Abstract
The linker for activation of T cells (LAT) is an adaptor protein that couples T cell receptor (TCR) engagement to downstream signaling cascades. LAT is important in early thymocyte development as LAT-deficient mice have a complete block at the DN3 stage. To study the role of LAT beyond the DN3 stage, we generated mice in which the lat gene was deleted by the Cre recombinase. Analysis of these mice showed that deletion of LAT after the DN3 stage allowed thymocytes to develop past the DN3 to DN4 checkpoint and to generate DP thymocytes. However, LAT-deficient DP thymocytes were severely defective in responding to stimulation via the TCR and failed to differentiate into SP thymocytes efficiently. Consequently, few LAT-deficient mature T cells could be found in the periphery. These T cells had undergone extensive homeostatic proliferation and expressed low levels of the TCR on their surface. In addition, our data suggested that expression of LAT was also essential for Foxp3 expression during Treg development and Treg maintenance in the periphery. Together our data indicate that in addition to its role in pre-TCR signaling, LAT also plays an essential role in thymocyte development during transition from the DP to SP stage.
Keywords: T cells, cell activation, cell differentiation, signal transduction
Introduction
LAT (linker for activation of T cells) is a membrane-associated adaptor protein that couples the engagement of T cell receptor (TCR) to Ras-MAPK activation and Ca2+ mobilization. Upon TCR engagement, LAT is phosphorylated by ZAP-70 tyrosine kinase on its membrane-distal tyrosine residues and directly interacts with Grb2, Gads, and PLC-γ1 (1–5). LAT binding of Gads recruits an important cytosolic adaptor protein, SLP-76, to the membrane, which can further interact with PLC-γ1, Vav1, and other signaling molecules(6, 7). Together, LAT and SLP-76 bring PLC-γ1 to the plasma membrane to be phosphorylated and activated. Activated PLC-γ1 then hydrolyzes PIP2 into IP3 and DAG. IP3 interacts with the IP3-receptor and induces intracellular Ca2+ mobilization, whereas DAG activates PKCs and binds to RasGRP1 to activate the Ras-MAPK pathway(8, 9). LAT also contributes to Ras-MAPK activation through the recruitment of Grb2, which in turn brings Sos to the plasma membrane to activate Ras(10).
The essential role of LAT in T cell development has been clearly demonstrated in LAT-deficient mice. Thymocyte development in LAT−/− mice is completely blocked at the CD25+CD44− DN3 stage, indicating an absolute requirement for LAT in pre-TCR mediated signal transduction. As a result, LAT−/− mice completely lack DP and SP thymocytes in the thymus and mature T cells in the periphery(11). Similar phenotypes were seen in mice with the four membrane-distal tyrosines, Y136, Y175, Y195, and Y235 mutated simultaneously, indicating the importance of these tyrosines in LAT function(12). Interestingly, mice with a mutation at the PLCγ1-binding site of LAT (Y136) have a partial bock in thymocyte development; however, they develop a polyclonal lymphoproliferative disease due to hyperactivation and expansion of CD4+ T cells(13–15). While both positive and negative thymocyte selection are impaired, the development of the naturally arising CD4+CD25+ regulatory T cells (Treg) is severely impaired in the LAT Y136F mice(16). Introduction of CD4+CD25+ Treg cells into these mice is able to prevent the development of the autoimmune disease(16). Interestingly, mice with mutations at the other three membrane-distal tyrosines (Y175, Y195, and Y235) also develop a similar disease(17). These data suggest that LAT-mediated signaling is important in T cell activation and the control of autoimmunity.
The early block of thymocyte development in LAT−/− mice makes it impossible to study the function of LAT beyond the DN3 stage. Thus, the role of LAT in the transition from DN3 to DP and, further, from DP to SP stages remains unclear. In addition, the function of LAT in TCR-mediated signaling events, such as Ras-MAPK activation and calcium mobilization, has mostly been demonstrated in Jurkat cell lines(2, 3). Therefore, its role in primary T cells remains to be determined. To address these questions, we generated LAT knock-in mice in which the lat gene could be deleted upon expression of the Cre recombinase. By using CD4Cre transgenic mice, we successfully deleted LAT between the DN3 and DP stages. Our data showed that deletion of LAT after the DN3 stage allowed for the development of DP thymocytes. However, these LAT-deficient DP thymocytes were not able to respond to stimulation from the TCR and failed to further differentiate into mature T cells. Moreover, our data showed that expression of LAT in the DP stage was also essential for Treg development, and that LAT might play an important role in Treg maintenance in the periphery.
Material and Methods
Generation of LAT knock-in mice
The targeted ES cells were injected into 129/Sv blastocysts to generate chimeric mice, which were subsequently crossed with Flp transgenic mice to generate LATf/+ mice. LATf/+ mice were backcrossed with C57BL/6 mice for at least six generations before analysis. Flp, CD4Cre, and β-actin-Cre mice were purchased from the Jackson Laboratory. LATf/f mice were crossed with CD4Cre+LAT−/− mice to generate CD4Cre+LATf/− and LATf/− mice. All mice were used in accordance with the National Institutes of Health guidelines. The experiments described in this study were reviewed and approved by the Duke University Institutional Animal Care Committee. Mice were housed in specific pathogen-free conditions.
Antibodies, FACS analysis, and sorting
Fluorescence-conjugated antibodies used in flow cytometry, such as anti-CD3, CD4, CD8, CD25, CD44, CD62L, CD5, CD69, HSA, TCRβ, TCRγδ, and Foxp3, were all purchased from eBioscience. For cell surface marker staining, single-cell suspensions were prepared from mouse thymi, lymph nodes, or spleens and were incubated with the 2.4G2 antibody (anti-Fcγ receptor) before staining with different antibody mixtures. Further intracellular staining of Foxp3 was performed using the Foxp3 staining buffer set (eBioscience) according to the instruction from the manufacturer. FACS data were acquired on FACSDiva (Beckton Dickinson) and analyzed with the Flowjo software. DP thymocytes were sorted on FACSDiva after staining with antibodies against CD4 and CD8.
