Abstract
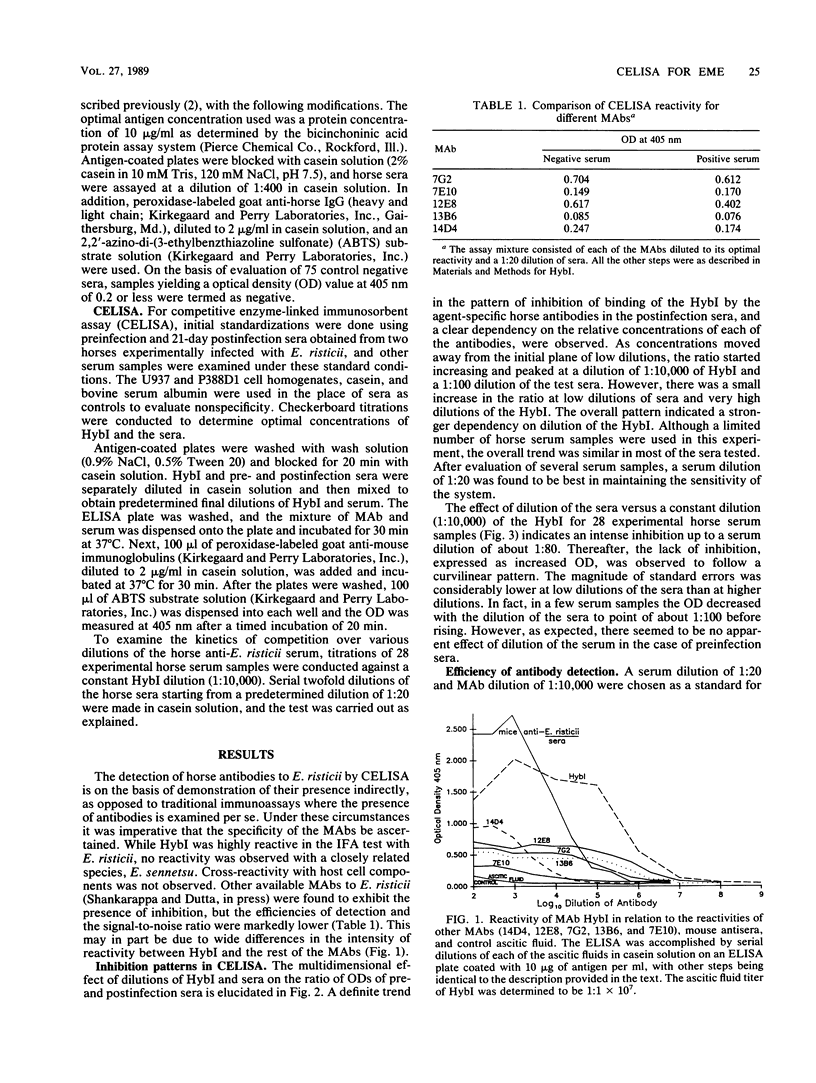
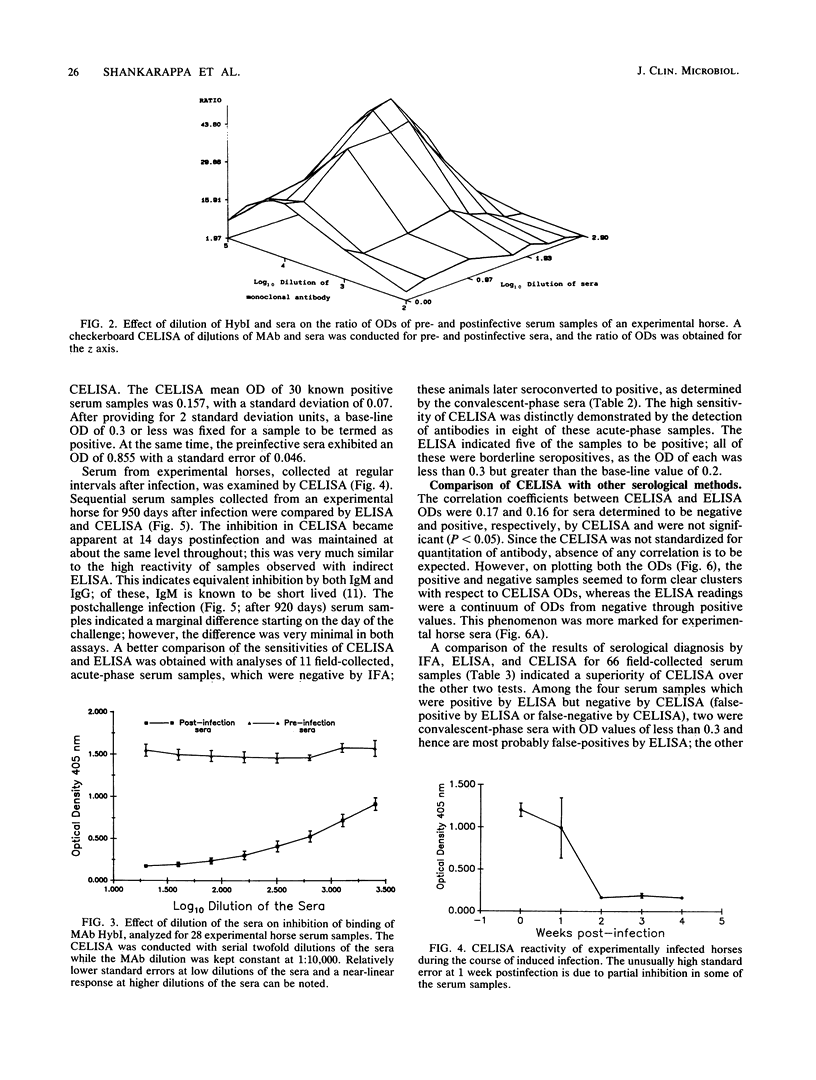
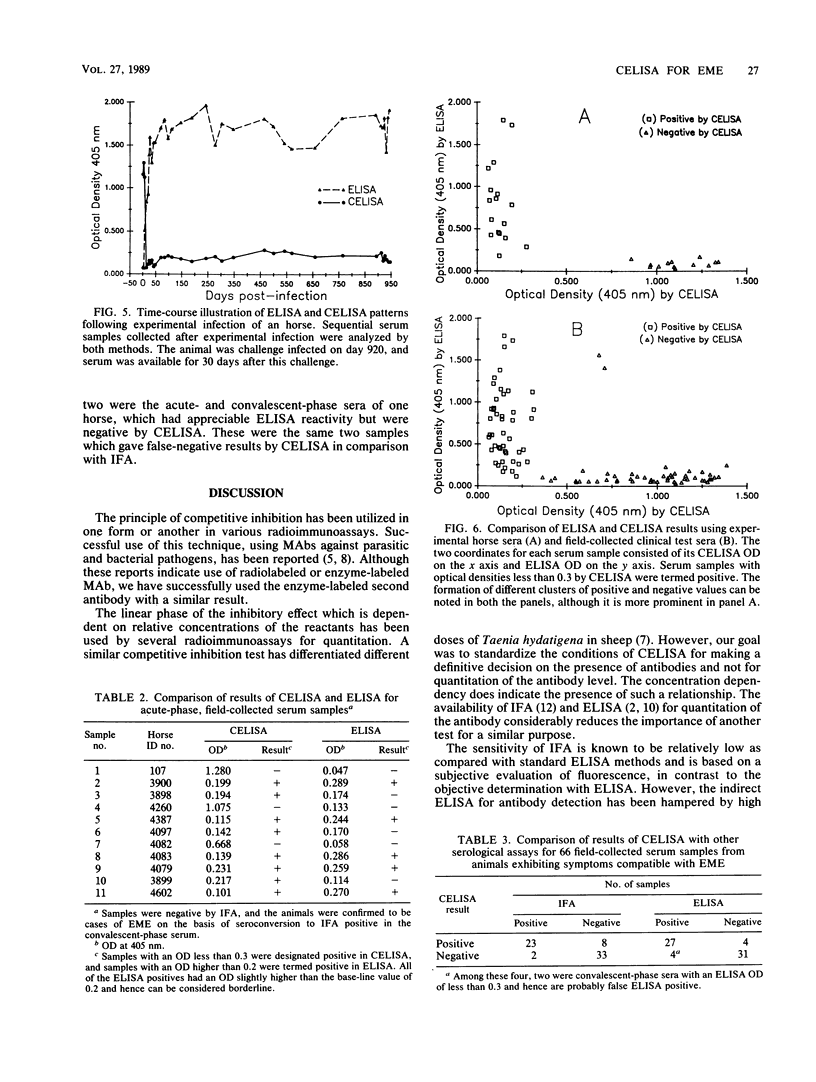
Competitive enzyme-linked immunosorbent assay (CELISA), mediated by a monoclonal antibody designated HybI, was developed for the diagnosis of equine monocytic ehrlichiosis. Inhibition of binding of HybI by the horse antibodies to Ehrlichia risticii was optimum at dilutions of 1:20 for serum and 1:10,000 for HybI. Mean optical densities (ODs) of positive and negative sera were 0.158 and 0.855, respectively. A comparison of ODs obtained by CELISA and indirect enzyme-linked immunosorbent assay (ELISA) indicated a marked tendency of positive and negative samples to cluster separately with respect to CELISA ODs, whereas the ELISA results displayed a continuum of ODs from negative to positive. Analysis of diagnosis by indirect fluorescent-antibody test (IFA), ELISA, and CELISA for 66 field-collected serum samples indicated that CELISA was superior to IFA and ELISA. Among 11 acute-phase serum samples negative by IFA which were obtained from horses that subsequently seroconverted, CELISA clearly demonstrated antibodies in 8 of these acute-phase sera, whereas 5 were borderline positive by ELISA. The presence of agent-specific humoral antibodies could be demonstrated conclusively by 