured by the dilution of DiD fluorescence by flow cytometry. Dot plots show mouse CD98 expression vs. DiD fluorescence by 2-color flow cytometry; fewer dots in the full-length CD69 or in the integrin-signaling-deficient CD98-CD69 chimera are indicative of the lack of proliferation in these samples. This experiment was repeated twice.
To study the function of these chimeras in primary B cells, we infected BM from Slc3a2f/fCD19-Cre+ mice with retroviruses containing bicistronic mRNAs encoding either human CD98hc or CD98-69 chimeras, followed by an internal ribosomal entry site and a GFP cassette. These transduced bone marrow cells were used to reconstitute lethally-irradiated recipients to create animals in which B cells that develop and continue to express a human CD98-CD69 chimera are marked by GFP fluorescence (Supplementary Fig. 9, 10a) (Fig. 6a). Staining with an antibody specific for mouse CD98hc enabled us to identify B cells that lack mouse CD98hc but express retrovirally encoded human CD98hc chimeras; these cells were found in the blood beginning as early as 3 weeks after BM transfer.
Furthermore, all B cells that expressed GFP also stained for either human CD98hc or CD69 (Supplementary Fig. 10b). Six weeks after BM transfer, we purified mouse CD98hc-null resting splenic B cells, labeled them with the membrane dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine (DiD) to track proliferation, and stimulated the cells with LPS. Four days later, we analyzed proliferation by dye dilution via flow cytometry (Fig. 6b). B cells reconstituted with full-length human CD98hc or with the integrin-interacting C98T98E69 chimera proliferated. In sharp contrast, the C98T69E98 chimera, which interacts with LAT-1 and mediates isoleucine transport but does not interact with integrins, failed to reconstitute proliferation. Thus, the integrin signaling portion of CD98hc is necessary and sufficient for proliferation of mature B cells.
Loss of CD98hc inhibits integrin-dependent events
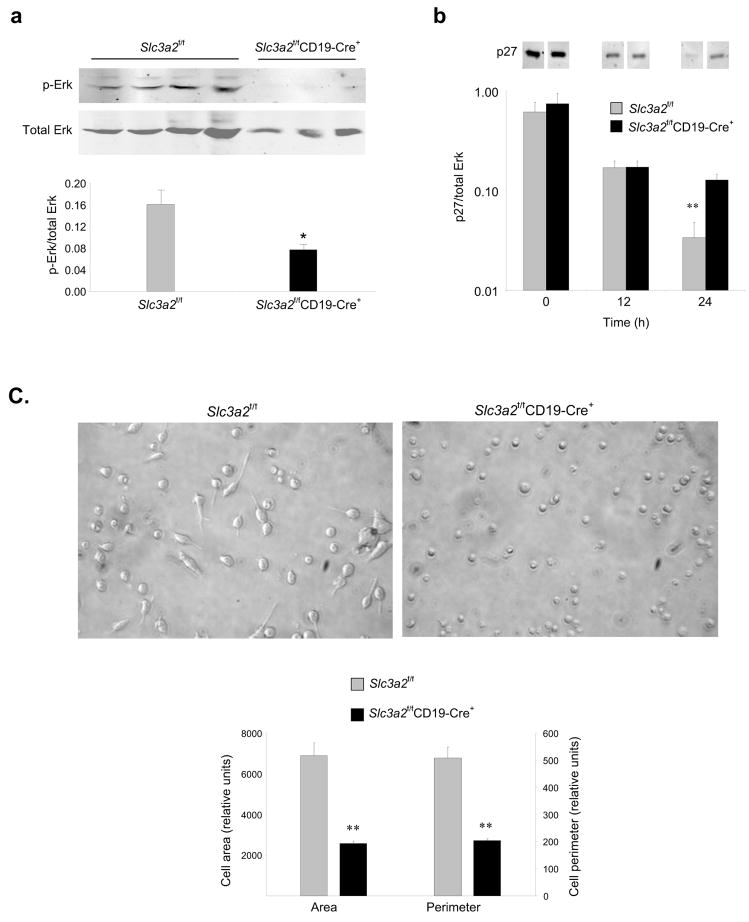
Integrin ligation leads to signals that result in cell spreading and migration 25. In addition, integrins synergize with certain tyrosine kinase receptors to prolong and sustain activation of the MAP kinase Erk1/2, which promotes cell proliferation 26 by downregulating cyclin-dependent kinase inhibitors27 and facilitating progression through the G1 phase of the cell cycle 28, 29. B cells from Slc3a2f/fCD19-Cre+ mice exhibited reduced Erk1/2 phosphorylation at 17 hours after BCR ligation (Fig. 7a). In sharp contrast, early (<1 hr) activation of Erk1/2, Akt, and Syk was intact in B cells lacking CD98hc (Supplementary Fig. 11a–c), as was expression of activation markers (Supplementary Fig. 11d). However, coincident with a failure to sustain Erk1/2 activation, Slc3a2f/fCD19-Cre+ B cells showed impaired downregulation of the p27 cyclin-dependent kinase inhibitor (Fig. 7b), consistent with the observed lack of proliferation. Thus, absence of CD98hc selectively impairs sustained activation of Erk1/2 after BCR ligation, an event known to depend on integrins in other cell types.