Western blotting
5×106 FACS-sorted DP cells were either left untreated or stimulated by crosslinking CD3 for 2 minutes, and were subsequently lysed in RIPA buffer. The postnuclear lysates were resolved on SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were then blotted with different primary antibodies. The anti-pErk, pPLCγ1, and PLCγ1 antibodies were purchased from Cell Signaling. Anti-Erk2 was purchased from Santa Cruz. The anti-LAT (11B12) and anti-pY (4G10) antibodies were from Upstate Biotechnology. For secondary antibodies, Alexa Fluor 680 anti-mouse-IgG (Molecular Probes) or IRDye 800 anti-rabbit-IgG (Rockland) were used accordingly. The membranes were scanned by the Licor Odyssey infrared imaging system.
Calcium flux
Thymocytes were first loaded with Indo-1 (Molecular Probes) in loading buffer (1× HBSS with 10 mM HEPES and 1% FBS) for 30 minutes and then stained with PE-anti-CD4 and PECy5-anti-CD8 antibodies. Calcium flux was initiated by the addition of biotinylated anti-CD3 (5 μg/ml) and anti-CD4 (1 μg/ml) or anti-CD8 (1 μg/ml) followed by crosslinking with streptavidin (25 μg/ml final concentration, Sigma). Ionomycin (2 μg/ml, Sigma) was used to induce TCR-independent calcium flux to ensure equal loading of Indo-1 dye. Calcium flux was assayed by monitoring the fluorescence emission ratio at 405/510 nm with BD FACStar and analyzed using the Flowjo software.
Results
Generation of LAT knock-in mice
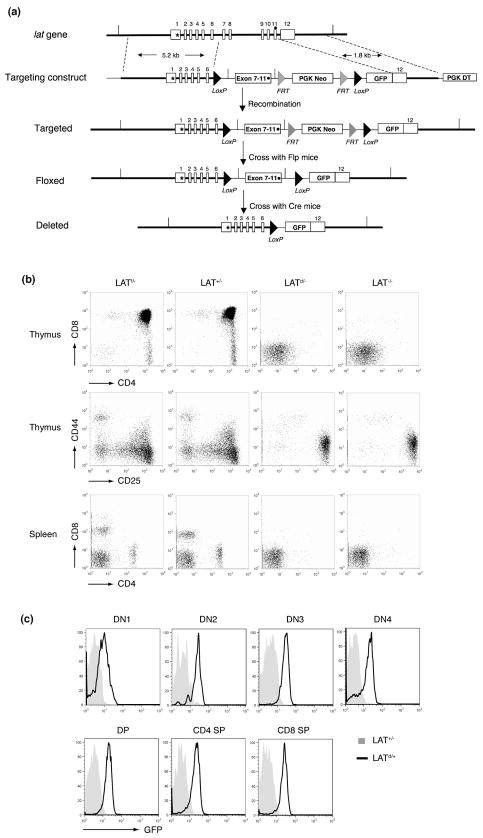
LAT-deficient mice have an early block in thymocyte development in the DN3 stage. To study the function of LAT in T cells beyond DN3, we generated mice in which the lat gene can be deleted conditionally upon expression of the Cre recombinase. As shown in Figure 1a, the lat gene consists of 12 exons with a stop codon at exon 11 and the 3′ untranslated region at exon 12. We replaced exons 7–11 with an artificial exon containing the corresponding cDNA fragment of these exons. These exons encode the C-terminal region of LAT, which has six membrane-distal tyrosine residues. These tyrosine residues are important for LAT function in T cell activation and development; mutation of these residues renders LAT non-functional (12, 18). To monitor deletion of the lat gene, we inserted an artificial exon containing a modified gfp gene that lacks its own start codon. Without deletion of the lat gene, GFP should not be expressed from the floxed alleles because the lat stop codon precedes it. However, upon expression of the Cre recombinase, the floxed lat sequence, including the stop codon, will be deleted, and a LAT-GFP fusion will be expressed from the deleted allele. Therefore, T cells should be GFP+ after deletion of LAT.
Figure 1. Generation of LAT knock-in mice.
(a). The LAT knock-in targeting strategy. Twelve lat exons are indicated. The initiation codon (*) is at the exon 1. The stop codon (·) is at the exon 11. Exon7–11 in the targeting construct was made from the corresponding lat cDNA fragment. The grey triangles represent the FRT sites. The black triangles represent the Loxp sites.
(b). T cell development in LATf/−, LAT+/−, LATd/− and LAT−/− mice. The thymi and spleens from 5-week old mice were analyzed by FACS. Top: the CD4 vs. CD8 profile of thymocytes. Middle: the CD25 vs. CD44 profile of the CD4−CD8− (DN) thymocytes. Bottom: the CD4 vs. CD8 profile of splenocytes.
(c). The GFP expression profile in different subsets of thymocytes from the age-matched LAT+/− and LATd/+ mice.
Targeted embryonic stem (ES) cells were used to generate mice with the floxed lat allele (LATf/+). To examine whether the floxed allele is functional, we crossed LATf/+ mice with LAT knockout mice (LAT−/−). Analysis of thymocyte development in LATf/− mice showed that T cells developed normally. Normal percentages of DP and SP thymocytes were seen (Figure 1b). Mature T cells were also present in the periphery (Figure 1b). T cells from LATf/− mice expressed normal TCR levels on their cell surface (data not shown). These data indicated that the floxed lat allele is functional during thymocyte development.
To show the loss of function after deletion, we first crossed LATf/+ mice with β-actin Cre transgenic mice, in which the Cre recombinase is ubiquitously expressed, to generate LATd/+ mice (d=deleted). We then crossed LATd/+ mice with LAT−/− mice to obtain LATd/− mice. Similar to LAT−/− mice, these LATd/− mice showed a profound block in thymocyte development at the DN3 stage and contained no mature T cells in the periphery (Figure 1b). These data indicated that the floxed lat allele can be successfully deleted upon Cre expression and that such deletion renders LAT non-functional during thymocyte development.
