Abstract
Evaluation of structural bone grafts risk of failure requires noninvasive quantitative predictors of functional strength. We hypothesized that a quantitative graft-to-host union biometric would correlate significantly with biomechanical properties as a surrogate for the risk of fracture. To test this, we developed a novel algorithm to compute the union between host callus and graft, which was termed the union ratio. We compared the union ratio of live autografts to devitalized allografts implanted into the mid-diaphysis of mouse femurs for 6 and 9 wk. Surprisingly, the autograft union ratio decreased from 0.228 ± 0.029 at 6 wk to 0.15 ± 0.011 at 9 wk (p < 0.05) and did not correlate with the torsional properties of the autografts . The allograft union ratio was 0.105 ± 0.023 at 6 wk but increased to 0.224 ± 0.029 at 9 wk (p < 0.05). As a single variable, the union ratio correlated significantly with ultimate torque (R 2 = 0.58) and torsional rigidity (R 2 = 0.51) of the allografts. Multivariable regression analyses of allografts that included the union ratio, the graft bone volume, the maximum and minimum polar moment of inertia, and their first-order interaction terms with the union ratio as independent variables resulted in significant correlations with the ultimate torque and torsional rigidity (adjusted R 2 = 0.80 and 0.89, respectively). These results suggest that, unlike live autografts, the union between the devitalized allograft and host contributes significantly to the strength of grafted bone. The union ratio has important clinical implications as a novel biometric for noninvasive assessment of functional strength and failure risk.
Key words: nonunion, bone, allograft, autograft, biomechanics, μCT
INTRODUCTION
Massive allografts (>5 cm), used to repair critically sized bone defects from tumors or trauma, commonly experience complications because of incomplete graft–host osseointegration, which leads to persistent nonunion.(1–3) Furthermore, fatigue fractures caused by accumulation and propagation of microdamage within the graft tissue might lead to catastrophic failure.(4) As a result, up to one half of large structural cortical allografts in children receiving allografts after bone tumor resection fail in the first 5 yr of the life of the graft.(1) Therefore, the development of adjuvant therapies to improve the longevity of the allografts will have a tremendous impact on the patients' quality of life and the economic burden of this problem. To date, however, there are no accepted quantifiable and noninvasive outcome measures of improved biomechanical strength for allograft healing in patients that could allow the unequivocal evaluation of the functional efficacy of these approaches in reasonably sized clinical trials. In preclinical animal models, the gold standard for functional outcome measures is the destructive evaluation of the biomechanical properties. However, such evaluation is not possible in the clinical setting, and noninvasive surrogate measures are needed to predict the biomechanical properties. Before such outcome measures can be used in clinical applications, they would first have to be developed and validated in preclinical animal models.
To that end, we recently used a previously described(5) mouse model of femoral reconstruction to study the differences in the biomechanics of live autografts and devitalized allografts.(6) Using μCT, we observed that devitalized allograft remodeling and incorporation into the host remained severely impaired compared with live autografts mainly due to the extent of callus formation around the graft and the rate and extent of the graft resorption. Biomechanical testing showed that autografts displayed greater ultimate torque and torsional rigidity compared with the allografts at 6 wk, but the biomechanical properties of allografts were equivalent to autografts by 9 wk.(6) However, the allografts' biomechanical properties deteriorated progressively at the later time points, consistent with accumulation of microdamage and graft resorption. Multivariate analyses that combined all of the established CT parameters of bone healing for both autografts and allografts could only predict 44% and 50% of the variability in torsional strength and rigidity of the grafted bones over time, respectively, which suggests that these CT variables fail to explain about one half of the variability in the torsional biomechanics. Based on categorical analysis of the mode of failure after biomechanical testing,(6) we hypothesized that the graft-to-host union, which was unaccounted for in the aforementioned correlations, would correlate significantly with torsional biomechanical properties.
Few previous reports have attempted to define a measure of cortical graft–host union based on histology or CT. For example, to determine the osteoconductive effects of porous poly(propylene fumarate) foam coating on the integration of intercalary cortical (tibial) allografts in a rat model, Lewandrowski et al.(7) described a histomorphometry technique for assessing allograft–host integration by tracing the perimeter of the graft and estimating the length of host-to-graft integration along that perimeter. The percentage of length that was connected was determined independently for both the proximal and distal ends of each graft and named the bonding index. Unfortunately, this method is limited by the destructive nature of this histological evaluation that does not permit one-to-one correlations of graft–host union with biomechanical properties. A previous report attempted to evaluate the union of cortices after fracture in response to PTH treatment using μCT imaging in a mouse tibial fracture model using planar midsagittal and midcoronal images and counting the number of bridged cortices.(8) This was adapted from an attempt at quantifying union on orthogonal 2D plain radiographs in a rat femoral fracture study investigating the effect of specific COX-2 inhibition on healing.(9) Unfortunately, these approaches were limited by the reliance on planar data and did not provide a true 3D estimation of the cortical union.