Figure 7.
Integrin signaling defects in B cells lacking CD98hc. (a) Late Erk signaling. Resting B cells (CD43−CD98−) were purified from splenocytes of 8–12 wk-old Slc3a2f/fCD19-Cre+ (n=4) or littermate control mice (n=3) and were stimulated with anti-IgM (30 ug/ml) and IL-4 (15 ng/ml) for 17 h. Cells were then immediatedly washed with ice-cold PBS, lysed, and Erk1/2 phosphorylation was analyzed by immunoblotting. Bands are present, albiet faint, in the Slc3a2f/fCD19-Cre+ lanes. (b) p27 downregulation. Purified B cells from Slc3a2f/fCD19-Cre+ or littermate control mice were stimulated with anti-IgM and IL-4 for 0, 12, or 24 h. Cells were washed and lysed, and expression of p27 was analyzed by immunoblotting (Intervening bands have been omitted for clarity). Bar graph summarizes staining of p27 normalized to total Erk. Error bars represent s.e.m. from n=3 mice per group for (a) and (b). Experiment for (a–b) was repeated once. (c) Integrin-dependent cell spreading. Purified B cells from Slc3a2f/fCD19-Cre+ or littermate control mice were stimulated with anti-CD40 and IL-4 for 24 h, and were plated on anti-LFA-1 for 16 h. Digital pictures show representative fields, and bar graphs summarize area and perimeter of 30 traced cells for each group. Experiment was repeated once.
To directly assess the effect of B cell CD98hc deficiency on another integrin-dependent function, we utilized a recently described B cell adhesion and spreading assay 30, 31. Activated B cells from Slc3a2f/fCD19-Cre+ mice failed to spread following direct antibody-mediated ligation of integrin α Lβ2 (LFA-1) (Fig. 7c). In sharp contrast, CD98hc-bearing B cells from Slc3a2f/fCD19-Cre− littermates spread extensively, as evidenced by a >2 fold increase in cell area and perimeter relative to the CD98hc-deficient B cells. In total, these data point to the potential importance of integrin signaling for B cell proliferation.
As an initial test of this provocative idea, we analyzed responses to crosslinking of the B cell receptor on B cells from mice engineered to lack all leukocyte integrins32. Even though some of these B cells expressed a small quantity of integrin β1, they exhibited decreased proliferation in response to BCR ligation (Supplementary Fig. 12), providing direct evidence of the involvement of integrins in B cell proliferation. The occurrence of some proliferation of these B cells raises the possibility that the loss of amino acid transport resulting from CD98hc deficiency may also contribute to the more profound defect in proliferation seen in CD98hc-deficient B cells.
