Summary
Currently, the standard treatment for osteogenesis imperfecta (OI) is bisphosphonate therapy. Recent studies, however, have shown delayed healing of osteotomies in a subset of OI patients treated with such agents. The current study sought to determine the effects of another therapy, RANKL inhibition, on bone healing and bone strength in the growing oim/oim mouse, a model of moderate-to-severe OI. Mice (73 oim/oim and 69 wildtype (WT)) were injected twice weekly with either soluble murine RANK (RANK-Fc) (1.5mg/kg) or saline beginning at 6 weeks of age. At 8 weeks of age, the animals underwent transverse mid-diaphyseal osteotomies of the right femur. Therapy was continued until sacrifice at 2, 3, 4 or 6 weeks post-fracture. At 6 weeks post-fracture, greater callus area (6.59±3.78mm2 vs 2.67±2.05mm2, p=0.003) and increased radiographic intensity (mineral density) (0.48 ± 0.14 vs. 0.30 ± 0.80, p=0.005) were found in the RANK-Fc vs saline oim/oim group, indicating a delay in callus remodeling. Despite this delay, mechanical tests at 6 weeks post-fracture revealed no significant differences in whole bone properties of stiffness and failure moment. Further, RANKL inhibition resulted in a greater failure moment and greater work to failure for the non-fractured contralateral WT bones compared to the non-fractured saline WT bones. Together, these results demonstrate that RANKL-inhibition does not adversely affect the mechanical properties of healing bone in the oim/oim mice, and is associated with increased strength in intact bone in the WT mice.
Keywords: osteogenesis imperfecta, fracture healing, receptor activator of nuclear factor-κB, RANKL, mice, anti-resorptives
Introduction
Osteogenesis imperfecta (OI) is a heritable connective tissue disorder with an incidence estimated at approximately 1:20,000 to 1:60,000 live births (50). It is characterized by heterogeneous mutations in the genes encoding for type I collagen, with clinical manifestations that include but are not limited to short stature, tissue fragility, skeletal deformities, joint laxity and death (46). In the last two decades, a number of observational studies of bisphosphonate-treated OI patients, most utilizing cyclic intravenous pamidronate, have demonstrated a decline in chronic bone pain, enhanced sense of well-being, and increased muscle strength with regular treatment (1, 2, 6, 17, 18, 28, 38, 45, 47-49, 52, 58). Recently, several randomized, clinical trials have also shown bisphosphonates to raise bone mineral density and lower the risk of fracture in OI patients though benefits in functional outcome are equivocal (16, 30, 51). Despite the advantages to bisphosphonate therapy, there are still issues regarding overall safety. A recent study found delayed healing in OI osteotomy patients treated with pamidronate, but no delay in fracture healing (39). Other issues found in pre-clinical studies surround possible delays in longitudinal growth (12), which is of particular concern to children and adolescents receiving these types of therapies. Bisphosphonates are also known to persist in bone tissue for many years (24), and it is unclear whether a maternal skeleton that contains bisphosphonates may lead to complications in fetal development (46). Optimal dose range and interval, as well as duration of treatment are also unknown. Consequently, new therapies that do not persist in the body for long periods in the body are being pursued.
One such group of compounds, known as RANKL inhibitors, bind to the receptor activator of nuclear factor-κB ligand (RANKL) which is produced by osteoblasts and by other cells. RANKL inhibitors inhibit the formation, activation and survival of osteoclasts (27). Osteoprotegerin (OPG) is a secretory glycoprotein which acts as a circulating decoy receptor of RANKL, effectively blocking its actions (53). Alterations of the RANKL/OPG ratio are implicated in bone destruction across a broad range of conditions (20). Randomized controlled clinical trials that evaluated the effects of a single dose of OPG-Fc fusion protein on biochemical bone markers in humans found marked and sustained suppression of bone resorption markers in a postmenopausal, osteoporotic population (3, 4), and also found decreased bone resorption marker levels in a myeloma and female skeletal metastatic population to a degree comparable to that of pamidronate (3, 4). Recently, a soluble, recombinant form of murine RANK (RANK-Fc), with properties similar to OPG, has been developed by combining the extracellular domain of the murine RANK molecule with the Fc region of human IgG1 (43). RANK-Fc binds to RANKL and prevents the substrate from ligating to physiologic membrane receptor RANK, thereby inhibiting the signal transduction process. In a murine study of SCID mice with prostate cancer, RANK-Fc was shown to decrease progression of cancer cells through inhibition of bone resorption (59).
RANKL inhibition holds great promise as an adjunct treatment for OI if it can be shown to reduce fracture rate in this population. Previous work by our group has demonstrated that alendronate therapy can increase bone mineral density (BMD) and reduce the incidence of fracture in this species of mouse (7). However, it is unclear how RANKL inhibition therapy would affect bone healing once fractures occur. Flick et al (14) evaluated RANKL inhibition in wildtype mice and found no significant delays by 28 days post-fracture. To address the effects of RANK-Fc therapy on fracture healing in OI, we utilized the oim/oim mouse, a model of OI that is deficient in proα2(I) collagen, resulting in production of type I collagen homotrimer. Animals homozygous for the oim mutation exhibit a moderate-to-severe OI phenotype characterized by skeletal fractures and deformities, osteopenia, and small body size (10). In the current study, oim/oim mice were treated with RANK-Fc and fracture healing was monitored longitudinally to test the hypothesis that such therapy would not result in delays in remodeling or in significant changes in whole bone mechanical properties.
Methods and Materials
Animals and RANK-Fc Therapy
All animal procedures were performed under an IACUC-approved protocol. Homozygous oim/oim mice and wildtype (WT) controls were purchased from Jackson Laboratory (Bar Harbor, ME) for breeding. Animals were housed in a light-controlled environment (12-hour light-dark cycles), given autoclaved tap water ad libitum, and were fed whole and powdered Rodent Diet #5001 (Purina, St. Louis, MO) that contained % 0.95 calcium, % 0.67 phosphorus, with 4.5 IU/gm vitamin D3 added. Mice were housed up to 4 mice/500 cm2 cage according to genotype and sex. Weaning was conducted at 3 weeks for WT mice and at 4 weeks for oim/oim mice. A total of 142 mice were divided into groups according to genotype, therapy, and post-fracture time until sacrifice (Figure 1). Four different post-fracture timepoints were selected based on healing rates in prior studies (34, 56): 2 weeks, 3 weeks, 4 weeks, and 6 weeks. In the 2, 3, and 4 week groups, n=5-6 per group. In all the 6 week groups, n=18-20 per group. Starting at six weeks of age, mice were injected subcutaneously with either soluble RANK-Fc (generously supplied by Amgen Inc., Thousand Oaks, CA) or saline, twice per week, for a period of 4, 5, 6 or 8 consecutive weeks (as determined prior to the start of the study). Experimental mice received RANK-Fc at a dose of 1.5mg/kg while saline controls received equivalent volumes based on weight. This dose of RANK-Fc was selected based on prior studies indicating ability to significantly reduce osteoclast numbers (14), and on pilot studies in our laboratory that showed increased bone density with treatment. All animals underwent osteotomy at 8 weeks of age, as described in the section below. Pre-treatment with experimental drug or saline control at 6 weeks of age, two weeks prior to osteotomy, was performed to mirror the clinical scenario where OI patients fracture while on anti-resorptive therapy.