In this study, we describe and validate a novel μCT-based algorithm to compute a 3D measure of union between host (bone and callus) and mouse femoral graft (autograft or allograft) based on the surface area of the graft onto which bone forms to connect the graft to the host. The ratio of connected graft area to total graft surface area is computed for each graft end and the lesser value for each graft is termed the union ratio. This technique is used to investigate the variation in the osseointegration of mouse femoral grafts to test the hypothesis that the union ratio correlates significantly with the torsional strength and rigidity of bone allografts. Last, we show the translational potential of this measurement technique using clinical CT data of a nonunion tibia fracture to show that, despite lower relative resolution, this technique could be useful, which justifies further validation in future clinical studies.
MATERIALS AND METHODS
Experimental model
Specimens analyzed in this study are a subset of a previous study that was performed with protocols approved by the University of Rochester Committee for Animal Resources.(6) Briefly, 4-mm intercalary defects in C57Bl/6 mouse femurs were reconstructed using either the live cortical bone graft from the same mouse (autograft) or a devitalized bone graft from a donor mouse (allograft) and secured in place with an intramedullary pin. Only mice that were killed at 6 (n = 7 autografts and 8 allografts) or 9 wk (n = 12 autografts and 7 allografts) after surgery were included in this study. Femurs were disarticulated from the hip and knee joints, and the intramedullary, stainless-steel pins were removed carefully. Specimens were moistened with saline and frozen at −20°C until thawed for μCT imaging and subsequent biomechanical testing.
Specimens were scanned at 13.9-μm resolution using the Explore Locus SP scanner (GE Healthcare Technologies, London, Ontario, Canada) at 80 kVp and 80 mA with 415 projections of 1700-ms integration time. GE MicroView software was used for measuring bone volume and cross-sectional organization. To compensate for slight variations in the scanner, a threshold was determined for each scan using a standardized automatic threshold-selection feature of the GE MicroView software that uses the Otsu method. This determines the threshold that maximizes the variance between the groups of pixels.(10) The selected threshold was consistently verified against the user's perception of the boundary of the mineralized bone. Manual segmentation of the graft and callus bone volumes (BVGraft, BVCallus) was performed on axial cross-sections of the grayscale images as previously described.(6) The cross-sectional polar moments of inertia (PMI) were computed for each slice throughout the grafted region. The maximum, minimum, and average PMI (PMIMax, PMIMin, PMIAve) were recorded for each specimen. In circular, prismatic shafts, the PMI correlates directly with torsional rigidity and inversely with the shear stress.(11) The torsional biomechanical properties of the grafted femurs were determined using the EnduraTec TestBench system (200-N.mm torque cell; Bose, Minnetonka, MN, USA) at a rate of 1°/s. Raw data from the testing was plotted as torque versus rotation (normalized to the measured gage-length) and used to determine the ultimate torque and torsional rigidity for each specimen. The torsional rigidity was determined as the maximum slope of the curve between the start of the test and the maximum torque. Specifically, we used a sliding window one fifth the width of this region to determine the maximum slope.
Union ratio algorithm
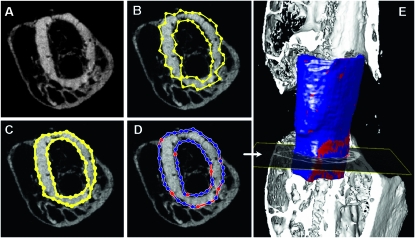
Custom software was written in MATLAB (The Mathworks, Natick, MA, USA) for the analysis of the union ratio from the μCT images. An active contouring algorithm(12) was adapted for the semiautomated generation of a shell around the graft. First, contours are drawn around the periosteal and endosteal surfaces of the bone graft in a single transverse μCT slice, which has been lightly low-pass filtered using a 2D Gaussian filter (σ = 1.8 pixels; Figs. 1A and 1B). The contour snaps to the edge of the graft based on the 2D gradient of the grayscale image using a Prewitt filter (Fig. 1C). Last, the contour dilates to a neighboring pixel with the darkest grayscale intensity along a four-pixel-long line drawn from each contour point perpendicular to the line that connects the two contour points on each side of the current contour point. Thus, the contour dilates into the void space between the graft and callus bone if it exists (Fig. 1D). Because the contour snaps to the gradient between contrasting pixels, the contour point will shift to the material of lesser radiopacity. Generally, this means it shifts off the dense cortical graft, onto either newly mineralized callus (woven bone is less radiopaque than organized lamellar bone) or onto unmineralized soft tissue adjacent to the graft. Cubic spline interpolation was used to smoothly join the contours. The contour from the previous slice is copied onto the next where the edge detection and void space search processes are repeated under operator supervision and modification, until the entire length of the graft is contoured to create a shell around the graft. The shell is meshed using triangular elements that are used to quantify the amount of graft area in contact with host bone or mineralized callus by summing one third of the area of each triangle element for each vertex that falls within a voxel with a grayscale value greater than the threshold used to define mineralized tissue. The proximal and distal halves of the graft are evaluated separately, and the lesser ratio of the connected surface area to total graft surface area is used in the analysis and assigned as the value of the union ratio to account for any variation in graft size in our standardized model.