CD98hc confers a selective advantage on B cells
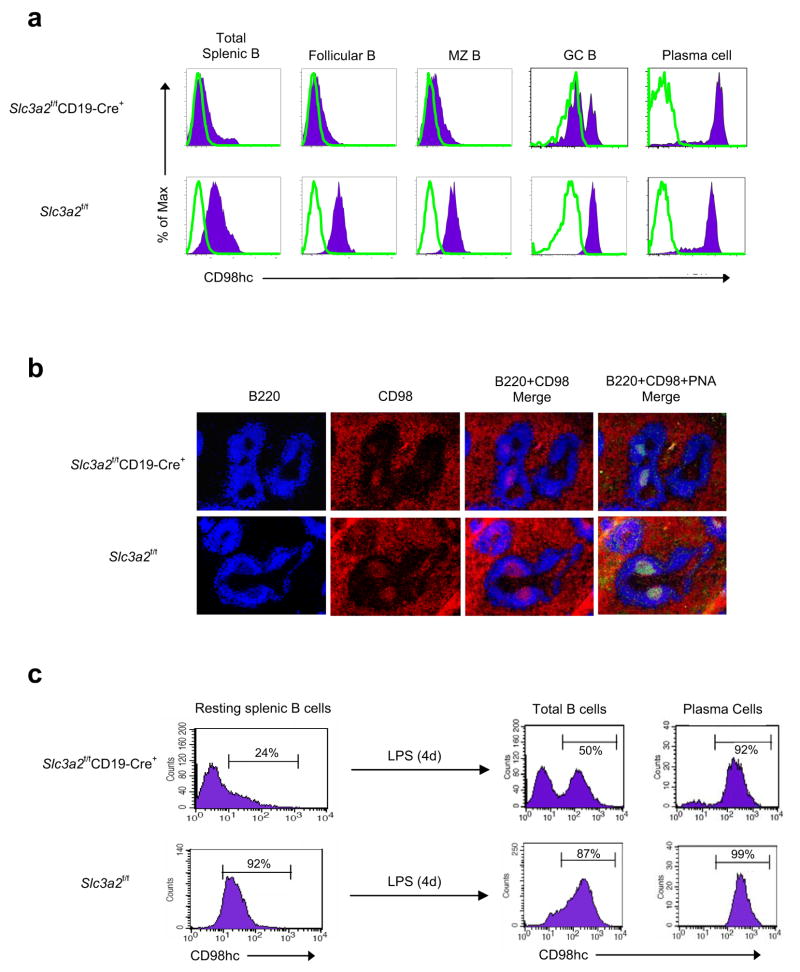
During T cell-dependent responses, antigen-specific B cells expand in germinal centers in competition for limited antigen 33. The preceding experiments indicated that CD98hc conferred a strong selective advantage during B cell expansion in vitro. To measure this advantage in vivo, we immunized Slc3a2f/fCD19-Cre+ mice with TNP-KLH, a T cell-dependent antigen, and analysed expression of CD98hc on splenic B cell subsets. In this experiment, CD98hc was present on <10% of resting splenic B cell subsets (Fig. 8a). However, during the germinal center response, B cells undergo a proliferative burst and are selected for the capacity to bind antigen with high affinity. At this stage, the percentage of CD98hc-expressing cells in the Slc3a2f/fCD19-Cre+ mice increased from <10% to 40% (Fig. 8a). Furthermore, following class switching, >99% of plasma cells in Slc3a2f/fCD19-Cre+ mice expressed detectable surface CD98hc (Fig. 8a). Thus, there were no detectable CD98-null plasma cells in Slc3a2fl/flCD19Cre mice, and these observations are consistent with our in vitro findings that CD98hc is required for the rapid proliferation of mature B cells and subsequent formation of plasma cells.
Figure 8.
Selection for CD98hc+ B cells during activation. (a) CD98hc expression on responding B cells in vivo. Cells were isolated from halves of spleens from Slc3a2f/fCD19-Cre+ and control mice immunized with the T cell-dependent antigen TNP-KLH in CFA. CD98hc expression (filled histogram) was measured on indicated B cell subsets by flow cytometry. Isotype control, open histograms. Data from one representative mouse of each genotype are shown (n=3 per group). (b) Selection of CD98hc+ B cells in vivo. Remaining halves of spleens were frozen, sectioned, and stained for B220 and CD98hc; germinal centers were verified with PNA staining. Data from one mouse are shown and are representative of 3 mice per group; experiment for (a-b) was repeated once. (c) Selection of CD98hc+ B cells in vitro. Resting splenic B cells were purified from Slc3a2f/fCD19-Cre+ mice without depletion of CD98hc+ cells or from control mice. Cells were cultured with LPS for 4 days, stained for B220, CD98hc, and CD138 (Syndecan-1, a plasma cell marker), and analyzed by flow cytometry. Representative data (n=3 per group) from one mouse are shown; entire experiment was repeated twice.
