Abstract
Aromatic compounds are among the most prevalent and persistent pollutants in the environment. Petroleum-contaminated soil and sediment commonly contain a mixture of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatics. Aromatics derived from industrial activities often have functional groups such as alkyls, halogens and nitro groups. Biodegradation is a major mechanism of removal of organic pollutants from a contaminated site. This review focuses on bacterial degradation pathways of selected aromatic compounds. Catabolic pathways of naphthalene, fluorene, phenanthrene, fluoranthene, pyrene, and benzo[a]pyrene are described in detail. Bacterial catabolism of the heterocycles dibenzofuran, carbazole, dibenzothiophene, and dibenzodioxin is discussed. Bacterial catabolism of alkylated PAHs is summarized, followed by a brief discussion of proteomics and metabolomics as powerful tools for elucidation of biodegradation mechanisms.
Keywords: Bioremediation, biodegradation, PAHs, polycyclic aromatic hydrocarbons
1. Introduction
Biodegradation is a viable bioremediation technology for organic pollutants. It has long known that microorganisms degrade environmental pollutants in various matrices and environments. Bioremediation utilizes the metabolic versatility of microorganisms to degrade hazardous pollutants. A goal of bioremediation is to transform organic pollutants into harmless metabolites or mineralize the pollutants into carbon dioxide and water [1]. A feasible remedial technology requires microorganisms being capable of quick adaptation to and efficient uses of pollutants of interest in a particular case in a reasonable period of time. Many factors influence microorganisms to use pollutants as substrates or cometabolize them. Therefore, understanding catabolic pathways and mechanisms and responsible enzymes is an effective means to define important factors for efficient cleanup of pollutants. Research has been conducted to understand bioremediation for environmental pollutants such as aromatic compounds that are among the most prevalent and persistent environmental pollutants.
Biodegradation is a very broad field and involves uses of a wide range of microorganisms to break chemical bonds. It has been well reviewed [1, 2], however, it is a very active field and new data are rapidly contributed to the literature. This review is focused on bacterial catabolic pathways of selected aromatic pollutants under aerobic culture conditions (Table 1). The selected aromatic pollutants include the PAHs naphthalene, fluorene, phenanthrene, fluoranthene, pyrene, and benzo[a]pyrene, the heterocycles dibenzofuran, carbazole, dibenzothiophene, and dibenzodioxin, and alkylated PAHs. Metabolomics and proteomics in elucidation of mechanisms of microbial degradation of aromatics are also briefly discussed.
Table 1.
Isolated bacteria capable of degrading aromatic compounds (incomplete list).
| Bacterial species | Strains | Aromatics | References |
|---|---|---|---|
| Achromobacter sp. | NCW | CBZ | [106] |
| Alcaligenes denitrificans | FLA | [73] | |
| Arthrobacter sp. | F101 | FLE | [41] |
| Arthrobacter sp. | P1-1 | DBT, CBZ, PHE | [57, 108] |
| Arthrobacter sulphureus | RKJ4 | PHE | [56] |
| Acidovorax delafieldii | P4-1 | PHE | [56] |
| Bacillus cereus | P21 | PYR | [87] |
| Brevibacterium sp. | HL4 | PHE | [56] |