14 days after infection. The results suggest that CELISA is more sensitive than IFA and ELISA and, owing to the marked differences between positive and negative samples, can be easily adapted for use in the field for detection of horse antibodies to E. risticii.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dutta S. K., Myrup A. C., Rice R. M., Robl M. G., Hammond R. C. Experimental reproduction of Potomac horse fever in horses with a newly isolated Ehrlichia organism. J Clin Microbiol. 1985 Aug;22(2):265–269. doi: 10.1128/jcm.22.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Rice R. M., Hughes T. D., Savage P. K., Myrup A. C. Detection of serum antibodies against Ehrlichia risticii in Potomac horse fever by enzyme-linked immunosorbent assay. Vet Immunol Immunopathol. 1987 Jan;14(1):85–92. doi: 10.1016/0165-2427(87)90077-8. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Lim P. L., Ho M. Y. Diagnosis of enteric fever by inhibition assay using peroxidase-labelled monoclonal antibody and Salmonella typhi lipopolysaccharide. Aust J Exp Biol Med Sci. 1983 Dec;61(Pt 6):687–704. doi: 10.1038/icb.1983.65. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of poliovirus: a hypothesis to explain the mechanism and the one-hit character of the neutralization reaction. Virology. 1976 Feb;69(2):500–510. doi: 10.1016/0042-6822(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Cruise K. M., Garcia E. G., Anders R. F. Hybridoma-derived antibody with immunodiagnostic potential for schistosomiasis japonica. Proc Natl Acad Sci U S A. 1981 May;78(5):3165–3169. doi: 10.1073/pnas.78.5.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse V. F., Shepard C. C., Redus M. D., Tzianabos T., McDade J. E. A comparison of the complement fixation, indirect fluorescent antibody, and microagglutination tests for the serological diagnosis of rickettsial diseases. Am J Trop Med Hyg. 1979 Mar;28(2):387–395. doi: 10.4269/ajtmh.1979.28.387. [DOI] [PubMed] [Google Scholar]
- Pretzman C. I., Rikihisa Y., Ralph D., Gordon J. C., Bech-Nielsen S. Enzyme-linked immunosorbent assay for Potomac horse fever disease. J Clin Microbiol. 1987 Jan;25(1):31–36. doi: 10.1128/jcm.25.1.31-36.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B., Cordes D. Rickettsial link with acute equine diarrhoea. Vet Rec. 1984 Oct 13;115(15):390–390. doi: 10.1136/vr.115.15.390-a. [DOI] [PubMed] [Google Scholar]
- Ristic M., Holland C. J., Dawson J. E., Sessions J., Palmer J. Diagnosis of equine monocytic ehrlichiosis (Potomac horse fever) by indirect immunofluorescence. J Am Vet Med Assoc. 1986 Jul 1;189(1):39–46. [PubMed] [Google Scholar]