Slc3a2f/fCD19-Cre+ mice had significantly fewer germinal center B cells and plasma cells than Slc3a2f/fCD19-Cre− mice one week after immunization (data not shown), likely due to the existence of far fewer CD98hc-expressing precursor cells capable of proliferating in germinal centers and forming plasma cells. However, 2–3 weeks after immunization, titers of antibodies specific for TNP-KLH in Slc3a2f/fCD19-Cre+ mice were similar to those in Slc3a2f/fCD19-Cre− mice, likely due to germinal center selection for the few pre-existing CD98hc-expressing antigen-specific B cells (Supplementary Fig. 13). This strong selection for CD98hc-expressing B cells can thus account for the relatively modest reduction in basal serum IgG concentrations in Slc3a2f/fCD19-Cre+ mice. Immunohistochemical staining for CD98hc in spleens of immunized mice confirmed that germinal center B cells express CD98hc in Slc3a2f/fCD19-Cre+ mice, in contrast to surrounding follicular B cells (Fig. 8b). In vitro analysis of B cells purified from Slc3a2f/fCD19-Cre+ mice without depletion of CD98-expressing cells provided further confirmation of the strong selective advantage conferred by CD98hc; after 4 days in culture, 99% of plasma cells expressed CD98hc (Fig. 8c).
CD98hc is upregulated 20–30 fold after B cell activation and we showed here that CD98hc is required for B cells to proliferate and to differentiate into plasma cells. Hence we propose that upregulation of CD98hc can serve as a checkpoint in the progression to humoral immunity. Consequently, CD98hc expression and function are potential targets for the modulation of antibody responses.
Discussion
CD98hc was one of the earliest lymphocyte activation antigens described 3, yet its role in the immune response remained obscure. Whereas studies in which T cells were treated with anti-CD98hc antibody in vitro20, 21, 34 suggested that CD98hc was involved in T cell activation, the role of CD98hc in lymphocytes in vivo was unknown. Here we have shown that CD98hc is absolutely required for the rapid B cell clonal proliferation necessary for their subsequent differentiation into antibody-secreting plasma cells. Consequently, increased CD98hc expression provides antigen-stimulated B cells with a profound selective advantage.
The linkage of CD98hc integrin-binding function to B cell proliferation suggests a new paradigm for the role of integrin signaling in lymphocytes. In addition to functioning in hematopoiesis, trafficking, and in formation of immune synapses 16, 35–39, our work indicates that integrin signaling is involved in clonal proliferation during immune responses. CD98hc performs two cellular functions; the first is amino acid transport, through interaction with one of several light chains4, 5. The extracellular domain of CD98hc is responsible for this function, and reconstitution with a chimeric protein containing only this domain of CD98hc was not sufficient to rescue proliferation of CD98-null B cells. The second major function of CD98hc is mediating integrin signaling6, 7. The transmembrane and cytoplasmic domains of CD98hc are necessary for interaction with β-integrin subunits, which leads to pp125FAK-dependent PI3 kinase activation of Akt and p130CAS-mediated Rac activation 7. Reconstitution with a chimeric protein that contained only these portions of CD98hc was sufficient to rescue proliferation of CD98hc-null B cells.
Integrins can cooperate with immunoreceptors, utilizing similar downstream signaling proteins, to promote lymphocyte proliferation and activation40. Thus, our findings establish a nexus of CD98hc-dependent integrin and immunoreceptor signaling pathways regulating proliferation in lymphocytes. CD98hc could function by allowing integrins to lower the threshold for cellular activation41 by efficiently organizing components of the immunological synapse; however we found that CD98hc was not required for early activation events. Rather, our data support a mechanism whereby CD98h-mediated integrin signals can extend the kinetics of immunoreceptor signals42 to the point of driving cell division and clonal expansion.
Integrins drive fibroblast proliferation by sustaining Erk activation. Without integrin-mediated adhesion, growth factor signals are transient and unable to downregulate CDK inhibitors such as p27, and cells fail to exit the G1 phase of the cell cycle26 28, 29. Our data indicate that a similar mechanism is operative in B cells. CD98hc-deficient B cells were able to generate early BCR signals, but could not sustain late Erk1/2 signaling or downregulate p27, and failed to divide. The inability of CD98hc-deficient B cells to spread on integrin-specific antibodies confirmed the integrin signaling defect of CD98hc-deficient B cells. Recent work using supported lipid bilayers or plate-bound antibodies30 to study formation of immunological synapses, showed that B cells spread rapidly upon BCR activation in an integrin-dependent manner 30, 43; we now report that this spreading is CD98hc-dependent. Thus, CD98hc could promote integrin-dependent changes in cell shape that might stabilize interactions with antigen-bearing cells.