FIG. 1.
Illustration of the graft-to-host union ratio algorithm. A user outlines the surface of the graft using contours on transverse μCT slices (A). The semiautomated algorithm developed using MATLAB then optimizes the manually defined contours drawn around the endosteal and periosteal surfaces (yellow lines) (B). The contours are first snapped to the graft boundary by edge detection (C) and then dilated into darker regions away from the graft surface, finding the gap between graft and callus, if it exists (red denotes voxels that are adjacent to host bone/callus and blue denotes voxels that are adjacent to host soft tissue) (D). The resulting 2D contour from one slice is copied to the next slice, and the edge detection and gap-finding operations are performed. This process is repeated on each slice until the entire graft is enclosed in contours. A smoothed 3D shell is generated from the contours using MATLAB's isosurface function (E). The footprint of bone penetrating the shell therefore defines connection areas between the graft and host or callus. The union ratio is defined as the lowest area of the connections (red regions) in either the proximal or distal one half of the graft divided by the corresponding surface area of the graft half.
Algorithm validation
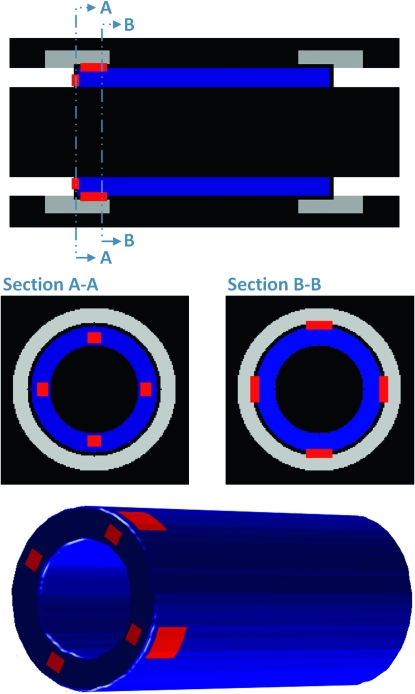
A digital model with standard hollow cylindrical geometry was created to validate the calculations used to measure the connected surface area. This model was generated as an idealized graft between two host ends, with geometrically defined connections simulating callus originating from the host tissue. The theoretically predetermined areas of connectivity that were used to validate the computational technique are shown in Fig. 2A. The hollow cylindrical model was generated with thickness of 15 pixels and outer diameter of 50 pixels, yielding a relative resolution similar to the resolution of the real μCT images (typical allograft cortical thickness was 180–200 μm (13–14 pixels) thick and ∼1.25–1.55 mm (90–110 pixels) in diameter. Predetermined areas of connectivity were created directly between the graft and host as rectangular prisms that either attached to the end surface of the graft (Fig. 2B) or intersected the periosteal surface of the graft connecting it to the callus (Fig. 2C). This idealized model was contoured, and the union area was computed as described for the experimental grafts.
FIG. 2.
Algorithm validation using a digital model. An idealized cylindrical graft (blue) between host cortical bone (white) and callus (light gray) was digitally generated in MATLAB and used to validate the union ratio measurement. The graft was given defined rectangular regions of union to the host directly (section A-A) as well as between the graft and the callus forming around it (section B-B). The theoretical union area (red regions) based on the idealized geometry projected onto the curved surface was 2173.2 pixels2. Using the contouring computational algorithm, the measured area was 2171.4 pixels2, resulting in a measurement error of only 0.08%.
Statistical analysis
Comparisons of autograft and allograft union ratio data at the different time points were performed using two-way ANOVA and Bonferroni posthoc multiple comparisons.