Figure 1. Experimental Design Flow Chart.
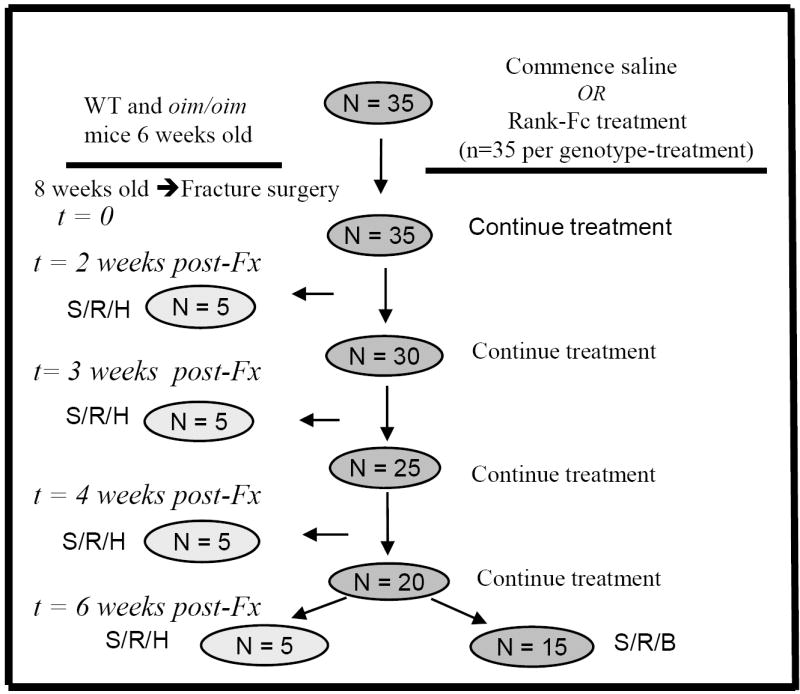
Flow chart detailing commencement and length of RANK-Fc (or saline) treatment, timepoint of fracture and sacrifice, and analyses performed at each timepoint. N = approximate number of animals at each timepoint for each genotype and treatment. S/R/H = sacrifice, radiographs obtained, and histology performed. S/R/B = sacrifice, radiographs obtained, and biomechanical studies performed. Fx=fracture.
Femoral Fracture Model
Given that oim/oim mice display severe bone fragility, we utilized a semi-open osteotomy model in an attempt to minimize fracture comminution. This technique is a modification of the semi-open diaphyseal rat femoral fracture model (5), and has been shown to provide reproducible results in prior studies of the oim/oim mice in our laboratory (57). All mice underwent surgery at 8 weeks of age. Wildtype mice were anesthetized with a cocktail of ketamine (50mg/kg), xylazine (10mg/kg), and ace promazine (2mg/kg) administered intraperitoneally (i.p.), while oim/oim received an i.p. injection of ketamine (35mg/kg) and ace promazine (1mg/kg), due to decreased capacity to tolerate anesthesia. The mouse’s right hip, thigh, and knee were prepared with Betadine (povidone-iodine) solution and a 1.0cm right medial parapatellar incision was made. In cases of oim/oim where fracture of the right femur was already present, the procedure was performed on the left lower extremity. The longitudinal fibers of the quadriceps mechanism were divided medially, and the patella was dislocated laterally to expose the femoral condyles. The femoral intramedullary canal was reamed with a 27gauge needle inserted between the condyles. The needle was introduced into the canal and driven in a retrograde fashion up the shaft of the femur to the level of the greater trochanter. Distally, the pin was cut flush with the cortex so as not to interfere with motion at the knee. Using a surgical scissor, an open transverse osteotomy was made through the mid-shaft of the femur. The wound was closed in layers with vicryl sutures in standard fashion. Lateral radiographs with the hindlimbs in external rotation and anteroposterior (AP) radiographs were taken post-operatively from a subset of mice to ensure correct positioning of the pin. These radiographs were subsequently graded for comminution. Mice were cared for after surgery according to standard AAALAC protocols and allowed unrestricted cage activity. Animals were sacrificed via CO2 inhalation.
Radioagraphic Image Acquisition and Image Analysis
Radiographs of the fracture site obtained on the day of fracture were graded for comminution according to the Winquist classification, where Winquist grades 1 - 4 represent increasing levels of comminution (55). Whole body radiographs were taken at the time of sacrifice. Femora were harvested bilaterally and the soft tissue was carefully removed. High resolution digital Faxitron radiographs (Faxitron X-Ray Corporation, Wheeling, IL) in the anterioposterior (AP) and mediolateral (ML) planes were obtained. Each image included an aluminum alloy step radiographic intensity standard for calibration purposes. Femora were then wrapped in gauze soaked in saline and kept frozen at -20° C until use.
AP and ML radiographs of the femora were analyzed using Image J software (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/). Each individual image was calibrated against known intensity and length standards using an aluminum alloy step intensity standard. Previous work by our group has demonstrated that radiographically-determined density values obtained using the aluminum alloy step intensity standard correlated well with bone mineral density (BMD) as determined by micro CT analysis (21). Several prior studies have also utilized this method (7, 35). Periosteal and external callus tissue was carefully outlined on both sides of the femur. Callus area for an individual femur was calculated as the sum of areas obtained from both the AP and ML images. Callus intensity for a particular femur was calculated as the average of all the callus intensities from both the AP and ML images.
Biomechanical Testing
The mechanical properties of the fractured and non-fractured contralateral femora were evaluated by four-point bend tests for all groups at the 6-week post-fracture timepoint (n =13-15 per group). The four-point bend test was chosen to ensure that failure occurred within the segment along the middle of the femoral shaft that contained the fracture. Tests were conducted at room temperature on a precision electromagnetic-based load frame (EnduraTEC ELF 3200, Bose Corporation, Minnetonka, MN). Prior to testing, bones were thawed completely and the intramedullary pins of the fractured femora were carefully removed. Femora were kept moist throughout the duration of the test. Femora were tested such that the anterior side was loaded in compression and the posterior side in tension. The posterior surface was placed on the lower supports, which were set 7.9mm apart for WT and 7.1mm apart for the oim/oim. The upper posts were 2.1mm apart for the WT and 1.9mm apart for the oim/oim. Load was applied at 0.05mm/sec until failure occurred.