An intriguing implication of this work relates to the origin of the adaptive immune system. CD98hc orthologues first appear in primitive vertebrates10 as do sequences in integrin β-cytoplasmic domains that permit CD98hc to interact with integrins 44. The coincidence of these events with the emergence of adaptive immunity 2, 10 suggests that the survival advantage conferred by adaptive immunity was among the factors that favored the maintenance of CD98hc and its ability to mediate integrin signaling. CD98hc is over-expressed in many cancers and mediates tumorigenesis 7, 45–47. In particular, recent work underscores the importance of integrin signaling25 in the development and maintenance of epithelial cancers 48, 49 and of CD98hc in potentiating the growth of cancer cells 7, 47. Thus the appearance of CD98hc in vertebrates, which enables an adaptive immune response, may lead to increased susceptibility to cancer.
Methods
Mice
Slc3a2-floxed mice were generated by flanking exons 1 and 2, which encode the transmembrane portion of CD98hc, with loxP sites 12. The neomycin selection cassette used to select ES clones positive for a Slc3a2-floxed allele was flanked by Flp sites and thus was excised when Slc3a2-floxed mice were bred with human β-actin FLPe deleter mice (Jackson Laboratories). Slc3a2f/fCD19-Cre+ mice were the result of crossing Slc3a2f/f with the CD19-Cre+ strain 13. Slc3a2f/+CD19-Cre+ offspring were identified by PCR and backcrossed with Slc3a2f/f mice to create mice heterozygous for CD19-Cre and homozygous for the Slc3a2 floxed allele (Slc3a2flf). Experiments in this paper utilize this littermate comparison for every experiment possible. For \Supplementary Figures 2 and 4, Slc3a2+/− × Slc3a2f/fCD19-Cre+ offspring were used. All mice were housed at the University of California San Diego animal facility, and all experiments were approved by the Institutional Animal Care and Use Committee (IACUC). Pan integrin-deficient mice were generated as previously described 32. Briefly, integrins were ablated by crossing mice carrying Itgb1flf, Itgavflf, Itbg2−/−, and Itgb7−/− genes with mice carrying an Mx1-Cre transgene. Cre expression in hematopoietic cells was induced by intraperitoneal injection of 250 μg polyI:C (Amersham Biosciences). 8 days after polyI:C injection, mice were used for B cell proliferation assays.
Flow cytometry of B cell subsets
BM cells were prepared by dissecting femur and tibia bones from adult (10–20 wk-old) Slc3a2f/fCD19-Cre+ mice and flushing with media using a 23-gauge needle. After passing through the 23-gauge needle twice, erythrocytes were lysed for 8 min at room temperature using ACK lysis buffer (Biowhittaker). Splenic single-cell suspensions were prepared by dissociating whole spleens using a 7ml tissue grinder (Kontes), and lysing erythrocytes as for BM. Peritoneal lavage cells were isolated from adult mice by flushing peritoneum with 10 ml complete medium in 1–2 ml batches. After counting, BM, spleen, or peritoneal cells were stained in 100 μl of staining buffer (PBS, 0.5% BSA) containing fluorochrome-conjugated antibodies (BD Biosciences) against mouse B220 (RA3-6B2), IgM (II/41), CD21 (7G6), CD23 (B3B4), CD98hc (RL388), GL7 (GL7), CD138 (281-2), and Fas (Jo2), at optimal concentrations. After incubation on ice for 30–45 min, followed by three washes in staining buffer, subsets were analyzed by flow cytometry using FACS Aria or FACSCalibur cytometers (BD Biosciences).
Immunohistochemistry
For analysis of CD98 selection after immunization, adult Slc3a2f/fCD19-Cre+ and littermate control (Slc3a2flf) mice were immunized with 100 μg TNP-KLH (Biosearch) emulsified in Complete Freund’s Adjuvant (CFA) (250 μl i.p.). Seven days later, spleens were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek U.S.A.) and frozen at −80°C. Eight μm sections were mounted on microscope slides, fixed for 10 min in cold acetone, blocked for 1 h with block buffer (0.5% BSA in PBS), and stained for 1hr at RT with peanut agglutinin (PNA)-FITC (Vector Labs) and anti-B220 (BRA3-6B2), CD5 (53-7.3), CD3 (145-2C11), CD98 (RL388) (all from BD Biosciences), or anti-MOMA-1 (BaChem, clone MOMA-1). After washing with PBS containing 0.5% Tween, sections were covered with Gel/Mount (Biomeda Corp) and sealed with glass coverslips. Images were acquired using Zeiss Axiocam M1 microscope (Zeiss) and Slidebook software (Intelligent Imaging Innovations).