To evaluate intraoperator and interoperator error in the estimation of the union ratio, a subset of two specimens from each group (eight specimens total) was randomly selected to be repeated by the first operator (D.G.R.) as well as performed and repeated by another trained operator (M.O.P.). The average percent error between measurements was calculated by the absolute value of the difference between measures divided by the average measurement. As described by Lodder et al.,(13) the CV is the SD between measurements normalized by the mean of the paired measurements, calculated as where a and b are the first and the second measurements, Ma and Mb are the mean values for the two groups, and n is the number of paired observations. Intraclass correlation coefficients (ICCs) to evaluate the concordance, or agreement, between measurements within and between operators were computed.(14) This is defined as the difference between the overall variation and the measurement variation, divided by the sum of the measurement and overall variation. The ICC ranges between 0 and 1, where 1 is perfect concordance.
As previously described,(6) univariate regression analysis was used to determine whether union ratio correlated with the ultimate torque and torsional rigidity of 6- and 9-wk specimens. We also determined the best single variable regressions for the allografts and autografts separately and together using MiniTab Release 12 (State College, PA, USA). Multivariate regression analysis was used to determine combinations of μCT parameters that correlated with the torsional mechanical properties using SAS 9.1 (SAS Institute, Cary, NC, USA).(6) Interactions between the union ratio and other structural variables were also studied. The regression model was set up such that interacting terms also include the variables by themselves. Bidirectional stepwise selection regression analysis was used to select significant regression terms.
RESULTS
Algorithm validation
To validate the semiautomated contouring algorithm and computation of the union ratio, we created a digital model that resembles a graft connected to host bone and callus by a footprint of defined dimensions (Fig. 2). The predetermined connected area that accounts for the curvature of the cylindrical model surface was computed to be 2173.2 pixels2. Using the contouring method and the MATLAB algorithm, the union area was determined to be 2171.4 pixels2, resulting in a measurement error of only 0.08%.
Union ratio of autografts and allografts
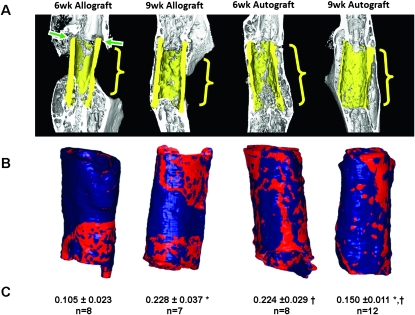
Figure 3 illustrates the typical differences in the union with host bone and callus between allografts and autografts at 6 and 9 wk. At 6 wk, the union ratio of autografts was nearly double that of allografts (p < 0.05). The areas of union were also more uniformly distributed along the length of the autografts compared with the allografts for which new bone formation was restricted to the host bone at the ends of the grafts (Fig. 3). At 9 wk, the allografts' union ratio was 2.2 times that of 6-wk allografts (p < 0.05), whereas the autografts' union ratio decreased 33% from 6 to 9 wk (p < 0.05).
FIG. 3.
Representative μCT sagittal sections of 6- and 9-wk allografts and autografts (A) with the corresponding union area maps and union ratio numerical values (B). The graft bone is highlighted in yellow. Red indicates areas where the graft is connected to the host. Note that the areas of union and nonunion (green arrows) correspond accurately to the measured union areas on the surface of the graft represented in red. Note also that union with the periosteal and endosteal surfaces, and the ends of the graft were all accounted for. The proximal and distal halves of the graft were evaluated separately, and the lowest value of the union area normalized by the surface area was reported as the union ratio (C) (mean ± SE). Significantly different means: † p < 0.05 between time points for each graft type and *p < 0.05 between graft types at each time point.
We also investigated the intra- and interoperator sources of error in the measurement of the union ratio. The average percent error between operators' measurements was 12%, and the CV was 9.7%. The intraoperator ICC was 0.930 for DGR and 0.949 for MOP, whereas ICC between different operators (D.G.R. and M.O.P.) was 0.926. These results indicate that the measurements were remarkably reproducible.
Correlations between union ratio and torsional properties
To estimate the effects of the union ratio on the torsional biomechanical properties, we performed univariate linear regression analyses. When autografts and allografts at all time points were grouped, the regression analysis identified weak, yet significant, associations between the union ratio and the torsional properties (Table 1). However, when analyzing the allograft data separately, the correlation was much stronger. In contrast, there were no significant associations between the autografts' union ratio and torsional properties. Taken together, these results suggest that the union ratio is a significant indicator of functional strength in the devitalized allografts that undergo no or little remodeling over the first 9 wk of healing, whereas it does not correlate with the biomechanical properties of autografts that undergo a robust remodeling(6) such that the union ratio actually decreases between 6 and 9 wk because of excessive graft resorption.
Table 1.