In addition, since GFP was inserted into the lat locus, GFP expression upon deletion of LAT should be able to mark cells that normally express LAT. To examine LAT expression during thymocyte development, we monitored GFP expression in different subsets of thymocytes in LATd/+ mice. Corresponding thymocyte subsets from LAT+/− mice were used as negative controls in FACS analysis. As shown in Figure 1c, the GFP fluorescence signal was detected as early as the DN1 stage, although the expression level was low when compared with other subsets of thymocytes. GFP expression was seen throughout the later stages of thymocyte development, from DN2 to SP. Mature T cells in the periphery of LATd/+ mice expressed GFP as well (data not shown). In contrast, mature B cells were GFP− (data not shown). These data showed that GFP can be expressed upon deletion of the lat gene and can be used as a marker to monitor the lat gene deletion on a single-cell basis.
LAT deletion after the DN3 stage allows development of DP thymocytes
To study LAT function during thymocyte development beyond DN3, we crossed LAT knock-in mice (LATf/f) with CD4Cre transgenic mice that had been bred onto the LAT−/− background (CD4Cre+LAT−/−) to generate CD4Cre+LATf/− mice. In these mice, expression of the Cre recombinase is driven by the CD4 proximal promoter and is initiated at the late DN3 stage(19, 20). Thymocyte development in CD4Cre+LATf/− mice should progress through the DN3 checkpoint and possibly beyond.
Thymi from 4-week-old CD4Cre+LATf/− mice were slightly smaller than those from their littermate controls (LATf/−), and their total cellularity was moderately decreased by approximately 25% (Figure 2b). FACS analysis of thymocytes showed that, in contrast to LAT−/− mice, which lack both DP and SP thymocytes, DP thymocytes were clearly present in CD4Cre+LATf/− mice (Figure 2a). The percentage of DP thymocytes in these mice was slightly increased compared with LATf/− controls (Figure 2a); the absolute number of DP thymocytes was decreased by approximately 20%3 (Figure 2b). The percentage of DN thymocytes in CD4Cre+LATf/− mice was slightly increased (Figure 2a), although the number of DN thymocytes was similar (Figure 2b). Despite a large number of DP thymocytes, very few SP thymocytes were present in the CD4Cre+LATf/− mice. Both the percentages and the numbers of SP thymocytes were dramatically reduced compared with LATf/− controls (Figure 2a, 2b). To confirm Cre-mediated deletion, we analyzed GFP expression in different thymocyte subsets of CD4Cre+LATf/− mice. As shown in Figure 2c, GFP+ cells appeared as early as the DN3 stage, although the percentage of GFP+ DN3 cells was very low. This is consistent with previous publications showing that Cre activity is initiated in the late DN3 stage in this CD4-Cre transgenic line(20). A small number of GFP+ DN4 or immature single positive (ISP) cells were detected as well. Most of the DP thymocytes clearly expressed GFP. To confirm the absence of LAT protein after deletion, GFP+ DP cells from CD4Cre+LATf/−mice were sorted by FACS, with DP cells from LATf/− mice also sorted as a control. Lysates of these cells were subjected to Western blotting by an anti-LAT antibody. As shown in Figure 2d, LAT protein was not detected in GFP+ DP cells from CD4Cre+LATf/− mice. These data indicated that, although Cre-mediated deletion of LAT started in as early as the DN3 stage, such deletion mainly occurred in and was nearly completed by the DP stage.
Figure 2. Thymocyte development in the CD4Cre+LATf/− mice.
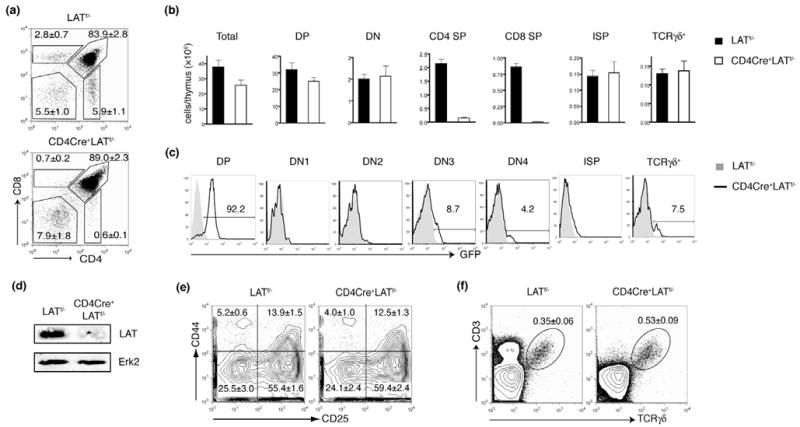
(a). The CD4 vs. CD8 expression profile of total thymocytes from 4-week-old LATf/− and CD4Cre+LATf/− littermates. The figure shown is one representative of five mice analyzed.
(b). The total numbers of different thymocyte populations from 4-week-old LATf/− and CD4Cre+LATf/− littermates. Five mice for each genotype were analyzed.
(c). GFP expression in different subsets of thymocytes.
(d). Western blot analysis of LAT expression in GFP+DP thymocytes. GFP+ DP thymocytes were sorted by FACS and lysed. Postnuclear lysates were analyzed by Western blotting. An anti-Erk2 blot was used as a loading control.
(e). The CD25 vs. CD44 expression in the DN thymocytes from the LATf/− and CD4Cre+LATf/− mice.
(f). The development of 33 T cells in CD4Cre+LATf/− mice. The numbers in each panel represent the percentages of the gated population.
We further examined thymocyte development before the DP stage. Analysis of DN thymocytes from CD4Cre+LATf/− and LATf/− mice by expression of CD44 and CD25 revealed that DN thymocyte development in CD4Cre+LATf/− mice was relatively normal as no obvious differences in the percentages of DN subsets were seen (Figure 2e). We also assessed the development of ISP thymocytes (CD4−CD8+TCRlo-intHSAhi), an intermediate stage between DN and DP(21, 22). Although very few CD8+ SP thymocytes were present in CD4Cre+LATf/−mice (Figure 2a), most of them were TCRlo-intHSAhi, a phenotype typical of ISP cells (Figure 3a). The number of ISP thymocytes in CD4Cre+LATf/− mice was comparable to that in control mice (Figure 2b). Normal development of thymocytes from DN to ISP was expected since the lat gene was only deleted in a small fraction of these two populations (Figure 2c).