| Burkholderia sp. | S3702, RP007, 2A-12TNFYE-5, BS3770 | PHE | [32, 50, 143] |
| Burkholderia sp. | C3 | PHE | [57] |
| Burkholderia cepacia | BU-3 | NAP, PHE, PYR | [10] |
| Burkholderia cocovenenans | PHE | [59] | |
| Burkholderia xenovorans | LB400 | BZ, BP | [133] |
| Chryseobacterium sp. | NCY | CBZ | [106] |
| Cycloclasticus sp. | P1 | PYR | [88] |
| Janibacter sp. | YY-1 | DBF, FLE, DBT, PHE, ANT, DD | [116] |
| Marinobacter | NCE312 | NAP | [30] |
| Mycobacterium sp. | PYR, BaP | [4, 68, 75, 76, 83, 93, 144] | |
| Mycobacterium sp. | JS14 | FLA | [68] |
| Mycobacterium sp. | 6PY1, KR2, AP1 | PYR | [78, 85, 139] |
| Mycobacterium sp. | RJGII-135 | PYR,BaA, BaP | [79] |
| Mycobacterium sp. | PYR-1, LB501T | FLA, PYR, PHE, ANT | [49, 67, 70, 77, 87, 145] |
| Mycobacterium sp. | CH1, BG1, BB1, KR20 | PHE, FLE, FLA, PYR | [40, 51, 52, 69] |
| Mycobacterium flavescens | PYR, FLA | [65, 82] | |
| Mycobacterium vanbaalenii | PYR-1 | PHE, PYR, dMBaA | [61, 126] |
| Mycobacterium sp. | KMS | PYR | [84] |
| Nocardioides aromaticivorans | IC177 | CBZ | [110] |
| Pasteurella sp. | IFA | FLA | [146] |
| Polaromonas naphthalenivorans | CJ2 | NAP | [153] |
| Pseudomonas sp. | C18, PP2, DLC-P11 | NAP, PHE | [27, 55, 56] |
| Pseudomonas sp. | BT1d | HFBT | [119] |
| Pseudomonas sp. | B4 | BP, CBP | [136] |
| Pseudomonas sp. | HH69 | DBF | [104] |
| Pseudomonas sp. | CA10 | CBZ, CDD | [109] |
| Pseudomonas sp. | NCIB 9816-4 | FLE, DBF, DBT | [113] |
| Pseudomonas sp. | F274 | FLE | [47] |
| Pseudomonas paucimobilis | PHE | [73] | |
| Pseudomonas vesicularis | OUS82 | FLE | [73] |
| Pseudomonas putida | P16, BS3701, BS3750, BS590-P, BS202-P1 | NAP, PHE | [33, 50] |
| Pseudomonas putida | CSV86 | MNAP | [125] |
| Pseudomonas fluorescens | BS3760 | PHE, CHR, BaA | [50, 147] |
| Pseudomonas stutzeri | P15 | PYR | [87] |
| Pseudomonas saccharophilia | PYR | [87] | |
| Pseudomonas aeruginosa | PHE | [148] | |
| Ralstonia sp. | SBUG 290
U2 |
DBF
NAP |
[103]
[152] |
| Rhodanobacter sp. | BPC-1 | BaP | [149] |
| Rhodococcus sp. | PYR, FLA | [65, 86] | |
| Rhodococcus sp. | WU-K2R | NAT, BT | [121] |
| Rhodococcus erythropolis | I-19 | ADBT | [120] |
| Rhodococcus erythropolis | D-1 | DBT | [122] |
| Staphylococcus sp. | PN/Y | PHE | [63] |
| Stenotrophomonas maltophilia | VUN 10,010 | PYR, FLA, BaP | [64, 90] |
| Stenotrophomonas maltophilia | VUN 10,003 | PYR, FLA, BaA, BaP, DBA, COR | [66, 95] |
| Sphingomonas yanoikuyae | R1 | PYR | [87] |
| Sphingomonas yanoikuyae | JAR02 | BaP | [100] |
| Sphingomonas sp. | P2, LB126 | FLE, PHE, FLA, ANT | [54, 71, 72, 150] |
| Sphingomonas sp. | DBF, DBT, CBZ | [105] | |
| Sphingomonas paucimobilis | EPA505 | FLA, NAP, ANT, PHE | [36, 151] |
| Sphingomonas wittichii | RW1 | CDD | [112] |
| Terrabacter sp. | DBF63 | DBF, CDBF, CDD, FLE | [48, 109, 117] |
| Xanthamonas sp. | PYR, BaP, CBZ | [93] |
PYR, pyrene; BaP, Benzo[a]pyrene; PHE, phenanthrene; FLA, fluoranthene; FLE, fluorene; ANT, anthracene; NAP, naphthalene; BaA, benz[a]anthracene; dMBaA, dimethylbenz[a]anthracene; DBA, dibenz[a,h]anthracene; COR, coronene; CHR, chrysene; DBF, dibenzofuran; CDBF, chlorinated dibenzothophene; HFBT, 3-hydroxy-2-formylbenzothiophene; BP, biphenyl; CBP, chlorobiphenyl; NAT, naphthothiophene; BT, benzothiophene; BZ, benzoate; ADBT, alkylated dibenzothiophene; CBZ, carbazole; DD, dibenzo-p-dioxin; CDD, chlorinated dibenzo-p-dioxin; MNAP, methyl naphthalene.