Structural mechanical properties that are dependent on geometry were measured. Failure moment was calculated according to the formula:
where a = distance in mm between the inner and outer posts, and Fmax = the maximum load the bone sustained during testing. Bending stiffness, a measure of the rigidity of the whole bone, was determined according to the following formula:
where m = the slope of the elastic region of the load-displacement curve, a = distance in mm between the inner and outer posts, and L = distance in mm between the outer posts. Total displacement equaled the displacement at the failure load. Since post-yield displacement is minimal in the four-point bend test, the degree of displacement before failure was taken as a measure of the specimen’s brittleness. Work to failure, a measure of the energy required to fracture the bone, was calculated as the area under the entire load-displacement curve. Mechanical properties of the fractured femur (failure moment, bending stiffness, total displacement, and work to failure) were compared to the corresponding mechanical property data from the non-fractured contralateral femur. Contralateral femora from the oim/oim groups that sustained spontaneous fractures were not mechanically tested.
Histology
Harvested fractured femora (n=3-5 per group) were fixed in 10% buffered neutral formalin for 24 hours, and decalcified in 10% EDTA (pH 7.2-7.4) for approximately two weeks. Intramedullary pins were then removed prior to embedding the specimens in paraffin. Sections 6μm thick were obtained along the coronal plane from anterior to posterior and stained with alcian blue to identify proteoglycans in cartilage as follows: Deparaffinized, rehydrated sections were incubated in 3% acetic acid for 3-5 minutes and stained with alcian blue solution for 30 minutes, followed by rinsing in distilled water. They were then counterstained with Kernechtrot for 5 minutes before rinsing, dehydrating and clearing in xylene. Analysis was performed with the BioQuant Image Analysis system (BIOQUANT Image Analysis Corporation Nashville, TN) using a threshold function. The amount of cartilage was measured by quantitation of the proteoglycan staining, which appeared as a deep blue color. The percentage of cartilage within the callus region was calculated as follows:
Since specimens from later timepoints lacked any discernable cartilage, only the 2 week post-fracture groups were analyzed quantitatively.
Statistical analysis
Statistical analyses were performed with SigmaStat software (SPSS Inc., Chicago, IL). For each measurement, the means and standard deviation were calculated for each group. For the biomechanical tests at 6 weeks post-fracture and for the histological analysis at two weeks post-fracture, two-way ANOVA tests were performed to test for the simultaneous effects of genotype and RANK-Fc treatment on the outcome variables. For the radiographic data, three-way ANOVA tests were performed to test for the simultaneous effects of genotype, RANK-Fc treatment, and time on the outcome variables. Bonferroni post-hoc tests were performed for comparison of groups with values significant at p<0.05.
Results
Radiographic image analysis
Post-Fracture Radiographs
The inital fractures created in the oim/oim mice had more comminution than those created in the WT mice (Table 1). The fractures created in the WT mice were generally stable, and there were no Winquist Grade III or IV fractures. In contrast, 17% of the RANK-Fc and 11% of the saline-treated oim/oim mice had Winquist Grade III fractures, and 5% of the saline-treated oim/oim mice had Winquist Grade IV fractures.
Table 1.
Winquist Classification of Femoral Fracture Comminution at T = 0 weeks
| Winquist I | Winquist II | Winquist III | Winquist IV | |
|---|---|---|---|---|
| WT RANK-FC (n=5) | 5 (100%) | 0 | 0 | 0 |
| WT Saline (n=8) | 7 (88%) | 1 (12%) | 0 | 0 |
| WT (Total n=13) | 12 (93%) | 1 (7%) | 0 | 0 |
| oim/oim RANK-Fc (n=18) | 8 (44%) | 7 (39%) | 3 (17%) | 0 |
| oim/oim Saline (n=37) | 25 (68%) | 6 (16%) | 4 (11%) | 2 (5%) |
| oim/oim (Total n=55) | 33 (60%) | 13 (24%) | 7 (13%) | 2 (3%) |
Bony union of the fracture occurred in all mice regardless of genotype or treatment by 6 weeks post-fracture. Callus area generally decreased from 2 weeks to 6 weeks post-fracture for the saline groups, indicating remodeling of the callus (Figure 2A). Among the 6 week groups, a significant difference between oim/oim RANK-Fc and oim/oim saline was detected, indicating a delay in callus remodeling. Significantly reduced callus area was observed for the 6 week WT saline group compared to the 2 week WT saline treated group. At six weeks post-fracture, the callus intensity was significantly greater in the oim/oim RANK-Fc group compared to the oim/oim saline group, indicating persistence of callus and delayed remodeling (Figure 2B). Callus intensity decreased from 4 weeks to 6 weeks post-fracture for the RANK-Fc treated WT groups. Qualitative differences in callus at six weeks post-fracture can be appreciated by comparison of radiographic images (Figure 2C).
Figure 2.