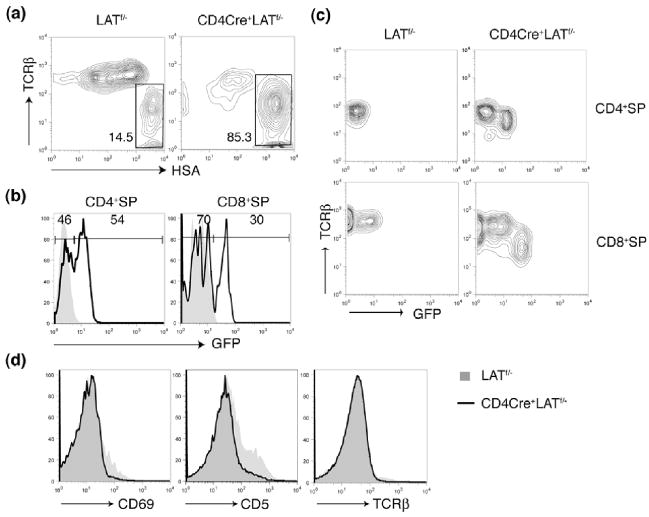
Figure 3. The defective positive selection in the CD4Cre+LATf/− mice.
(a). TCRβ vs. HSA expression on CD4−CD8+ thymocytes from 4-week old LATf/− and CD4Cre+LATf/− littermates.
(b). GFP expression in the CD4+SP and CD8+SP thymocytes (gated on HSAneg-lo population) in the LATf/− and CD4Cre+LATf/− mice. The shadowed area represents cells from LATf/− mice.
(c). GFP vs. surface TCRβ expression in the CD4+SP and CD8+SP thymocytes (gated on HSAneg-lo population).
(d). Expression of CD69, CD5, and TCRβ on DP thymocytes from the LATf/− and CD4Cre+LATf/− mice.
In addition, we examined the development of γδ T cells, which are absent in LAT-deficient mice(11). The divergence of γδ and αβ T cells is first evident in the DN2 stage and is largely complete by the DN3 stage(23–25). Thus, CD4Cre-mediated LAT deletion is not expected to significantly affect the development of γδ T cells. As predicted, the numbers of CD3+TCRγδ+ thymocytes in CD4Cre+LATf/− mice were similar to those in control mice (Figure 2b), although the percentage of γδ T cells was increased slightly due to the decreased number of total thymocytes in these mice (Figure 2f). While the majority of the γδ-TCR+ thymocytes remained GFP− (Figure 2c), there was a small population of GFP+ γδ-TCR+ thymocytes (~7.5%). It is possible that Cre is also expressed in γδ thymocytes even though it is driven by the CD4 proximal promoter. Another possibility is that these GFP+ γδ-TCR+ thymocytes were derived from DN3 cells in which there was some residual LAT protein after deletion of the lat gene.
Altogether, the data above showed that deletion of LAT by CD4-Cre was able to drive thymocyte development past the DN3 checkpoint and resulted in relatively normal development of DP thymocytes. Deletion of the lat gene in these DP cells was nearly complete as indicated by both the expression of GFP and the absence of LAT protein. CD4Cre+LATf/− mice can be used to study the role of LAT in DP thymocytes development and signaling.
Absence of LAT in DP thymocytes blocks further maturation
The signals initiated by the interaction between αβ TCRs and MHC-peptide complexes drive DP thymocytes through both positive and negative selection to develop into SP thymocytes(26, 27). The existence of LAT-deficient DP thymocytes in the CD4Cre+LATf/− mice enabled us to investigate whether LAT is required during the transition from DP to SP. In order to assess this, we next examined the development of SP thymocytes in CD4Cre+LATf/− mice. Unlike DP cells, very few SP thymocytes were generated in these mice (Figure 2a). The number of CD4+SP cells was reduced more than 10 fold while the number of mature CD8+SP (CD4−CD8+TCRhiHSAlo-int) cells decreased by approximately 20 fold (Figure 2b). Among the CD4−CD8+ thymocytes, more than 85% were TCRlo-intHSAhi ISP cells (Figure 3a). Interestingly, although the vast majority of DP thymocytes expressed GFP, about 46% of mature CD4+ and 70% of mature CD8+ SP thymocytes were GFP− (Figure 3b). These GFP− SP thymocytes were likely derived from a few DP thymocytes that had escaped lat deletion. Although our data strongly suggested that LAT played an important role during the transition from DP to SP, we did detect some GFP+CD4+ or GFP+CD8+ SP thymocytes in these mice (Fig.3b). While we would not exclude the possibility that GFP+ (LAT-deficient) DP cells are still capable of developing into SP cells at a reduced efficiency, these GFP+ SP thymocytes are likely derived from GFP− DP or SP cells with continued Cre-mediated deletion. Interestingly, surface expression of the TCR on these GFP+ SP thymocytes was considerably lower than that on their GFP− counterparts (Figure 3c).
The absence of LAT completely abrogates TCR-mediated Erk activation and calcium mobilization in Jurkat cells(2, 3). If LAT plays a similar role in DP thymocytes to that observed in Jurkat cells, LAT-deficiency should severely impair positive selection. To examine positive selection of thymocytes in CD4Cre+LATf/− mice, we analyzed the surface expression of CD5, CD69, and TCRβ on DP thymocytes. Upregulation of these markers is considered to be one of the characteristics of positive selection(28–32). While a small percentage of DP thymocytes from LATf/− mice upregulated CD5, CD69, or TCRβ, these cells were greatly reduced in the GFP+ DP thymocytes from CD4Cre+LATf/− mice (Figure 3d). Together, these data clearly demonstrated that deletion of LAT in DP thymocytes impaired positive selection and blocked the development of SP thymocytes. Therefore, in addition to its essential role in pre-TCR-mediated signaling in DN thymocytes, LAT is also indispensable for TCR-dependent maturation of DP thymocytes.