Antibody analyses
For antigen-specific antibody responses, adult Slc3a2f/fCD19-Cre+ and control littermate mice were injected i.p. with 50 μg TNP-LPS (Sigma) in 250 μl PBS (T cell-independent antigen), or 100 μg TNP-KLH (Biosearch) emulsified in 250 μl CFA (T cell-dependent antigen). Blood serum was collected by centrifugation of tail vein bleed (100–200 μl with 1–2 mM EDTA solution as an anticoagulant) before (pre-immune) and at 1, 2, and 3 wk after immunization. TNP-specific antibody concentrations in blood sera were assessed by direct ELISA with TNP-OVA as the coating antigen and AP-conjugated polyclonal anti-mouse IgG (Sigma), polyclonal anti-mouse IgM (Sigma), or anti-mouse IgG3 (Clone R40-82, BD Biosciences) as the detection antibody. Due to inconsistencies in commercially available anti-TNP standards, ELISA data in Fig. 2 and Supplementary Fig. 13 were quantified by multiplying the O.D. reading out of 3–4 dilutions of sample that was in the linear portion of the assay with the dilution factor to obtain values shown in bar graphs. Circulating IgG and IgM concentrations from naive mice and from in vitro splenic B cell stimulations were measured by sandwich ELISA with polyclonal anti-mouse IgG (Sigma) or anti-mouse IgM (Sigma), detection antibodies as for direct ELISA, and standard curves obtained with purified polyclonal IgG or IgM.
In vitro proliferation and differentiation of resting B cells
Resting B cells (defined as CD43−) were purified from spleen cell suspensions of Slc3a2f/fCD19-Cre+, pan integrin-deficient, or control mice by depletion of CD43+ cells (and depletion of CD98hc+ B cells for Slc3a2f/fCD19-Cre+ mice or depletion of β-1 integrin+ cells in pan integrin-deficient mice) using B cell magnetic beads (Dynal). Purity, as assessed by anti-B220 (RA3-6B2) or anti-CD43 (eBioR2-60) staining and flow cytometry, was routinely 95–98%. Resting B cells were plated (400,000 per well) in 48-well plates and stimulated for 3–5 days with 20 μg/ml Ultrapure LPS (InVivoGen), 30 μg/ml F(Ab′)2 goat anti-mouse IgM (Jackson Immunoresearch), 2 μg/ml anti-CD40 (BD Biosciences, Clone 3/23), or 50 ng/ml recombinant murine IL-4 (Peprotech). Unstimulated resting cells were cultured with media alone. Anti-CD138 (eBioscience, clone DL-101), anti-IgG3 (BD Biosciences, clone R40-82) and anti-B220 (eBioscience, clone RA3-6B2) staining and flow cytometry were used to assess class-switching and differentiation to plasma cells. For measuring proliferation, purified resting B cells were labeled with 2 μM carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes), and analyzed by flow cytometry for dilution of fluorescence from cell division at 3–5 days. In Supplementary Fig. 12 histograms of unstimulated cells are shown, as the extent of CFSE labelling varied between samples. For some differentiation or proliferation experiments cells were also stained with anti-CD98 (eBioscience clone RL388). Propidium iodide exclusion and automated enumeration by flow cytometry was used for analyzing expansion of cell populations. It should be noted that in this in vitro culture system, many of the cells die or are lost during harvesting or staining, and a smaller subset divide rapidly.