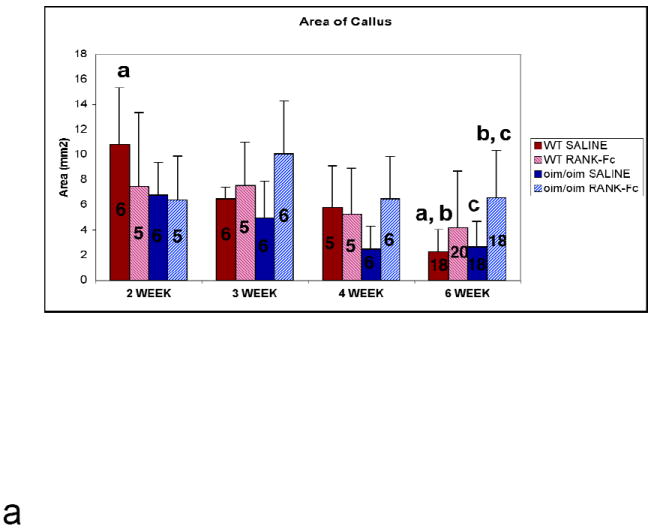
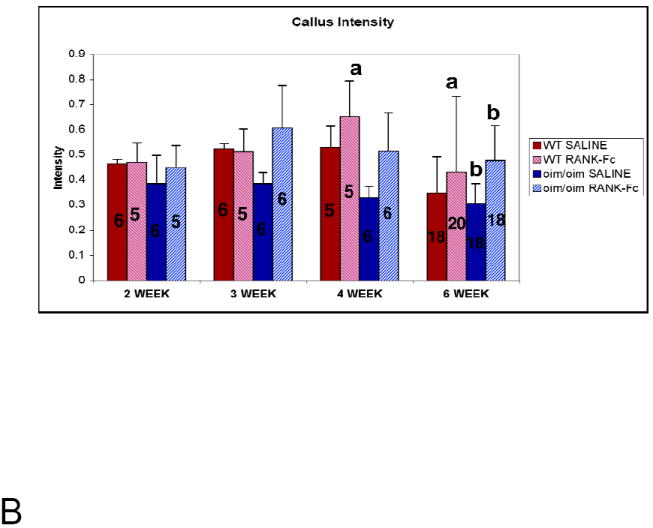
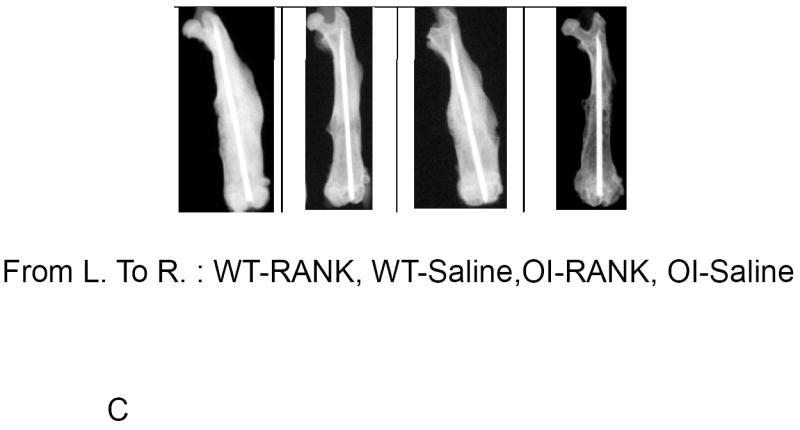
(A) Quantification of Callus Area by Radiographic Image Analysis. Saline-treated groups show a general decline in callus area from 2 weeks to 6 weeks post-fracture, whereas RANK-Fc treated groups demonstrate persistence of callus, with significantly greater callus area at 6 weeks post-fracture for the oim/oim RANK-Fc group compared to control. (Values shown are means ± standard deviation; values that are significantly different from each other are noted with matching lower case letters. Number per group is shown within each bar). (B) Quantification of Radiographic Intensity of Callus Callus intensity is significantly greater at 6 weeks post-fracture for the oim/oim RANK-Fc group compared to controls. (Values shown are means ± standard deviation; values that are significantly different from each other are noted with matching lower case letters. Number per group is shown within each bar). (C) Radiographs of Fractured Femora at 6 weeks Post-Fracture AP radiographs of femora taken 6 weeks post-fracture. Treatment with RANK-Fc leads to qualitative, visible differences in healing bone most readily observed in the oim/oim mice as increased callus area and intensity. (From left to right: WT RANK-Fc, WT Saline, oim/oim RANK-Fc, oim/oim Saline).
Mechanical testing
Oim/oim Bones
In the specimens tested, failure occurred consistently through the fracture site. Functional assessment by mechanical testing revealed that RANK-Fc had no significant effect on failure moment and bending stiffness as compared to saline controls. Failure moment was lower in fractured versus contralateral non-fractured oim/oim bones in both saline and RANK-Fc treated mice (Figure 3), suggesting that the fractured oim/oim bones were not completely healed at 6 weeks. Bending stiffness was also lower in the fractured versus non-fractured oim/oim bones for mice treated with RANK-Fc. (Figure 4). There were no differences in total displacement for the RANK-Fc or saline-treated fractured oim/oim bones versus their contralateral non-fractured bones (Figure 5), and no significant differences in work to failure (Figure 6).
Figure 3. Failure Moment of Fractured and Non-Fractured Femora (6 weeks post-fracture).
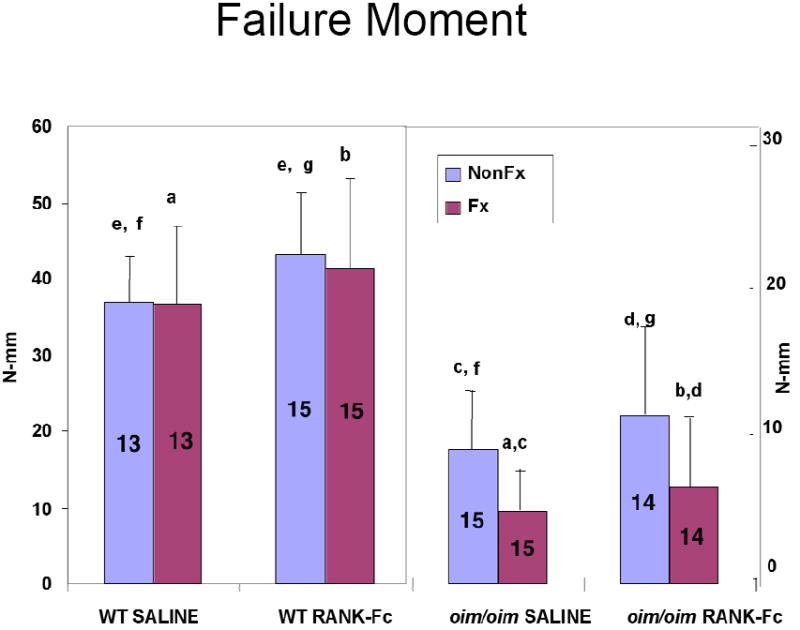
Both RANK-Fc and saline treatment resulted in a lower failure moment for the fractured compared to the contralateral non-fractured oim/oim bones indicating incomplete healing. For the non-fractured contralateral WT bones, RANK-Fc treatment resulted in a greater failure moment. (Values shown are means ± standard deviation; values that are significantly different from each other are noted with matching lower case letters. Number per group is shown within each bar).
Figure 4. Bending Stiffness of Fractured and Non-Fractured Femora (6 weeks post-fracture).
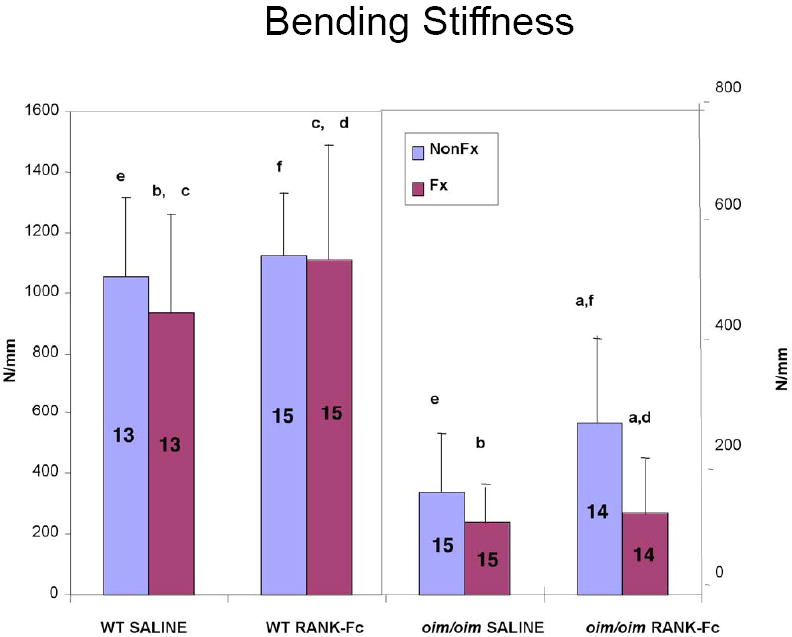
RANK-Fc treatment resulted in a lower bending stiffness in the fractured compared to the non-fractured oim/oim bones, results reflective of the fact that the fractured oim/oim bones were not completely healed at 6 weeks. For the fractured WT bones, RANK-Fc treatment resulted in a greater bending stiffness compared to saline WT. (Values shown are means ± standard deviation; values that are significantly different from each other are noted with matching lower case letters. Number per group is shown within each bar).