Coefficients of Determination (R 2) and p Values for Univariate Linear Regression of Graft Union Ratio With Ultimate Torque and Torsional Rigidity
| Group | Ultimate torque | Torsional rigidity | ||
| R2 | p* | R2 | p | |
| Autografts and allografts | 0.12 | <0.04 | 0.15 | <0.02 |
| Autografts | 0.15† | NS | 0.05† | NS |
| Allografts | 0.58 | <0.001 | 0.51 | <0.003 |
* p values for the two-sided test of the null hypothesis that the slope of the regression line is zero.
† Inverse linear correlations (i.e., negative slope).
NS, p > 0.05.
To account for other variables that contribute to the biomechanical properties of the grafts, we studied multivariate correlations between μCT parameters and torsional properties as previously described.(6) When included as an independent variable, the union ratio was a significant, predictive variable that increased the regression coefficients for rigidity and strength of 6- and 9-wk autografts and allografts as a group.
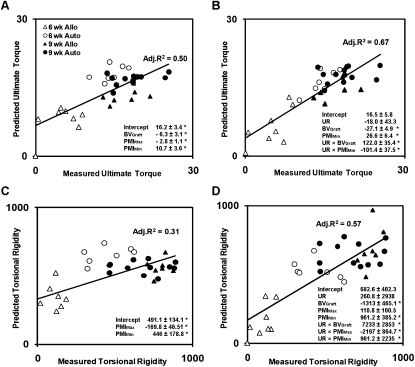
To determine the union ratio's ability to improve the correlation between structural measures and mechanics, multivariable regression was performed twice, once without the union ratio and again with the union ratio as an independent and interacting term. Without union ratio, BVGraft, PMIMax, and PMIMin were found to correlate with TUlt, yielding an adjusted R 2 = 0.50 (Fig. 4A) and PMIMax and PMIMin were found to correlate with TR, yielding an adjusted R 2 = 0.31 (Fig. 4C). Including union ratio in the regression model improved the correlation coefficients. The ultimate torque correlated significantly with the combination of union ratio, BVGraft, PMIMin, and the interaction terms union ratio × BVGraft and union ratio × PMIMin (adjusted R 2 = 0.67; Fig. 4B). The torsional rigidity correlated significantly with union ratio, BVGraft, BVCallus, PMIMax, PMIMin, and the interaction terms union ratio × PMIMax and union ratio × PMIMin (adjusted R 2 = 0.57; Fig. 4D).
FIG. 4.
Multivariable linear regression analysis of geometric μCT-based parameters including bone volume, PMI, and union ratio. The regression analysis was performed without union ratio (A and C) and with union ratio (B and D) for the combined set of autografts (Auto) and allografts (Allo). Adjusted R 2 and the significant regression coefficients are indicated on each graph with their ±SE. *Independent variables or the interaction terms are significant (p < 0.05).
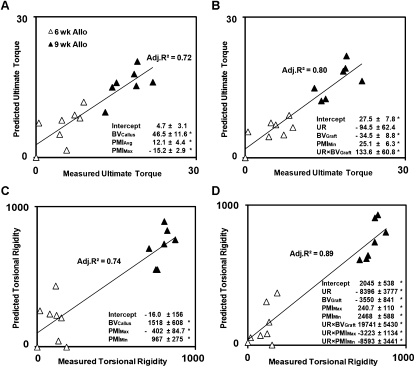
When allografts were analyzed separately without including the union ratio in the multivariate regression analysis, BVCallus, PMIAve, and PMIMax correlated with the ultimate torque with an adjusted R 2 = 0.72 (Fig. 5A). When the union ratio was included in the model, union ratio, PMIMin, BVGraft, and union ratio × BVGraft correlated with the ultimate torque, increasing the adjusted R 2 to 0.80 (p < 0.05; Fig. 5B). Likewise, the correlation with the torsional rigidity of allografts significantly improved with the addition of the union ratio from an adjusted R 2 from 0.74 to 0.89 (p < 0.05), with the combination of the union ratio, BVGraft, PMIMax, and PMIMin and the interaction terms with the union ratio: union ratio × BVGraft, union ratio × PMIMax, and union ratio × PMIMin (Figs. 5C and 5D).
FIG. 5.
Multivariable linear regression analysis of geometric μCT-based parameters including bone volume, PMI, and union ratio for allografts only. The regression analysis was performed without union ratio (A and C) and with union ratio (B and D) for allografts only. Adjusted R 2 and the significant regression coefficients are indicated on each graph with their ±SE. *Independent variables or the interaction terms are significant (p < 0.05).