Retroviral CD98hc-chimera reconstitution and BM transplant
The BM transplant protocol was generously shared by S. Rowland in the lab of R. Pelanda (Denver, CO). Briefly, donor mice were treated with 4 μg per mouse of 5-Fluorouracil (Adrucil, from Sicor pharmaceuticals) in 200 μl PBS, i.p. Three days later, ecopack293 packaging cells (Imgenex) were transfected with pCl-Eco (Imgenex) and with one of 4 retroviral constructs (C98T98E98, C98T69E98, C98T98E69, or C69T69E69 in an MSCV-IRES-GFP backbone) 50 and cultured for 48 hours. On day 4, donor mice were sacrificed, and BM cultured overnight in complete medium containing IL-3 (25 ng/ml), IL-6 (50 ng/ml), and SCF (50 ng/ml) (all from Peprotech). Viral supernatants were collected from packaging cells on day 5 and used to spin-fect BM at 2800 rpm, room temperature for 90 min on days 5 and 6. BM cells were harvested on day 7 and injected (250,000 per mouse) in 100 μl PBS i.v. Small samples were retained and stained for Sca-1 (eBioscience, clone D7), a marker of stem cells. In all samples, 24–29% of Sca-1+ populations were GFP+ before injection. 6–8 wk after transplant, (mouse)CD98hc-deficient resting B cells were purified from spleens of recipient mice, labeled, and stimulated with LPS. Proliferation and plasma cell differentiation was assessed as described above, but using the membrane-dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine DiD (Molecular Probes) that emits in a range distinct from GFP.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants AR27214, HL31950, and HL0780784. C.C.F. was a post-doctoral fellow of the Arthritis Foundation and J.C. is a post-doctoral fellow of the National Multiple Sclerosis Society (FG1802-A-1). The authors thank M. Slepak for excellent technical assistance, D. Rose (V.A. Hospital) for helpful advice on analyzing antibody responses, M. Cato (Burnham Insititute) for ELISPOT advice and protocols, and R. Zent (Vanderbilt University) for generously sharing Slc3a2+/− mice.
Footnotes
Additional Methods
Information on hematology, ELISPOT, spreading assay, signaling, and statistical analysis can be found online in Supplementary Methods.
Author Contributions
This project was conceived by M. Ginsberg, J. Cantor, and C. Féral, with advice and collaboration from R. Rickert. Experiments were done by J. Cantor with the following exceptions: immunohistochemical analysis of spleen sections and flow cytometry for B cell subsets were performed by C. Browne in the lab of R. Rickert, and R. Ruppert tested B cell proliferation in pan integrin-deficient mice in the lab of R. Fässler. J. Cantor and M. Ginsberg wrote the manuscript with editorial input from R. Rickert and C. Féral. The CD19-Cre mouse was provided by R. Rickert.
Author Information
The authors declare no competing financial interests.
References
- 1.Burnet M. The Clonal Selection Theory of Acquired Immunity. Cambridge University Press; Cambridge: 1959. [Google Scholar]
- 2.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–22. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Kehrl JH, Fauci AS. Identification, purification, and characterization of antigen-activated and antigen-specific human B lymphocytes. Trans Assoc Am Physicians. 1983;96:182–7. [PubMed] [Google Scholar]
- 4.Bertran J, et al. Stimulation of system y(+)-like amino acid transport by the heavy chain of human 4F2 surface antigen in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1992;89:5606–10. doi: 10.1073/pnas.89.12.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torrents D, et al. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem. 1998;273:32437–45. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- 6.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–5. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- 7.Feral CC, et al. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci U S A. 2005;102:355–60. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham RT. Mammalian target of rapamycin: immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr Opin Immunol. 1998;10:330–6. doi: 10.1016/s0952-7915(98)80172-6. [DOI] [PubMed] [Google Scholar]
- 9.Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–72. doi: 10.1016/j.smim.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uinuk-Ool T, et al. Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc Natl Acad Sci U S A. 2002;99:14356–61. doi: 10.1073/pnas.212527699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsumura H, et al. The targeted disruption of the CD98 gene results in embryonic lethality. Biochem Biophys Res Commun. 2003;308:847–51. doi: 10.1016/s0006-291x(03)01473-6. [DOI] [PubMed] [Google Scholar]
- 12.Feral CC, et al. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J Cell Biol. 2007;178:701–11. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–8. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J Immunol. 2003;171:5921–30. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- 15.Brakebusch C, et al. Beta1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity. 2002;16:465–77. doi: 10.1016/s1074-7613(02)00281-9. [DOI] [PubMed] [Google Scholar]