LAT deletion abrogates TCR-mediated signaling in DP thymocytes
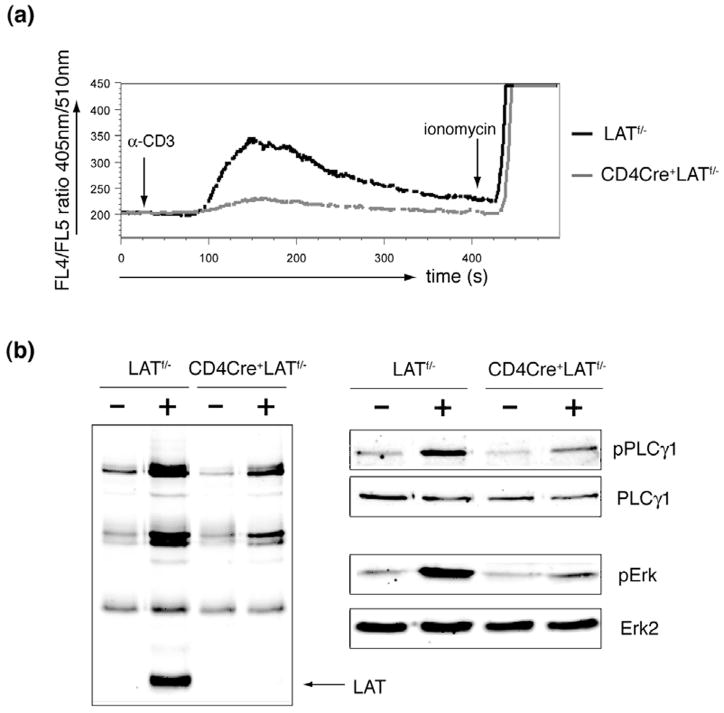
The developmental block during the DP to SP transition in CD4Cre+LATf/− mice is likely caused by the inability of LAT-deficient DP thymocytes to respond to TCR engagement. In LAT-deficient Jurkat cells, TCR-mediated Erk activation and calcium flux are defective. To investigate the function of LAT in thymocytes, we first examined TCR-mediated calcium flux in DP thymocytes. As shown in Figure 4a, GFP+ DP thymocytes from CD4Cre+LATf/− mice failed to mobilize calcium upon crosslinking of CD3. These thymocytes were able to respond normally to ionomycin treatment, suggesting that their calcium release apparatus was intact. Next, we performed biochemical analysis of TCR-mediated signaling events. GFP+ DP thymocytes were sorted from CD4Cre+LATf/− mice. DP thymocytes from LATf/− mice were also sorted as a control. The post-sort purity was >99% (data not shown). These thymocytes were stimulated with anti-CD3 before lysis. As shown in Figure 4b, while the total tyrosine phosphorylation of proteins in the GFP+ CD4Cre+LATf/− DP thymocytes was relatively normal, phosphorylated LAT was absent. Phosphorylation of PLCγ1 in these cells was diminished, which was consistent with the calcium data. Erk activation was also diminished as evidenced by its dramatically reduced phosphorylation. These data indicated that TCR-mediated Erk activation and calcium mobilization were both defective in LAT-deficient DP thymocytes. The lack of proper activation necessary for positive-selection following engagement of the TCR in CD4Cre+LATf/− DP thymocytes likely prevented them from further differentiating into SP thymocytes.
Figure 4. Defective TCR-mediated signaling in LAT-deficient DP thymocytes.
(a). Calcium flux. Thymocytes from the LATf/− and CD4Cre+LATf/− mice were loaded with Indo-1 and then stimulated by crosslinking CD3. Ionomycin was also added at the indicated time point. Calcium in DP thymocytes was monitored by flow cytometry and represented as the ratio of fluorescence at 405nm and 510nm.
(b). Tyrosine phosphorylation of proteins. GFP− LATf/− and GFP+ CD4Cre+LATf/− DP thymocytes were sorted by FACS and stimulated for 2 minutes by anti-CD3 crosslinking. Postnuclear lysates were analyzed by Western blotting with anti-pTyr, pPLC-γ1, and pErk. PLCγ1 and Erk2 blots were shown as loading controls.
Few mature LAT-deficient T cells exist in the periphery of CD4Cre+LATf/− mice
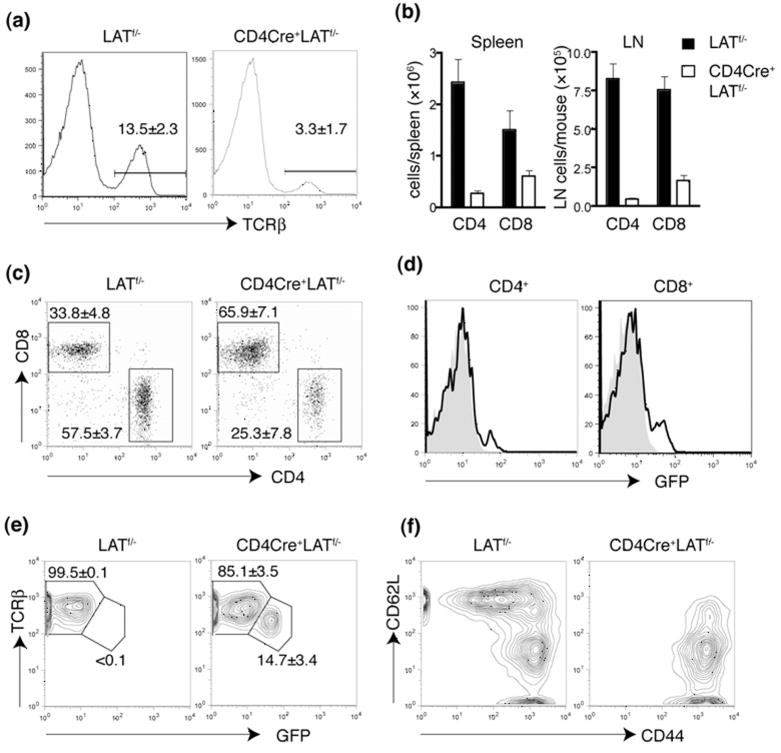
We next examined the T cell compartment in the periphery of CD4Cre+LATf/− mice. As expected, the percentage of T cells was greatly reduced in both spleens (Figure 5a) and lymph nodes (data not shown). The numbers of CD4+ and CD8+ T cells were also significantly decreased (Figure 5b). Interestingly, there were more CD8+ T cells than CD4+ T cells in the periphery of CD4Cre+LATf/− mice compared with LATf/− mice (Figure 5c). Most of these CD4+ and CD8+ T cells appeared to be GFP− (Figure 5d), an indication that these cells escaped Cre-mediated deletion of LAT. Interestingly, compared with the GFP− T cell population, GFP+ T cells had a lower surface expression of TCRβ (Figure 5e).