2. Aromatic Compounds in the Environment
Aromatic compounds can be defined as organic molecules containing one or more aromatic rings, specifically benzene rings, for example. Different aromatic compounds co-exist as complex mixtures in petroleum refinery and distillation sites. There are three major categories: polycyclic aromatic hydrocarbons (PAHs), heterocyclics, and substituted aromatics. PAHs are a group of chemicals that contain two or more fused aromatic rings in linear, angular, or cluster arrangements [3, 4]. Physical and chemical properties of PAHs vary with the number of rings and hence their molecular weight. Chemical reactivity, aqueous solubility and volatility of PAHs decrease with increasing molecular weight. As a result, PAHs differ in their transport, distribution and fate in the environment and their effects on biological systems. The US EPA has identified 16 PAHs as priority pollutants. Some of these PAHs are considered to be possible or probable human carcinogens, and hence their distributions in the environment and possible exposure to humans have been of concerns [5]. High-molecular-weight PAHs are paid particular attention as they are recalcitrant [4]. In general, PAHs are relatively stable and recalcitrant in soils and less easy to degrade than many other organic compounds. They are difficult to remove from contaminated soil using the treatments that have been used successfully to clean soils contaminated with more degradable or volatile organic compounds such as alkanes [6]. Three major sources of PAHs are petrogenic, pyrogenic and biogenic. Petrogenic PAHs are from petroleum and petroleum-derived products, and are often marked as in abundance of alkyl-substituted PAHs such as alkyl naphthalenes, alkyl phenanthrenes and alkyl dibenzothiophenes. Pyrogenic PAHs are produced from combustion processes and are comprised of predominantly unsubstituted PAHs. Biogenic aromatic compounds including aromatic amino acids, lignin compounds and their derivatives are of biotransformation origin.
PAHs may accumulate in high concentrations in terrestrial environments near coal gasification sites and tar oil distillation plants [7]. Major sources of PAHs are incomplete combustion of organic materials, gas production, wood treatment facilities, and waste incineration [3, 8–10]. PAHs are formed naturally during thermal geologic reactions associated with fossil-fuel and mineral production, and during burning of vegetation in forest and bush fires [11, 12]. Anthropogenic sources, particularly fuel combustion, automobiles, spillage of petroleum products, and waste incinerators are significant sources of PAHs into the environment. Tobacco cigarette smoking is a significant source of PAH exposure to smokers and secondary smokers. PAHs generated during anthropogenic combustion activities are primarily transported via atmospheric deposition [13, 14]. Petroleum refining and transport activities are major contributors to localized loadings of PAHs into the environment. Such loadings may occur through discharge of industrial effluents and through accidental release of raw and refined products [9].
Heterocyclic compounds including dibenzothiophene and carbazole are components of creosote, crude oils, and shale oils and often co-exist in the environment with PAHs and other aromatic compounds [15]. Dibenzothiophene is a sulfur heterocyclic compound and is quite persistent in the environment. Little information about dibenzothiophene toxicity is available in the literature. Carbazole, a nitrogen heterocycle, is carcinogenic and toxic [16]. Dibenzofuran and its substituted analogues are found in several woody plants as stress chemicals, so called phytoalexins [17]. However, most of the environmental concerns with dibenzofuran are related to its halogenated analogues, especially its chloro/bromo derivatives.
Persistent organic pollutants (POPs) are among the most concerned environmental pollutants because they persist in the environment, bioaccumulate through the food web, and pose a risk of causing adverse effects to the environment and human health. POPs are also referred to as persistent, bioaccumulative and toxic chemicals (PBTs). POPs include aldrin, brominated flame retardants, chlordane, DDT, dieldrin, endrin, mirex, organometallic compounds such as tributyltin, PAHs, heptachlor, hexachlorobenzene, polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and toxaphene. PCDD/Fs are formed unintentionally from human activities. One of the main sources of PCDD/Fs is municipal waste incinerators [18]. PCDD/Fs formed are absorbed on the fly ash, and then enter into the environment. Therefore, the fly ash has been considered as a harmful waste causing environmental pollution. Yang et al. [19] suggested an efficient catalytic detoxification method for PCDD/Fs in fly ash.
Alkyl PAHs (e.g., methylnaphthalene) have increasingly become an environmental concern. Because alkyl substitution causes a substantial decrease of water solubility, alkyl PAHs tend to be bioaccumulative. They are abundant in fossil fuels, crude oil and petroleum derived products. Boylan and Tripp [20] identified a large number of aromatic hydrocarbons (e.g., alkyl-benzenes and naphthalenes) in seawater extracts of several crude oils and kerosene. Alexander et al. [21] suggested that methylnaphthalenes may be useful indicators of thermal maturity of sedimentary organic matter. Methylnaphthalenes in a petroleum oil fraction can be used as active ingredient of repellent to control mosquitoes [22]. Alkylbenz[a]anthracene, especially 7,12-dimethylbenz[a]anthracene, is a potent carcinogen in rodent skin and mammal cells [23].