- 16.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–12. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 17.Fenczik CA, et al. Distinct domains of CD98hc regulate integrins and amino acid transport. J Biol Chem. 2001;276:8746–52. doi: 10.1074/jbc.M011239200. [DOI] [PubMed] [Google Scholar]
- 18.Tangye SG, Hodgkin PD. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology. 2004;112:509–20. doi: 10.1111/j.1365-2567.2004.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol. 2004;5:55–63. doi: 10.1038/ni1016. [DOI] [PubMed] [Google Scholar]
- 20.Freidman AW, Diaz LA, Jr, Moore S, Schaller J, Fox DA. The human 4F2 antigen: evidence for cryptic and noncryptic epitopes and for a role of 4F2 in human T lymphocyte activation. Cell Immunol. 1994;154:253–63. doi: 10.1006/cimm.1994.1075. [DOI] [PubMed] [Google Scholar]
- 21.Diaz LA, Jr, et al. Int Immunol. 1997;9:1221–31. doi: 10.1093/intimm/9.9.1221. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Herrera J, Sanchez-Madrid F, Diez AG. Differential expression of the 4F2 activation antigen on human follicular epithelium in hair cycle. J Invest Dermatol. 1989;92:247–50. doi: 10.1111/1523-1747.ep12276789. [DOI] [PubMed] [Google Scholar]
- 23.Zent R, et al. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J Biol Chem. 2000;275:5059–64. doi: 10.1074/jbc.275.7.5059. [DOI] [PubMed] [Google Scholar]
- 24.Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans. 2007;35:1187–90. doi: 10.1042/BST0351187. [DOI] [PubMed] [Google Scholar]
- 25.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–60. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 27.Motti ML, et al. Loss of p27 expression through RAS-->BRAF-->MAP kinase-dependent pathway in human thyroid carcinomas. Cell Cycle. 2007;6:2817–25. doi: 10.4161/cc.6.22.4883. [DOI] [PubMed] [Google Scholar]
- 28.Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 29.Walker JL, Assoian RK. Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 2005;24:383–93. doi: 10.1007/s10555-005-5130-7. [DOI] [PubMed] [Google Scholar]
- 30.Lin KB, et al. The rap GTPases regulate B cell morphology, immune-synapse formation, and signaling by particulate B cell receptor ligands. Immunity. 2008;28:75–87. doi: 10.1016/j.immuni.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Arana E, et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 2008;28:88–99. doi: 10.1016/j.immuni.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 33.Wolniak KL, Shinall SM, Waldschmidt TJ. The germinal center response. Crit Rev Immunol. 2004;24:39–65. doi: 10.1615/critrevimmunol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 34.Warren AP, et al. Convergence between CD98 and integrin-mediated T-lymphocyte co-stimulation. Immunology. 2000;99:62–8. doi: 10.1046/j.1365-2567.2000.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu Y, Rose DM, Ginsberg MH. Integrins in the immune system. Adv Immunol. 1999;72:325–80. doi: 10.1016/s0065-2776(08)60024-3. [DOI] [PubMed] [Google Scholar]
- 36.Sims TN, Dustin ML. The immunological synapse: integrins take the stage. Immunol Rev. 2002;186:100–17. doi: 10.1034/j.1600-065x.2002.18610.x. [DOI] [PubMed] [Google Scholar]
- 37.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 38.Lo CG, Lu TT, Cyster JG. Integrin-dependence of lymphocyte entry into the splenic white pulp. J Exp Med. 2003;197:353–61. doi: 10.1084/jem.20021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–34. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 40.Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 41.Batista FD, et al. The role of integrins and coreceptors in refining thresholds for B-cell responses. Immunol Rev. 2007;218:197–213. doi: 10.1111/j.1600-065X.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 42.Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK. Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell. 1999;10:3197–204. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleire SJ, et al. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–41. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 44.Prager GW, Feral CC, Kim C, Han J, Ginsberg MH. CD98hc (SLC3a2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem. 2007 doi: 10.1074/jbc.M702877200. [DOI] [PubMed] [Google Scholar]
- 45.Esteban F, et al. Relationship of 4F2 antigen with local growth and metastatic potential of squamous cell carcinoma of the larynx. Cancer. 1990;66:1493–8. doi: 10.1002/1097-0142(19901001)66:7<1493::aid-cncr2820660710>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 46.Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun. 1999;262:720–5. doi: 10.1006/bbrc.1999.1051. [DOI] [PubMed] [Google Scholar]
- 47.Henderson NC, et al. CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J Biol Chem. 2004;279:54731–41. doi: 10.1074/jbc.M408700200. [DOI] [PubMed] [Google Scholar]
- 48.White DE, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 49.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–94. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Parijs L, et al. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–8. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.