Figure 5. Abnormal T cells in the periphery of CD4Cre+LATf/− mice.
(a). Reduced number of mature T cells in the CD4Cre+LATf/− mice.
(b). The total numbers of CD4+ and CD8+ T cells in spleen and lymph nodes.
(c). The CD4 vs. CD8 expression profile of TCRβ+ splenocytes.
(d). GFP expression in splenic CD4+ and CD8+ T cells from the LATf/− (Grey) and CD4Cre+LATf/− (Solid line) mice.
(e). TCRβ expression in the splenic CD8+ T cells.
(f). The CD44 vs. CD62L expression profile of the splenic CD8+ T cells from the LATf/− and CD4Cre+LATf/− mice.
Even though the number of peripheral T cells was significantly reduced in CD4Cre+LATf/− mice, it was higher than expected considering the severe block in thymocyte development from the DP to SP stage. Since most of the T cells in the periphery were GFP−, it is possible that they had undergone a tremendous homeostatic expansion driven by the lymphopenic environment in CD4Cre+LATf/− mice. To test this hypothesis, we analyzed the surface expression of CD44 and CD62L on these peripheral T cells. As shown in Figure 5f, the majority of CD8+ T cells in the CD4Cre+LATf/− spleen were CD44hiCD62Llo, a characteristic phenotype of T cells that had undergone homeostatic expansion. A similar phenotype was also observed in CD4+ T cells from these mice (data not shown). These results suggested that the mature T cells in CD4Cre+LATf/− mice likely developed from the few thymocytes that escaped LAT deletion and greatly expanded in the lymphopenic periphery.
Development of regulatory T cells in CD4Cre+LATf/− mice
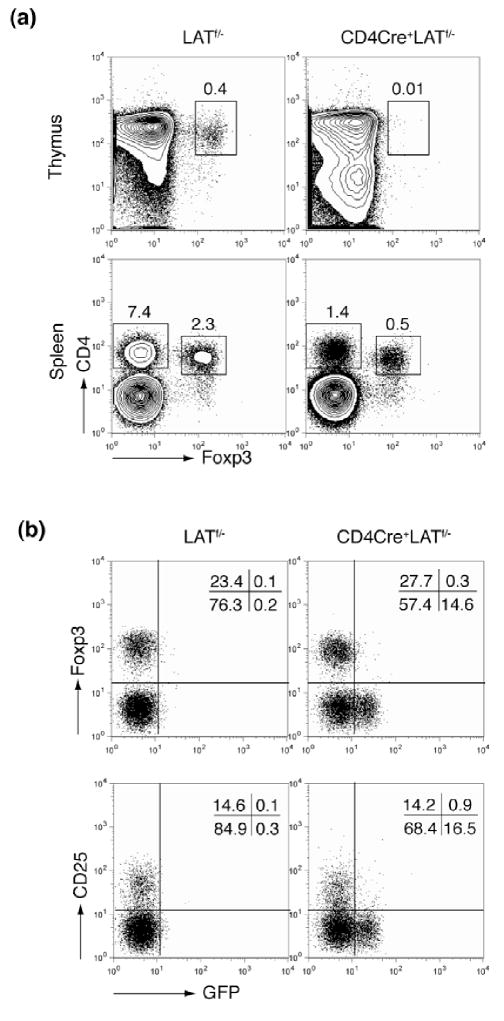
LAT Y136F mice, in which the PLCγ1-binding site of LAT was mutated, lack naturally arising CD4+CD25+Foxp3+ regulatory T cells (Treg) (16), suggesting that the LAT-PLCγ1 interaction plays an important role in Treg development. To examine the role of LAT in the development of regulatory T cells, we examined the expression of Foxp3 in the LAT knock-in mice. While a small but significant population of CD4+Foxp3+ thymocytes existed in the LATf/− mice, few, if any, could be found in age-matched CD4Cre+LATf/− mice (Figure 6a). This result was consistent with the severe defect seen in thymocyte development from the DP to SP stage, suggesting that LAT expression in DP thymocytes is also required for Foxp3 expression and Treg development.
Figure 6. Development of T regulatory cells in the CD4Cre+LATf/− mice.
(a). Foxp3 expression in thymocytes and splenocytes of LATf/− and CD4Cre+LATf/− mice (10 weeks old).
(b). Expression of Foxp3 and CD25 in GFP+ and GFP− CD4+ splenic T cells.
Although the CD4Cre+LATf/− mice lacked CD4+Foxp3+ thymocytes, their peripheral CD4+ T cells contained a similar percentage of Foxp3+ cells (~25%) when compared with the LATf/− controls (Figure 6a). More strikingly, while both GFP− and GFP+ CD4+ mature T cells existed in CD4Cre+LATf/− mice, the Foxp3+ Treg cells were exclusively found in the GFP− compartment, indicating that they had escaped LAT deletion. The same observation was made using CD25 as a marker for the naturally arising Treg cells (Figure 6b). It is intriguing that GFP+ T cells could be found in the periphery but the Treg population was missing from these cells. We reason that, due to the severe thymocyte development block from DP to SP, the peripheral GFP+ T cells had likely arisen from the continued deletion of LAT in the GFP−T cells. If this were true, a similar percentage of Foxp3+ cells should have been expected among the CD4+GFP+ T cells as well. Therefore, the complete absence of Treg cells from the CD4+GFP+ T cell population suggested that LAT likely plays an important role in the maintenance of the Treg population.