Figure 5. Total Displacement of Fractured and Non-Fractured Femora (6 weeks post-fracture).
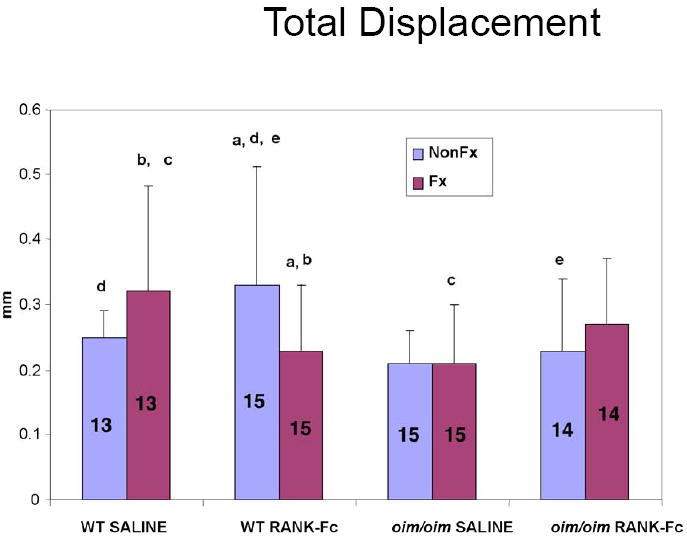
For the fractured WT bones, RANK-Fc treatment resulted in diminished total displacement compared to saline WT. RANK-Fc treatment also resulted in a lower displacement in the fractured vs non-fractured WT bone. (Values shown are means ± standard deviation; values that are significantly different from each other are noted with matching lower case letters. Number per group is shown within each bar).
Figure 6. Work to Failure of Fractured and Non-Fractured Femora (6 weeks post-fracture).
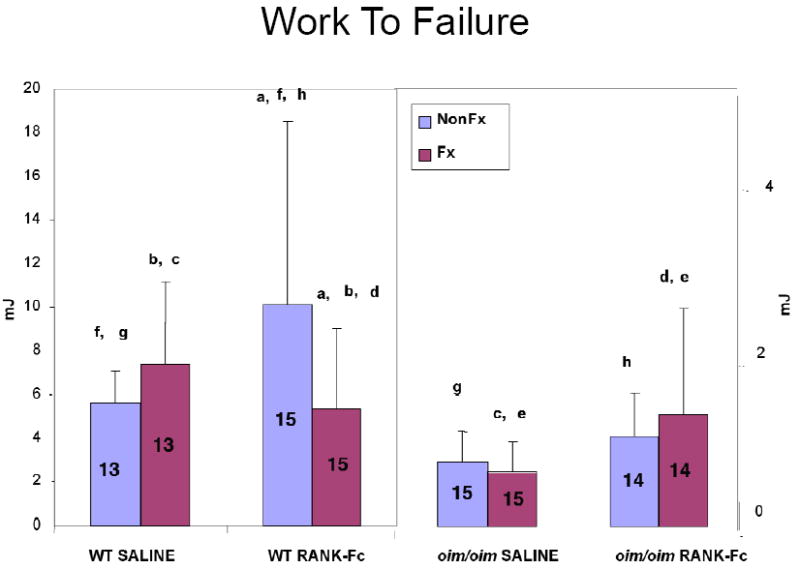
RANK-Fc treatment resulted in a lower work to failure in the fractured vs non-fractured WT bone, but for the non-fractured contralateral WT bones, RANK-Fc treatment resulted in a greater work to failure compared to the non-fractured WT saline. A non-significant (p=0.07) increase in work to failure for the oim/oim RANK-Fc group vs the oim/oim saline group is noted. (Values shown are means ± standard deviation; values that are significantly different from each other are noted with matching lower case letters. Number per group is shown within each bar).
WT Bones
Similar to the oim/oim bones, failure occurred consistently through the fracture site. Failure moment was greater for the WT RANK-Fc non-fractured bone versus the saline non-fractured bone (Figure 3). For the fractured WT bones, bending stiffness was greater in the RANK-Fc treatment group compared to saline controls (Figure 4), but total displacement was diminished (Figure 5). In contrast, total displacement increased for the WT non-fractured RANK-Fc bones compared to the non-fractured saline bones (Figure 5). Work to failure was greater for the WT RANK-Fc non-fractured bone compared to the saline non-fractured group (Figure 6), but was diminished for the WT RANK-Fc fractured bone compared to the saline fractured group (Figure 6). However, no significant differences were observed in work to failure for the RANK Fc fractured WT bones compared to saline non-fractured bones, demonstrating that after fracture repair, RANK-Fc bones were equally as strong a normal, non-fractured WT bones.
Histological analysis
No significant differences in quantity of callus cartilage was found for the RANK-Fc treated groups (10.22% ± 7.48 for WT; 10.36 % ± 4.89 for oim/oim) compared to the saline treated groups (6.49% ± 3.44 for WT; 5.04% ± 1.81 for oim/oim) at two weeks post-fracture, indicative of no delays in early endochondral bone healing.
Discussion
Fracture healing is a multifaceted physiologic process intended to restore the functional and biomechanical competency of the injured bone, as well its original geometry. A complex array of cytokines, growth factors, and other chemical messengers steer the process in a spatio-temporal manner through three major phases: inflammatory, reparative, and remodeling. After hematoma formation and the initial inflammatory response, osteoblasts predominate, laying down new bone via intramembranous ossification as well as endochondral ossification (11). In the remodeling phase that follows, woven bone is replaced by lamellar bone, with osteoclasts responsible for resorption and osteoblasts in charge of laying down new matrix. A recent study on fracture healing in mice revealed that expression profiles for OPG, RANKL, and macrophage colony-stimulating factor (M-CSF), three key signaling molecules that modulate osteoclast formation, function and survival, are tightly coupled during the healing process (26). Kon et al (26) have shown that RANKL expression levels are virtually undetectable in intact bones but can be induced with trauma, peaking on day 3 and day 14 after fracture but remaining strongly induced throughout the period of fracture healing. It would not be unreasonable, therefore, to predict that RANKL inhibition, if conducted early in the fracture healing process and continuously, will exert a measurable effect. The results of this study do indeed provide evidence that RANKL inhibition with RANK-Fc can delay remodeling in oim/oim mice, contrary to our original hypothesis. In spite of this, however, adverse affects on the mechanical properties of the healing oim/oim bone were not found.