DISCUSSION
Despite the high incidence of bone fractures and the clinical development of safe and effective anabolic/osteogenic therapies for bone healing (i.e., teriparatide, BMP-2), the lack of a noninvasive outcome measure of biomechanical healing of fractured bone continues to limit our ability to define nonunions and evaluate new therapies for unmet clinical needs. Previously we attempted to correlate established μCT parameters with torsional properties in the murine femoral auto and allograft model and found that we could at best predict 50% of the biomechanical properties of the mouse grafted femurs.(6) This poor correlation is largely explained by the fact that none of the established μCT parameters are not capable of quantifying the extent of cortical bone union between the graft and the host, which intuitively should be directly related to strength of the bone. Therefore, we developed and validated a novel algorithm to quantitatively estimate the union between graft and host bone based on μCT data. Our results highlighted the differences in healing caused by graft type, as well as the changes in union and osseointegration patterns over time. Furthermore, one-to-one correlations showed that the union ratio was a significant predictive variable of the biomechanical properties of the devitalized allografts but not the live autografts.
Quantifying the union ratio of live autografts and devitalized allografts corroborated previously published qualitative observations regarding the biology and biomechanics of healing in both cases.(5,6) Histological evidence showed that devitalized allografts induce a foreign body reaction that encases the graft in a fibrous layer initially, which can be gradually overcome with progression of the creeping callus from the host bone that typically remains restricted to the graft ends.(5) Our results now show that the mitigation of nonunion by 9 wk, when the callus finally penetrates the fibrous capsule and integrates with the devitalized allograft, significantly increases the ultimate torque and torsional rigidity.
In the case of autografts, the union ratio did not independently correlate with torsional properties, whereas the allografts' union ratio significantly correlated with the torsional properties (Table 1). We hypothesize that these results reflect fundamental biological differences in the healing of live autografts and the devitalized allografts that arise from the contribution of periosteal cells in live autografts that are absent in devitalized allografts. We have previously shown that autograft repair is facilitated by both endochondral bone formation at the host–graft junction and by intramembranous bone formation along the entire length of the graft as early as 2 wk after transplantation and undergoes significant remodeling by 4 wk.(5) This results in the formation of a new bone collar that bridges the entire length of the autograft by 4 wk, which is also apparent in this study at 6 and 9 wk in Fig. 3. We hypothesize that this new bone collar begins to assume a significant share of the in vivo loading, and therefore, the autograft begins to experience significant stress-shielding and undergoes rapid and substantial resorption (by up to 57%) by 6 wk,(6) thus rendering its contribution to mechanical properties of the femur negligible. Therefore, whether the remaining graft has a high degree of union to the new cortical shell plays little role in the overall mechanical strength. In contrast, devitalized allografts completely rely on endochondral bone formation initiated by the host at the host–graft cortical junction, with no evidence of periosteal bone formation along the length of the allograft and no appreciable graft resorption. The result is significant callus formation that is limited to the host–graft junction and whose union with the allograft is crucial to load transmission and mechanical strength.
Furthermore, our multivariate correlations do not account for the complete cortical bridging observed in 100% of the autografts at 6 and 9 wk, which likely makes a significant contribution to the biomechanical properties. The development of a measure of this type of union could potentially contribute to the ability to predict the mechanical stability of healing bone autografts.
Previously published studies have attempted to estimate fracture and graft union using histological and stereological techniques(7) and 2D plain radiographs.(9) Unfortunately, these approaches are prone to inaccuracies because they do not account for the 3D nature of the cortical healing. Recent reports have attempted to use high-resolution μCT imaging to characterize fracture nonunion.(8,15) These studies defined measures of union based on counting the number of bridged cortices in planar sections(8) or relied on qualitative 3D rendering of the fracture sites to show union or the lack thereof in response to the treatment.(15) Therefore, our study not only reports the development of a novel quantitative measure of union, but to the best of our knowledge, it is also the first to report direct correlations between the graft and host degree of union and the biomechanical properties of the reconstructed bone, which could have important applications in longitudinal preclinical and clinical studies of bone repair and grafting.
The union ratio has significant clinical implications as a novel quantitative biometric that merits further study in large animal preclinical studies using clinical CT scanners. Various preclinical and clinical studies have been performed to treat bone injuries with adjuvant treatments to enhance healing and bone formation around allografts, their incorporation and remodeling, and their biomechanical properties and durability. Such treatments include the use of BMPs and other growth factors,(16–19) co-engraftment with mesenchymal stem cells,(20,21) the use of locally administered gene therapy,(22–25) engineered bone graft substitutes,(26,27) and recently, the use of the bone anabolic factor such as PTH,(28–31) to name a few. The evaluation of the repair quality and osseointegration in preclinical animal models can be accomplished by destructive biomechanical testing. However, the evaluation of clinical patients has to date been mostly based on nonquantitative radiographic outcomes because destructive biomechanical testing is not an option.