3. Aerobic Bacterial Catabolic Pathways for Selected PAHs
3.1. Naphthalene
Naphthalene has often been used as a model compound to investigate the ability of bacteria to degrade PAHs because it is the simplest and the most soluble PAH [24]. Therefore, information of bacterial degradation of naphthalene has been used to understand and predict pathways in the degradation of three- or more ring PAHs. Many bacteria that have been isolated and utilize naphthalene as a sole source of carbon and energy belong to the genera Alcaligenes, Burkholderia, Mycobacterium, Polaromonas, Pseudomonas, Ralstonia, Rhodococcus, Sphingomonas, and Streptomyces (Table 1) [3, 10, 25–36, 152, 153].
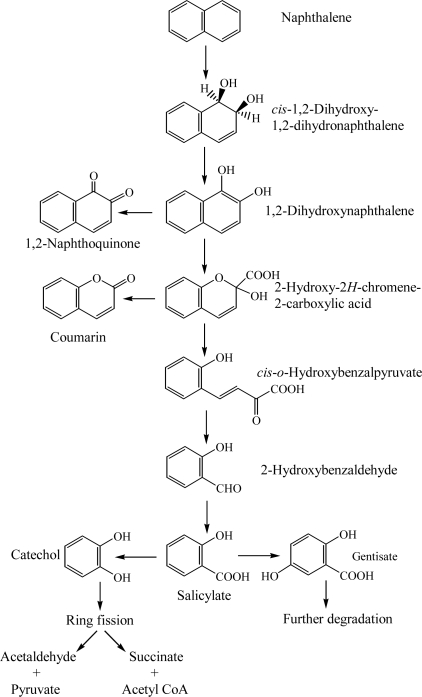
Degradation of naphthalene starts through the multicomponent enzyme, naphthalene dioxygenase, attack on the aromatic ring to form cis-(1R, 2S)-dihydroxy-1,2-dihydronaphthalene (cis-naphthalene dihydrodiol) (Scheme 1) [24, 37]. The cis-naphthalene dihydrodiol formed by naphthalene dioxygenase is subsequently dehydrogenated to 1,2-dihydroxynaphthalene by a cis-dihydrodiol dehydrogenase [24, 25]. Subsequently, 1,2-dihydroxynaphthalene is metabolized to salicylate via 2-hydroxy-2H-chromene-2-carboxylic acid, cis-o-hydroxybenzalpyruvate, and 2-hydroxy-benzaldehyde (Scheme 1) [24, 26, 27, 33]. Also, 1,2-dihydroxynaphthalene is nonenzymatically oxidized to 1,2-naphthaquinone [25]. Salicylate is typically decarboxylated to catechol, which is further metabolized by ring fission in meta- and ortho-pathways. Fuenmayor et al. [29] reported that salicylate is converted to gentisate by salicylate-5-hydroxylase. Recently, Jouanneau et al. [31] purified the salicylate 1-hydroxylase from Sphingomonas sp. strain CHY-1 and characterized its biochemical and catalytic properties.
Scheme 1.
Proposed catabolic pathways of naphthalene by bacteria [24–27, 33].
The bacterial degradation of naphthalene has been well characterized for the catabolic enzyme system encoded by the plasmid NAH7 in Pseudomonas putida G7 [27, 37, 38]. NAH7 has two operons that contain the structural genes for naphthalene degradation. One operon contains the gene for the upper catabolic pathway encoding the enzymes necessary for the conversion of naphthalene to salicylate. The second operon contains the gene for the lower catabolic pathway encoding the enzymes necessary for the metabolism of salicylate through the catechol meta-cleavage pathway to pyruvate and acetaldehyde [24, 27, 37, 38]. The entire sequence structure of plasmid NAH7 (82,232 bp) was determined by Sota et al. [154]. Also, the complete 83,042 bp sequence of the circular naphthalene degradation plasmid pDTG1 from Pseudomonas putida NCIB 9816–4 was determined by Dennis and Zylstra [155]. Parales et al. [39] reported that aspartate 205 in the catalytic domain of naphthalene dioxygenase is a necessary residue in the major pathways of electron transfer to mononuclear iron at the active site.