Although the mice utilized in this study fracture spontaneously, this is the first large-scale study that establishes a fracture healing model for oim/oim mice whereby fractures can be monitored over a known time course. In a closed femoral fracture healing model in the mouse, Manigrasso et al (34) found that peak fracture callus volume was evident at 10-14 days post-fracture, with fracture bridging apparent by 3 weeks, which is approximately 1 week faster than rats. Bone remodeling, as evidenced by histologic sampling, was observed as early as 3 weeks post-fracture, though mechanical testing indicated that significant increases in structural or material strength did not occur until 6-12 weeks after fracture (34). In the current study, radiographic image analysis indicates that RANK-Fc administration does not prevent the initiation or formation of callus, since all groups demonstrated callus formation by 2 weeks. This corroborates findings by Flick et al (14) who showed that uncoupling the bone formation/resorption processes by RANKL inhibition had no adverse effect on callus formation. Our results also show that callus area and density were significantly greater in the RANK-Fc treated oim/oim group at 6 weeks post-fracture compared to time-equivalent saline controls, suggesting delays in remodeling, possibly due to reduced osteoclast activity. Wildtype mice, however, showed no differences with RANK-Fc treatment compared to saline controls. A study from our laboratory has also found enhanced callus area and density in fractured oim/oim mice treated with the anti-resorptive alendronate compared to saline controls (57). Similarly, other studies on fracture healing in animals treated with bisphosphonates demonstrated either no influence on callus size or increased callus size, but none have shown evidence of decreased callus (8, 19, 22, 25, 31, 33, 41, 44).
Restoration of mechanical stability is usually considered the most important functional outcome in fracture healing, making evaluation of bone strength critical to analysis (9, 29, 32, 44). In fracture healing studies of bisphosphonate therapy, mechanical parameters were usually unaltered (25, 33, 42, 44) or even improved (8, 19, 31, 32) compared to controls. In this experiment, there were no significant differences in the mechanical properties of the treated oim/oim mice bone compared to saline controls. This is likely due to the larger callus which may serve as compensation for weaker bone, and is actually an adaptation governed by a feedback mechanism (13). An unexpected finding in our data was that in WT mice, treatment with RANK-Fc actually left fractured bones stiffer but more brittle (as evidenced by the decreased total displacement) compared to WT controls. Similarly, with alendronate treatment, intact WT bone was found to become more brittle (37). It is uncertain what the underlying mechanism is for the increased brittleness, but possibilities include osteoclastic inhibition that allows for excess woven bone at the fracture site with minimal progression to the structurally and mechanically superior lamellar bone, as discussed by Jepsen et al (23) when considering possible contributors to increased bone brittleness in mice. Alternatively, there could be increased mineralization density in the intact cortical bone, as was found in the alendronate-treated bone. A recent study found that soluble OPG was able to reverse woven bone to a lamellar structure in the cortices of OPG knockout mice (36), lending support to the increased mineralization density as the primary contributor to increased brittleness. Unfortunately, in the current study, limited sample size for histology prevented systematic quantitative assessment of the woven bone at the fracture site, and therefore it is not possible to drawn firm conclusions concerning the composition of the fracture callus.
Notwithstanding this limitation, and despite the increased brittleness, there was no difference in work to failure for the RANK-Fc fractured WT bones compared to the saline non-fractured WT bones, demonstrating that RANK-Fc fractured bones are equally as strong as normal, non-fractured bones, an important clinical outcome. RANK-Fc was also shown to improve bone properties in non-fractured WT bone, including increasing total displacement, in contrast to the effects seen in alendronate-treated WT bone where displacement was decreased (37).
In the Flick et al study (14), it was observed that RANKL inhibition seemed to generate an increase in persistent, uncalcified cartilage matrix. Here, we show that the percentage of cartilage in the fracture callus was not significantly different in mice treated with RANK-Fc compared to those treated with saline at 2 weeks post-fracture though our sample size was limited to 3-5 specimens per group. Further, there was not a measurable amount of calcified cartilage left at 3, 4 or 6 weeks post-fracture, an indication of callus remodeling.
We are aware of some limitations to this study, including the choice of fracture model. As oim/oim bone is inherently brittle and prone to fracture easily, a surgical procedure that results in consistent and reproducible fractures is of paramount importance. Though other models besides the open osteotomy utilized here are commonly used in murine fracture healing studies, none have been applied to oim/oim mice. The selection of the osteotomy model for this study was based on prior experience that showed it enabled creation of fractures that minimized the risk of comminution in these extremely fragile animals. In spite of this, increased comminution was still evident in the initial oim/oim mice fractures, regardless of treatment, and could possibly contribute to the finding of increased callus area at six weeks post-fracture. Since this study did not follow healing to completion in all the mice, an experiment that allows for a protracted recuperative period would be able to provide more information on the long-term effects of RANK-Fc on healing. In humans, it usually takes 1-4 years to replace callus with functionally competent lamellar bone (15), whereas in dogs remodeling of the fracture site may be completed in approximately 32 weeks (40). The time to complete fracture healing in mice has not been established, but it is apparent from the presence of callus at the terminal timepoint in this study that it is greater than 6 weeks.
Another limitation concerns the number of mice per group for the comparison of mechanical properties. Only one parameter of the fractured bones was found to be significantly different between a RANK-Fc treated group and a saline group of the same genotype - the increased stiffness of the fractured RANK-Fc WT bones compared to fractured saline controls. Although a power analysis had been performed prior to the current study based on data from three point bend tests on intact bones (37), the experimental variation in the four point bend data was greater than expected, possibly due to the variability in the fracture healing process. Consequently, it is possible that some of the other differences in mechanical properties may not have reached statistical significance due to inadequate sample size in each group, such as the non-statistical increase in work to failure (p=0.07) for the fractured RANK-Fc treated oim/oim bones compared to the fractured saline controls. One further limitation concerns the inability to extract material properties from the mechanical testing data in this study. A change in either cross-sectional area or material properties, or both, could contribute to the observed increase in stiffness for the fractured WT bones in the RANK-Fc treatment group compared to saline controls. Unfortunately, normalization of structural properties to bone geometry was not possible here, as the non-uniform distribution of callus made it very difficult to assess cross-sectional properties. However, in a prior study where we investigated the effects of alendronate on non-fractured mouse bone, we found that increased stiffness in the WT bone occurred with increased bone diameter but no difference in material properties (35).