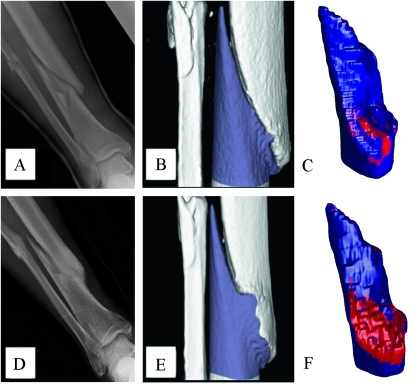
To show the potential clinical utility of our algorithm on CT scans of clinical resolution, we retrospectively analyzed clinical CT images of an anonymous patient with a prolonged nonunion (>4 mo) tibial fracture, which was subsequently nonsurgically treated with teriparatide. We used our custom MATLAB software to contour the segment of bone on one side of the fracture site similarly to contouring around the murine graft. The surface area forming union to the other side of the fracture was estimated by the software. After 4 mo of treatment, the patient had a 2.8-fold increase in the mineralized union area connecting the fractured segments, which underscored the functional outcome of the patient being able to finally bear weight on the healing leg (Fig. 6). This example in no way suggests that the technique is ready for clinical applications. Adapting the union ratio to evaluate clinical union, especially with different treatments, is substantially more complicated because the actual geometry of every graft and fracture vary and will require additional development of techniques and tools to suppress hardware artifacts, for example, and investigate the effects of lower clinical CT resolution. Furthermore, making the clinical translation is still going to be quite challenging, because clinical grafts and fractures are not always simple and reproducible, and torsion testing for “validation” is not an option for human patients. Eventually, when validated in additional preclinical and clinical studies, the union ratio can potentially help overcome a significant hurdle in longitudinal clinical trials by providing a quantitative CT-based union biometric that identify patients at risk for nonunion complications.
FIG. 6.
Estimating the union area from clinical CT data of human patients. Clinical X-rays and CT scan data of an anonymous patient's fractured tibia before and after 4 mo of teriparatide therapy were obtained retrospectively from the University of Rochester Department of Orthopaedics, in compliance with institutional review board research exemption. The tibial nonunion 4.5 mo after fracture is apparent from plain X-ray (A). The nonunion was confirmed by 3D reconstruction of the patient's CT as evidenced by the space between the proximal (white) and distal (blue) ends of the fracture (B), which yielded a union area (red) of 4.2 cm2 (C). The effects of teriparatide on fracture healing are shown by X-ray (D) and 3D CT (E and F) and were quantified as a 2.8-fold increase in union area.
ACKNOWLEDGMENTS
The authors thank Laura Yanoso for help with μCT, Drs. Susan Bukata and Lee Kaback for the clinical CT data, and Dr. Christopher Beck for advice on statistics. This work was funded by grants from the Orthopedic Research Education Foundation, the Musculoskeletal Transplant Foundation, the Wallace H. Coulter Foundation, the National Institutes of Health (AR053459, DE017096, AR054041, AR51469, and AR48681), and research grants from DePuy, J&J. For inquires regarding distribution of the MATLAB code of the program written to perform this analysis, please contact the corresponding author.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Brigman BE, Hornicek FJ, Gebhardt MC, Mankin HJ. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res. 2004:232–239. doi: 10.1097/01.blo.0000127132.12576.05. [DOI] [PubMed] [Google Scholar]
- 2.Mohler DG, Yaszay B, Hong R, Wera G. Intercalary tibial allografts following tumor resection: The role of fibular centralization. Orthopedics. 2003;26:631–637. doi: 10.3928/0147-7447-20030601-13. [DOI] [PubMed] [Google Scholar]
- 3.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001:87–98. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 5.Tiyapatanaputi P, Rubery PT, Carmouche J, Schwarz EM, O'Keefe RJ, Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22:1254–1260. doi: 10.1016/j.orthres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds DG, Hock C, Shaikh S, Jacobson J, Zhang X, Rubery PT, Beck CA, O'Keefe RJ, Lerner AL, Schwarz EM, Awad HA. Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts. J Biomech. 2007;40:3178–3186. doi: 10.1016/j.jbiomech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Lewandrowski KU, Bondre S, Hile DD, Thompson BM, Wise DL, Tomford WW, Trantolo DJ. Porous poly(propylene fumarate) foam coating of orthotopic cortical bone grafts for improved osteoconduction. Tissue Eng. 2002;8:1017–1027. doi: 10.1089/107632702320934119. [DOI] [PubMed] [Google Scholar]
