Abstract
New insights are emerging about the interactions between brain cells and carbon nanotubes, which could eventually lead to the development of nanoengineered neural devices.
Neurons are electrically excitable cells that transmit and process information in the nervous system. Recently, it has been shown that neurons continue to grow when placed on carbon nanotubes, and can still carry electrical signals when stimulated by the nanotubes1-6 (Fig. 1). As a result, several nanotube-based neural applications are being developed, such as neural prosthesis for restoring neurological sensory- and motor-functions, and tools for monitoring or modifying neural activity. However, little is known about the details of the interactions between the nanotubes and the neurons. On page 126 of this issue, Laura Ballerini from the University of Trieste and co-workers7 from Italy, Switzerland, Belgium and Ireland report that carbon nanotubes alter the electrophysiological responses of neurons by forming tight contacts with their membranes.
Figure 1.
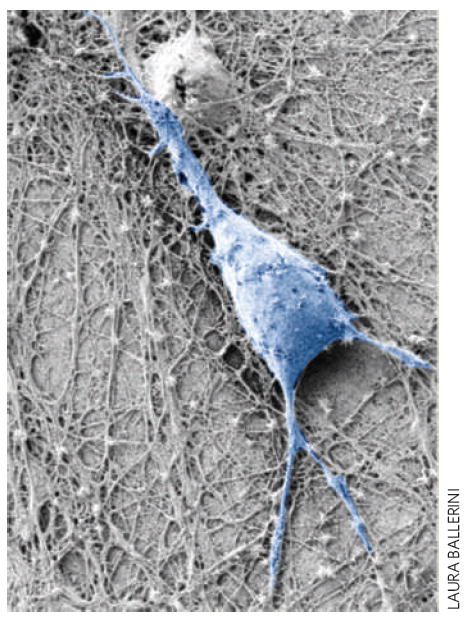
Scanning electron micrograph of a neuron (pseudo-coloured in blue) growing on a carbon nanotube layer. New experiments indicate that carbon nanotubes enhance the excitability of neurons by forming tight contacts with the cell membrane so that electrical activity is diverted through the nanotubes.
A neuron consists of a central part known as the soma or cell body, and long processes called axons and dendrites. In general, each neuron has multiple dendrites that carry signals into the soma, and a single axon that carries signals away from the soma towards the next cell. Driven by electrochemical gradients, the movement of ions across the ion channels found on the soma and the axon generate precisely timed electrical signals called action potentials, which normally travel along the axon in one direction — away from the soma and towards the next neuron.
However, in neurons with extensive dendrites near the soma, such as some neurons found in the hippocampus part of the brain, the action potentials can sometimes travel in the opposite direction. This ‘backpropagation’ from the soma induces calcium-mediated action potentials in the dendrites and this contributes to an after-depolarization event8,9 — an induced depolarization of the membrane potential immediately following an action potential that is larger than normal — at the soma. A decision to fire an action potential occurs when the inputs from the dendrites exceed the firing threshold. The relative contribution of the after-depolarization event to this decision to fire depends on the degree of coupling between the dendrites and soma, which, in turn, determines the strength of the after-depolarization effect.
Ballerini and co-workers7 addressed the effect of nanotubes on the electrical properties of isolated neurons by examining the presence of after-depolarization events in the cell soma. They injected a brief current pulse into the soma to force the neurons to fire a regular train of six action potentials and measured the presence of membrane depolarization in the soma at the end of the last action potential. Compared with control neurons grown on glass substrates, hippocampal neurons grown on carbon nanotube substrates showed significantly enhanced after-depolarization.
When the experiment was repeated with neurons grown on a conductive substrate such as tin oxide and a non-conductive substrate containing nanoscale features similar to carbon nanotubes, the neurons did not show significantly enhanced after-depolarization, demonstrating that this effect is specific to carbon nanotubes. Furthermore, as expected, the after-depolarization depended on the degree of dendritic branching: dorsal root ganglion neurons, which have minimal dendritic branching, never displayed after-depolarization at any spiking frequency when grown on the same carbon nanotube surfaces.
Modelling the membrane voltage input–output relationship between the soma and dendrites, the European team found that the nanotubes may be effectively short-circuiting the dendrites and soma — that is, diverting the electrical activity through the nanotubes — and this may be sufficient to account for the enhanced after-depolarization of neurons grown on nanotubes. Furthermore, in a coupled-network version of the single-neuron model, the enhanced after-depolarization effect could significantly prolong the spontaneously recurring action-potential burst events in the network. In effect, the nanotubes were able to modulate the physiology of the neurons. Transmission electron micrographs of neurons grown on nanotubes showed tight contacts between the nanotubes and the cell membrane, suggesting that specific changes in the electrical behaviour of the cell may be due to these intimate interactions.
This new work represents the ‘tip of the iceberg’ in that it hints at the potential of carbon nanotubes for interfacing with neurons, and eventually the nervous system. Although the observations and the electrotonic hypothesis are interesting, it remains unclear how this phenomena may contribute to a putative nano-engineered neural device: for example, calcium currents induced by backpropagation in dendrites of adult hippocampal neurons rarely result in calcium action-potentials, because the currents are too small9. It will take the degree of experimentation and modelling illustrated in this paper to answer these questions and engineer meaningful nanotube–neural devices.
Understanding the mechanisms that underlie the effect of carbon nanotubes on neural cells will be critical for designing functional neural devices based on empirical data and engineering principles, rather than qualitative trial-and-error approaches. The engineering challenges involved demand this, but the potential impacts of successfully interfacing carbon nanotube devices with neural cells will be well worth it.
References
- 1.Massobrio G, Massobrio P, Martinoia S. Nano Lett. 2008;8:4433–4440. doi: 10.1021/nl802341r. [DOI] [PubMed] [Google Scholar]
- 2.Malarkey EB, et al. Nano Lett. 2008;8:3538–3542. doi: 10.1021/nl8017912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keefer EW, et al. Nature Nanotech. 2008;3:434–439. doi: 10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Andrews RJ. Acta Neurochir Suppl. 2007;97:537–545. doi: 10.1007/978-3-211-33081-4_62. [DOI] [PubMed] [Google Scholar]
- 5.Mazzatenta A, et al. J Neurosci. 2007;27:6931–6936. doi: 10.1523/JNEUROSCI.1051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen-Vu TD, et al. Small. 2006;2:89–94. doi: 10.1002/smll.200500175. [DOI] [PubMed] [Google Scholar]
- 7.Cellot G, et al. Nature Nanotech. 2009;4:126–133. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- 8.Kepecs A, Lisman Net Comp Neural Sys. 2003;14:103–118. [PubMed] [Google Scholar]
- 9.Chen S, Yaari Y. J Physiol. 2008;586:1351–1363. doi: 10.1113/jphysiol.2007.148171. [DOI] [PMC free article] [PubMed] [Google Scholar]