In conclusion, RANK-Fc delays bone remodeling in growing oim/oim mice but does not diminish mechanical strength. We do not know what the effects of RANK-Fc would be on more mature mouse bone, and what, if any, systemic side effects may be attributed to chronic RANK-Fc administration. However, no toxicities have been found with OPG administration, which acts similarly via a RANKL inhibitory mechanism (54). Future fracture healing studies utilizing this therapy should further address issues of long-term effects, particularly in light of the two randomized, controlled trials utilizing an OPG-Fc fusion protein which demonstrate that RANKL inhibition is an efficient modality to treat altered bone metabolism in humans (3, 4).
Acknowledgments
This study was supported by NIH AR48337 (NPC) and utilized the NIH Musculoskeletal Core Facility at HSS (AR46121). This study was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR12538-01 from the National Center for Research Resources, NIH.
References
- 1.Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, Tato L. Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res. 2003;18:126–30. doi: 10.1359/jbmr.2003.18.1.126. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee I, Shortland GJ, Evans WD, Gregory JW. Osteogenesis imperfecta and intravenous pamidronate. Arch Dis Child. 2002;87:562–3. doi: 10.1136/adc.87.6.562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res. 2001;16:348–60. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- 4.Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, Harousseau JL, Lipton A, Mariette X, Williams CD, Nakanishi A, Holloway D, Martin SW, Dunstan CR, Bekker PJ. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–92. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 5.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 6.Brumsen C, Hamdy NA, Papapoulos SE. Long-term effects of bisphosphonates on the growing skeleton. Studies of young patients with severe osteoporosis. Medicine (Baltimore) 1997;76:266–83. doi: 10.1097/00005792-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Camacho NP, Raggio CL, Doty SB, Root L, Zraick V, Ilg WA, Toledano TR, Boskey AL. A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcif Tissue Int. 2001;69:94–101. doi: 10.1007/s002230010045. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002;17:2237–46. doi: 10.1359/jbmr.2002.17.12.2237. [DOI] [PubMed] [Google Scholar]
- 9.Chao EY, Inoue N, Elias JJ, Aro H. Enhancement of fracture healing by mechanical and surgical intervention. Clinical Orthopaedics & Related Research. 1998:S163–78. doi: 10.1097/00003086-199810001-00018. [DOI] [PubMed] [Google Scholar]
- 10.Chipman SD, Sweet HO, McBride DJ, Jr, Davisson MT, Marks SC, Jr, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1993;90:1701–5. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 12.Evans KD, Lau ST, Oberbauer AM, Martin RB. Alendronate affects long bone length and growth plate morphology in the oim mouse model for Osteogenesis Imperfecta. Bone. 2003;32:268–74. doi: 10.1016/s8756-3282(02)00974-2. [DOI] [PubMed] [Google Scholar]
- 13.Fleisch H. Can bisphosphonates be given to patients with fractures? J Bone Miner Res. 2001;16:437–40. doi: 10.1359/jbmr.2001.16.3.437. [DOI] [PubMed] [Google Scholar]
- 14.Flick LM, Weaver JM, Ulrich-Vinther M, Abuzzahab F, Zhang X, Dougall WC, Anderson D, O’Keefe RJ, Schwarz EM. Effects of receptor activator of NFkappaB (RANK) signaling blockade on fracture healing. J Orthop Res. 2003;21:676–84. doi: 10.1016/S0736-0266(03)00011-1. [DOI] [PubMed] [Google Scholar]
- 15.Frost HM. The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop Relat Res. 1989:294–309. [PubMed] [Google Scholar]
- 16.Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tato L, Viapiana O, Adami S. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res. 2005;20:758–63. doi: 10.1359/JBMR.041232. [DOI] [PubMed] [Google Scholar]
- 17.Giraud F, Meunier PJ. Effect of cyclical intravenous pamidronate therapy in children with osteogenesis imperfecta. Open-label study in seven patients. Joint Bone Spine. 2002;69:486–90. doi: 10.1016/s1297-319x(02)00434-7. [DOI] [PubMed] [Google Scholar]
- 18.Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–52. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 19.Goodship AE, Walker PC, McNally D, Chambers T, Green JR. Use of a bisphosphonate (pamidronate) to modulate fracture repair in ovine bone. Ann Oncol. 1994;5(Suppl 7):S53–5. [PubMed] [Google Scholar]
- 20.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. Jama. 2004;292:490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 21.Huang A, Raggio C, Fritton J, Camacho N. Comparison of radiographic and micro-CT determined parameters in mouse bone specimens. 51st Annual Meeting of the Orthopaedic Research Society. 2005 [Google Scholar]
- 22.Hyvonen PM, Karhi T, Kosma VM, Liimola-Luoma L, Hanhijarvi H. The influence of dichloromethylene bisphosphonate on the healing of a long bone fracture, composition of bone mineral and histology of bone in the rat. Pharmacol Toxicol. 1994;75:384–90. doi: 10.1111/j.1600-0773.1994.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 23.Jepsen KJ, Pennington DE, Lee YL, Warman M, Nadeau J. Bone brittleness varies with genetic background in A/J and C57BL/6J inbred mice. J Bone Miner Res. 2001;16:1854–62. doi: 10.1359/jbmr.2001.16.10.1854. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, Beneton MN, Gertz BJ, Sciberras DG, Holland SD, Orgee J, Coombes GM, Rogers SR, Porras AG. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res. 1997;12:1700–7. doi: 10.1359/jbmr.1997.12.10.1700. [DOI] [PubMed] [Google Scholar]
- 25.Koivukangas A, Tuukkanen J, Kippo K, Jamsa T, Hannuniemi R, Pasanen I, Vaananen K, Jalovaara P. Long-term administration of clodronate does not prevent fracture healing in rats. Clin Orthop Relat Res. 2003:268–78. doi: 10.1097/00003086-200303000-00036. [DOI] [PubMed] [Google Scholar]
- 26.Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, Gerstenfeld LC, Einhorn TA. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–14. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 27.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 28.Lee YS, Low SL, Lim LA, Loke KY. Cyclic pamidronate infusion improves bone mineralisation and reduces fracture incidence in osteogenesis imperfecta. Eur J Pediatr. 2001;160:641–4. doi: 10.1007/s004310100844. [DOI] [PubMed] [Google Scholar]
- 29.Lenehan TM, Balligand M, Nunamaker DM, Wood FE., Jr Effect of EHDP on fracture healing in dogs. J Orthop Res. 1985;3:499–507. doi: 10.1002/jor.1100030413. [DOI] [PubMed] [Google Scholar]