- 8.Gardner MJ, van der Meulen MC, Carson J, Zelken J, Ricciardi BF, Wright TM, Lane JM, Bostrom MP. Role of parathyroid hormone in the mechanosensitivity of fracture healing. J Orthop Res. 2007;25:1474–1480. doi: 10.1002/jor.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86-A:116–123. doi: 10.2106/00004623-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man. Cyber. 1979;9:62–66. [Google Scholar]
- 11.Shigley JE, Mischke CR. 6th ed. New York, NY, USA: McGraw-Hill; 2001. Mechanical Engineering Design. [Google Scholar]
- 12.Kass M, Witkin A, Terzopoulos D. Snakes: Active contour models. Int J Comput Vis. 1988;1:321. [Google Scholar]
- 13.Lodder MC, Lems WF, Ader HJ, Marthinsen AE, van Coeverden SC, Lips P, Netelenbos JC, Dijkmans BA, Roos JC. Reproducibility of bone mineral density measurement in daily practice. Ann Rheum Dis. 2004;63:285–289. doi: 10.1136/ard.2002.005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- 15.Dickson GR, Geddis C, Fazzalari N, Marsh D, Parkinson I. Microcomputed tomography imaging in a rat model of delayed union/non-union fracture. J Orthop Res. 2008;26:729–736. doi: 10.1002/jor.20540. [DOI] [PubMed] [Google Scholar]
- 16.Chen JB, Yu Y, Yang JL, Morgan DA, Walsh WR. BMP-7 and CBFA1 in allograft bone in vivo bone formation and the influence of gamma-irradiation. J Biomed Mater Res A. 2007;80:435–443. doi: 10.1002/jbm.a.30913. [DOI] [PubMed] [Google Scholar]
- 17.Golden JD, Jones AL, Bucholz RW, Bosse MJ, Lyon TR, Webb LX, Valentin-Opran A. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. J Bone Joint Surg Am. 2008;90:1168–1169. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 18.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1431–1441. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 19.Krause F, Younger A, Weber M. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. J Bone Joint Surg Am. 2008;90:1168. [PubMed] [Google Scholar]
- 20.Soltan M, Smiler D, Prasad HS, Rohrer MD. Bone block allograft impregnated with bone marrow aspirate. Implant Dent. 2007;16:329–339. doi: 10.1097/ID.0b013e31815c8ef4. [DOI] [PubMed] [Google Scholar]
- 21.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hidaka C, Goshi K, Rawlins B, Boachie-Adjei O, Crystal RG. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine. 2003;28:2049–2057. doi: 10.1097/01.BRS.0000091661.11228.C3. [DOI] [PubMed] [Google Scholar]
- 23.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 24.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O'Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, Ulrich-Vinther M, Soballe K, Guldberg RE, Lin AS, O'Keefe RJ, Zhang X, Schwarz EM. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–218. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Tan KK, Tan GH, Shamsul BS, Chua KH, Ng MH, Ruszymah BH, Aminuddin BS, Loqman MY. Bone graft substitute using hydroxyapatite scaffold seeded with tissue engineered autologous osteoprogenitor cells in spinal fusion: Early result in a sheep model. Med J Malaysia. 2005;60(Suppl C):53–58. [PubMed] [Google Scholar]
- 27.Mihelic NE. Repair of a severely comminuted distal femur fracture using surgical-grade calcium sulfate bone graft substitute. Orthopedics. 2004;27(Suppl):s127–s128. doi: 10.3928/0147-7447-20040102-09. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22:495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa T, Yamagiwa H, Hayami T, Liu Z, Huang KY, Tokunaga K, Murai T, Endo N. Human PTH (1-34) induces longitudinal bone growth in rats. J Bone Miner Metab. 2002;20:83–90. doi: 10.1007/s007740200011. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto T, Shigetomi M, Ohno T, Matsunaga T, Muramatsu K, Tanaka H, Sugiyama T, Taguchi T. Sequential treatment with intermittent low-dose human parathyroid hormone (1-34) and bisphosphonate enhances large-size skeletal reconstruction by vascularized bone transplantation. Calcif Tissue Int. 2007;81:232–239. doi: 10.1007/s00223-007-9056-7. [DOI] [PubMed] [Google Scholar]
- 31.Abe Y, Takahata M, Ito M, Irie K, Abumi K, Minami A. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1-34) in a rat spinal arthrodesis model. Bone. 2007;41:775–785. doi: 10.1016/j.bone.2007.06.025. [DOI] [PubMed] [Google Scholar]