Discussion
In this study, we generated LAT knock-in mice and used them to study the role of LAT during thymocyte development and TCR-mediated signaling. In these knock-in mice, the GFP sequence was inserted into the lat locus to monitor deletion of LAT on a single cell basis. This approach allowed us to identify T cells with LAT deletion and to study the effect of this deletion on thymocyte development and TCR-mediated signaling. By using the CD4Cre transgenic mice, we successfully deleted LAT after the DN3 stage. Because LAT−/− mice have a severe block of thymocyte development at the DN3 stage, this strategy allowed us to study the role of LAT beyond DN3. Our data showed that deletion of LAT after the DN3 stage effectively rescued the development of DP thymocytes but severely blocked the generation of SP thymocytes, eventually resulting in a significant loss of mature T cells in the periphery. Our data indicate that, in addition to its essential role in pre-TCR mediated signaling, LAT also plays an important role in TCR signaling in DP thymocytes and is required during thymocyte differentiation from DP to SP.
In CD4Cre+LATf/− mice, there was a severe block in thymocyte development from DP to SP; however, some GFP+ SP thymocytes still remained. As shown in Figure 3b, approximately 50% of the CD4+ and 30% of the CD8+ SP thymocytes in CD4Cre+LATf/− mice were GFP+. Meanwhile, GFP+ mature T cells could be found in the periphery as well. It is not clear from our data whether GFP+ LAT-deficient DP thymocytes have completely lost their capability of further differentiation. There are three possibilities for the existence of these GFP+ SP thymocytes. First, these cells might have developed from GFP− DP thymocytes, in which the lat gene was deleted after differentiation into SP cells. Even though our GFP reporter system allows us to identify cells that have deleted LAT, it is impossible to pinpoint exactly when such deletion occurs. This possibility is supported by the fact that we consistently observed a higher percentage of GFP+ cells in CD4+SP than in CD8+SP thymocytes, because the CD4 promoter that controls Cre expression should remain active in CD4+SP thymocytes. The second possibility is that the LAT protein might have a long half-life. Even after deletion of the lat gene, there might be a sufficient amount of LAT protein left to drive further thymocyte development. The third possibility is that the LAT-deficient DP thymocytes might still have some ability, although limited, to differentiate into SP thymocytes. TCR-mediated signaling, such as Erk activation and Ca2+ flux, might not be totally dependent on LAT. As shown in Figure 4a, TCR-dependent calcium flux in GFP+ DP thymocytes was extremely weak; however, it was not completely abolished. Similarly, weak activation of TCR-dependent Erk was also observed in these cells (Figure 4b). Such residual signals might enable a few GFP+ DP thymocytes to further differentiate.
What caused the aforementioned residual TCR-dependent signals in GFP+ DP thymocytes remains to be determined. In the LAT-deficient Jurkat cell line (JCaM2), TCR-dependent calcium mobilization and Erk are completely abolished(2, 3). We are more inclined to argue that a low level of LAT expression, which might be difficult to detect by Western blotting, may be present in these GFP+ DP thymocytes. However, it remains possible that other TCR-mediated LAT-independent signals play a role (33).
Mature T cells found in the periphery of the CD4Cre+LATf/− mice were CD44hiCD62Llo, a phenotype that is likely a consequence of lymphopenia-driven homeostatic expansion. Interestingly, while there were about the same number of GFP+ as GFP− SP cells in the thymus, GFP− mature T cells clearly dominated in the periphery. Thus, the GFP− mature T cells in the CD4Cre+LATf/− mice may have expanded much more vigorously than their GFP+ counterparts. On the other hand, it is also possible that GFP+ mature T cells might be defective in cell survival upon LAT deletion. An adoptive transfer approach will be taken to further address this possibility in the future.
We noticed that GFP+ SP thymocytes in CD4Cre+LATf/− mice expressed a considerably lower level of surface TCR than their GFP− counterparts. Similar phenomenon was observed in peripheral mature T cells as well. Previous studies have also linked the down-regulation of TCR expression with a defect in LAT-mediated signaling. In knock-in mice that express LAT with a point mutation at Y136, TCR and CD3 expression on the peripheral T cells is also much lower than on normal T cells(13, 14). Moreover, deficiency of SLP-76, which also plays an essential role in mediating TCR-dependent signaling, similarly causes down-regulation of TCR expression on the surface of peripheral T cells(34). Thus, normal signaling downstream of TCR engagement appears to positively regulate the expression of the TCR through mechanisms that are yet to be explored.
Given that LAT plays an essential role in SP thymocyte development, it was not surprising that the CD4Cre+LATf/− mice lacked CD4+Foxp3+ thymocytes. However, the exclusive lack of such CD4+CD25+ Treg cells in the peripheral CD4+GFP+ population was intriguing. Accumulating studies using TCR transgenic models have demonstrated that, compared with conventional T cells, Treg cells likely require higher TCR/MHC-peptide affinity for positive selection(35–38). As a result, the Treg development may be more dependent on LAT. Further supporting this hypothesis, CD4+CD25+ Treg cells are absent in both the thymus and the periphery in LAT Y136F mice, while the positive selection for conventional T cells is only partially defective(15, 16). Therefore, the residual TCR signaling in the GFP+ DP thymocytes, whether due to LAT-independent signaling pathways or the incomplete deletion of LAT protein, might have enabled them to develop into conventional T cells, but not Treg cells. On the other hand, our data also pointed to a possible role of LAT in the homeostasis of regulatory T cells. As we argued earlier, the GFP− Treg could have continued to delete LAT to become GFP+ Treg, whose survival may have been affected by the loss of LAT. More analysis needs to be done to address this issue.
The phenotype of our CD4Cre+LATf/− mice carried a striking resemblance to the SLP-76 conditional knockout mice that have recently been described (34). While SLP-76−/− mice suffer a severe block in thymocyte development at the DN3 stage and fail to generate any DP and SP thymocytes just like the LAT−/− mice(39–41), depletion of SLP-76 after the DN3 stage in CD4CreSLP76f/f mice also results in the re-appearance of DP thymocytes. SLP-76 deficient DP thymocytes fail to respond to TCR stimulation and are defective in both positive and negative selection. As a result, few SP thymocytes and mature T cells develop in the CD4CreSLP76F/F mice. Altogether, these data strongly suggest that the LAT-GADS-SLP76 complex is absolutely required to transduce signals from the TCR that would lead to positive and negative selection of DP thymocytes.