- 30.Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, Hill SC, Gerber LH, Marini JC. Controlled Trial of Pamidronate in Children With Types III and IV Osteogenesis Imperfecta Confirms Vertebral Gains but Not Short-Term Functional Improvement. J Bone Miner Res. 2005;20:977–86. doi: 10.1359/JBMR.050109. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Mori S, Li J, Kaji Y, Akiyama T, Kawanishi J, Norimatsu H. Long-term effect of incadronate disodium (YM-175) on fracture healing of femoral shaft in growing rats. J Bone Miner Res. 2001;16:429–36. doi: 10.1359/jbmr.2001.16.3.429. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Mori S, Kaji Y, Mashiba T, Kawanishi J, Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res. 1999;14:969–79. doi: 10.1359/jbmr.1999.14.6.969. [DOI] [PubMed] [Google Scholar]
- 33.Madsen JE, Berg-Larsen T, Kirkeby OJ, Falch JA, Nordsletten L. No adverse effects of clodronate on fracture healing in rats. Acta Orthop Scand. 1998;69:532–6. doi: 10.3109/17453679808997793. [DOI] [PubMed] [Google Scholar]
- 34.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004;18:687–95. doi: 10.1097/00005131-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy EA, Raggio CL, Hossack MD, Miller EA, Jain S, Boskey AL, Camacho NP. Alendronate treatment for infants with osteogenesis imperfecta: demonstration of efficacy in a mouse model. Pediatr Res. 2002;52:660–70. doi: 10.1203/00006450-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–74. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misof BM, Roschger P, Baldini T, Raggio CL, Zraick V, Root L, Boskey AL, Klaushofer K, Fratzl P, Camacho NP. Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse. Bone. 2005;36:150–8. doi: 10.1016/j.bone.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Montpetit K, Plotkin H, Rauch F, Bilodeau N, Cloutier S, Rabzel M, Glorieux FH. Rapid increase in grip force after start of pamidronate therapy in children and adolescents with severe osteogenesis imperfecta. Pediatrics. 2003;111:e601–3. doi: 10.1542/peds.111.5.e601. [DOI] [PubMed] [Google Scholar]
- 39.Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004;19:1779–86. doi: 10.1359/JBMR.040814. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Hara Y, Tagawa M, Tamura M, Yuge T, Fukuda H, Nigi H. Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. J Bone Miner Res. 1998;13:942–9. doi: 10.1359/jbmr.1998.13.6.942. [DOI] [PubMed] [Google Scholar]
- 41.Nyman MT, Gao T, Lindholm TC, Lindholm TS. Healing of a tibial double osteotomy is modified by clodronate administration. Arch Orthop Trauma Surg. 1996;115:111–4. doi: 10.1007/BF00573453. [DOI] [PubMed] [Google Scholar]
- 42.Nyman MT, Paavolainen P, Lindholm TS. Clodronate increases the calcium content in fracture callus. An experimental study in rats. Archives of Orthopaedic & Trauma Surgery. 1993;112:228–31. doi: 10.1007/BF00451880. [DOI] [PubMed] [Google Scholar]
- 43.Oyajobi BO, Anderson DM, Traianedes K, Williams PJ, Yoneda T, Mundy GR. Therapeutic efficacy of a soluble receptor activator of nuclear factor kappaB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 2001;61:2572–8. [PubMed] [Google Scholar]
- 44.Peter CP, Cook WO, Nunamaker DM, Provost MT, Seedor JG, Rodan GA. Effect of alendronate on fracture healing and bone remodeling in dogs. J Orthop Res. 1996;14:74–9. doi: 10.1002/jor.1100140113. [DOI] [PubMed] [Google Scholar]
- 45.Plotkin H, Rauch F, Bishop NJ, Montpetit K, Ruck-Gibis J, Travers R, Glorieux FH. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000;85:1846–50. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 46.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–85. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 47.Rauch F, Plotkin H, Travers R, Zeitlin L, Glorieux FH. Osteogenesis imperfecta types I, III, and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocrinol Metab. 2003;88:986–92. doi: 10.1210/jc.2002-021371. [DOI] [PubMed] [Google Scholar]
- 48.Rauch F, Plotkin H, Zeitlin L, Glorieux FH. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res. 2003;18:610–4. doi: 10.1359/jbmr.2003.18.4.610. [DOI] [PubMed] [Google Scholar]
- 49.Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110:1293–9. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowe DW, S J. In: Metabolic Bone Disease and Clinically Related Disorders. Avioli LV, Krane SM, editors. San Diefo: Academic Press; 1998. pp. 651–683. [Google Scholar]
- 51.Sakkers R, Kok D, Engelbert R, van Dongen A, Jansen M, Pruijs H, Verbout A, Schweitzer D, Uiterwaal C. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet. 2004;363:1427–31. doi: 10.1016/S0140-6736(04)16101-1. [DOI] [PubMed] [Google Scholar]
- 52.Shapiro JR, McCarthy EF, Rossiter K, Ernest K, Gelman R, Fedarko N, Santiago HT, Bober M. The effect of intravenous pamidronate on bone mineral density, bone histomorphometry, and parameters of bone turnover in adults with type IA osteogenesis imperfecta. Calcif Tissue Int. 2003;72:103–12. doi: 10.1007/s00223-001-1055-5. [DOI] [PubMed] [Google Scholar]
- 53.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 54.Smith BB, Cosenza ME, Mancini A, Dunstan C, Gregson R, Martin SW, Smith SY, Davis H. A toxicity profile of osteoprotegerin in the cynomolgus monkey. Int J Toxicol. 2003;22:403–12. doi: 10.1177/109158180302200512. [DOI] [PubMed] [Google Scholar]
- 55.Winquist RA, Hansen ST., Jr Comminuted fractures of the femoral shaft treated by intramedullary nailing. Orthop Clin North Am. 1980;11:633–48. [PubMed] [Google Scholar]
- 56.Yang KH, Park SJ. Stimulation of fracture healing in a canine ulna full-defect model by low-intensity pulsed ultrasound. Yonsei Med J. 2001;42:503–8. doi: 10.3349/ymj.2001.42.5.503. [DOI] [PubMed] [Google Scholar]
- 57.Yang X, M P G B, Riggo G, Diwan D, Camacho NP. The effect of alendronate on fracture healing of wild type and osteogenesis imperfecta mice. Transactions of the Forty-Seventh Annual Meeting of Orthopaedic Research Society. 2001 March; [Google Scholar]
- 58.Zacharin M, Bateman J. Pamidronate treatment of osteogenesis imperfecta--lack of correlation between clinical severity, age at onset of treatment, predicted collagen mutation and treatment response. J Pediatr Endocrinol Metab. 2002;15:163–74. doi: 10.1515/jpem.2002.15.2.163. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Dai J, Yao Z, Lu Y, Dougall W, Keller ET. Soluble receptor activator of nuclear factor kappaB Fc diminishes prostate cancer progression in bone. Cancer Res. 2003;63:7883–90. [PubMed] [Google Scholar]