Although our studies clearly demonstrated the important role of LAT during thymocyte development after the DN3 stage, its role in mature T cells still remains to be determined. There were only a small number of GFP+ T cells present in the periphery of CD4Cre+LATf/− mice, which makes it extremely difficult to study the role of LAT in mature T cells. Moreover, such T cells, which were CD44hiCD62LloTCRlo and had undergone homeostatic proliferation, are not likely to be ideal cells for future study. Additional work is being done to effectively delete LAT in normally developed T cells, and studies on these cells will shed more light on the role of LAT in mature T cell activation, survival, and homeostasis.
Acknowledgments
This work was supported by National Institutes of Heath grants AI048674 and AI056156.
References
- 1.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 2.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. International immunology. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 4.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 5.Asada H, Ishii N, Sasaki Y, Endo K, Kasai H, Tanaka N, Takeshita T, Tsuchiya S, Konno T, Sugamura K. Grf40, A novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. The Journal of experimental medicine. 1999;189:1383–1390. doi: 10.1084/jem.189.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yablonski D, Kadlecek T, Weiss A. Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Molecular and cellular biology. 2001;21:4208–4218. doi: 10.1128/MCB.21.13.4208-4218.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Motto DG, Koretzky GA, Weiss A. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 1996;4:593–602. doi: 10.1016/s1074-7613(00)80485-9. [DOI] [PubMed] [Google Scholar]
- 8.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 9.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nature immunology. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 10.Buday L, Egan SE, Rodriguez Viciana P, Cantrell DA, Downward J. A complex of Grb2 adaptor protein, Sos exchange factor, and a 36-kDa membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells. The Journal of biological chemistry. 1994;269:9019–9023. [PubMed] [Google Scholar]
- 11.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 12.Sommers CL, Menon RK, Grinberg A, Zhang W, Samelson LE, Love PE. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. The Journal of experimental medicine. 2001;194:135–142. doi: 10.1084/jem.194.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, Sainty D, He HT, Malissen B, Malissen M. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 14.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacana E, Menon RK, Shores EW, Samelson LE, Love PE. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 15.Sommers CL, Lee J, Steiner KL, Gurson JM, Depersis CL, El-Khoury D, Fuller CL, Shores EW, Love PE, Samelson LE. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. The Journal of experimental medicine. 2005;201:1125–1134. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. The Journal of experimental medicine. 2006;203:119–129. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunez-Cruz S, Aguado E, Richelme S, Chetaille B, Mura AM, Richelme M, Pouyet L, Jouvin-Marche E, Xerri L, Malissen B, Malissen M. LAT regulates gammadelta T cell homeostasis and differentiation. Nature immunology. 2003;4:999–1008. doi: 10.1038/ni977. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. The Journal of biological chemistry. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 19.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 20.Wolfer A, Wilson A, Nemir M, MacDonald HR, Radtke F. Inactivation of Notch1 impairs VDJbeta rearrangement and allows pre-TCR-independent survival of early alpha beta Lineage Thymocytes. Immunity. 2002;16:869–879. doi: 10.1016/s1074-7613(02)00330-8. [DOI] [PubMed] [Google Scholar]
- 21.Paterson DJ, Williams AF. An intermediate cell in thymocyte differentiation that expresses CD8 but not CD4 antigen. The Journal of experimental medicine. 1987;166:1603–1608. doi: 10.1084/jem.166.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald HR, Budd RC, Howe RC. A CD3- subset of CD4-8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+ cells. Eur J Immunol. 1988;18:519–523. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- 23.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25:105–116. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nature immunology. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 25.Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging beta- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53–64. doi: 10.1016/j.immuni.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 27.Mariathasan S, Jones RG, Ohashi PS. Signals involved in thymocyte positive and negative selection. Semin Immunol. 1999;11:263–272. doi: 10.1006/smim.1999.0182. [DOI] [PubMed] [Google Scholar]
- 28.Lanier LL, Allison JP, Phillips JH. Correlation of cell surface antigen expression on human thymocytes by multi-color flow cytometric analysis: implications for differentiation. J Immunol. 1986;137:2501–2507. [PubMed] [Google Scholar]
- 29.Fowlkes BJ, Edison L, Mathieson BJ, Chused TM. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. The Journal of experimental medicine. 1985;162:802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 31.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. The Journal of experimental medicine. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hugo P, Boyd RL, Waanders GA, Petrie HT, Scollay R. Timing of deletion of autoreactive V beta 6+ cells and down-modulation of either CD4 or CD8 on phenotypically distinct CD4+8+ subsets of thymocytes expressing intermediate or high levels of T cell receptor. International immunology. 1991;3:265–272. doi: 10.1093/intimm/3.3.265. [DOI] [PubMed] [Google Scholar]
- 33.Chau LA, Madrenas J. Phospho-LAT-independent activation of the ras-mitogen-activated protein kinase pathway: a differential recruitment model of TCR partial agonist signaling. J Immunol. 1999;163:1853–1858. [PubMed] [Google Scholar]
- 34.Maltzman JS, Kovoor L, Clements JL, Koretzky GA. Conditional deletion reveals a cell-autonomous requirement of SLP-76 for thymocyte selection. The Journal of experimental medicine. 2005;202:893–900. doi: 10.1084/jem.20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature immunology. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 36.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature immunology. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 37.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 38.Yu P, Haymaker CL, Divekar RD, Ellis JS, Hardaway J, Jain R, Tartar DM, Hoeman CM, Cascio JA, Ostermeier A, Zaghouani H. Fetal Exposure to High-Avidity TCR Ligand Enhances Expansion of Peripheral T Regulatory Cells. J Immunol. 2008;181:73–80. doi: 10.4049/jimmunol.181.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 40.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 41.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt FW, Geha RS. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]