3.2. Fluorene
Fluorene having three rings is a major constituent of fossil fuels and coal derivatives. Several bacteria able to use fluorene as their sole source of carbon and energy have been isolated and are in the genera of Arthrobacter, Brevibacterium, Burkholderia, Mycobacterium, Pseudomonas and Sphingomonas (Table 1) [26, 40–45]. Three major catabolic pathways are shown in Scheme 2. The initial 1,2-dioxygenation of fluorene forms fluorene-1,2-diol that is further transformed to 3-chromanone via 2-hydroxy-4-(2-oxo-indan-1-ylidene)-2-butenoic acid, 1-formyl-2-indanone, 2-indanone-1-carboxylic acid, and 2-indanone [41, 46]. The second pathway begins at an initial 3,4-dioxygenation of fluorene leading to salicylate formation through 2-hydroxy-4-(1-oxo-indan-2-ylidene)-2-butenoic acid, 2-formyl-1-indanone, 1-indanone-2-carboxylic acid, 1-indanone, 2-chromanone, and 3-(2-hydroxy-phenyl)-propionic acid [41, 43]. The third pathway starts from C-9 monooxygenation in Brevibacterium sp. DPO1361 and Pseudomonas sp. F274. This pathway is only productive if a subsequent angular carbon dioxygenation occurs, leading to the formation of phthalate that is further transformed to protocatechuate (Scheme 2) [40, 42, 44, 45, 47]. Several genes involved in the degradation of fluorene to phthalate were characterized in Terrabacter sp. DBF63 by Habe et al. [48].
Scheme 2.
Proposed catabolic pathways of fluorene by bacteria [26, 40, 41, 43, 45, 46].
Compound designations: 1, fluorene; 2, fluorene-1,2-diol; 3, 2-hydroxy-4-(2-oxo-indan-1-ylidene)-2-butenoic acid; 4, 1-formyl- 2-indanone; 5, 2-indanone-1-carboxylic acid; 6, 2-indanone; 7, 3-chromanone; 8, 9-fluorenol; 9, 9-fluorenone; 10, 3,4-dihydroxy-9-fluorenone; 11, 4-hydroxy-9-fluorenone; 12, 2’,3’-dihydroxy-biphenyl-2-carboxylic acid; 13, 8-hydroxy-3,4-benzocoumarin; 14, phthalate; 15, protocatechuic acid; 16, 4-carboxy-2-hydroxymuconate- 6-semialdehyde; 17, 2-pyrone-4,6-dicarboxylic acid; 18, 4-carboxy-2-hydroxymuconic acid; 19, fluorene-3,4-diol; 20, 2-hydroxy-4-(1-oxo-indan-2-ylidene)-2-butenoic acid; 21, 2-formyl-1-indanone; 22, 1-indanone-2-carboxylic acid; 23, 1-indanone; 24, 3-hydroxy- 1-indanone; 25, 2-chromanone; 26, 3-(2-hydroxy-phenyl)-propionic acid; 27, salicylic acid.
3.3. Phenanthrene
Phenanthrene, a three aromatic ring system, is found in high concentrations in PAH-contaminated sediments, surface soils, and waste sites [49]. Bacterial degradation of phenanthrene has been extensively studied. A variety of bacterial strains in Acidovorax, Arthrobacter, Brevibacterium, Burkholderia, Comamonas, Mycobacterium, Pseudomonas, and Sphingomonas have been isolated and have the ability to utilize phenanthrene as a sole carbon and energy source (Table 1) [10, 26, 32, 34, 36, 40, 49–59].
Phenanthrene contains bay- and K-regions able to form an epoxide, which is suspected to be an ultimate carcinogen [56, 60]. Therefore, it is used as a model substrate for studies on the catabolism of bay- and K-region containing carcinogenic PAHs such as benzo[a]pyrene, benzo[a]anthracene, and chrysene [56]. In general, bacterial degradation of phenanthrene is initiated by 3,4-dioxygenation to yield cis-3,4-dihydroxy-3,4-dihydrophenanthrene, which undergoes enzymatic dehydrogenation to 3,4-dihydroxyphenanthrene (Scheme 3) [57, 58, 61]. The diol is subsequently catabolized to naphthalene-1,2-diol through both ortho-cleavage to form 2-(2-carboxy-vinyl)-naphthalene-1-carboxylic acid and meta-cleavage to form 4-(1-hydroxy-naphthalen-2-yl)-2-oxo-but-3-enoic acid [57].
Scheme 3.
Proposed catabolic pathways of phenanthrene by bacteria [24, 49, 55, 57, 58, 63, 139, 150].
Compound designations: 1, phenanthrene; 2, cis-1,2-dihydroxy-1,2-dihydrophenanthrene; 3, 1,2- dihydroxyphenanthrene; 4, 3-hydroxy-3H-benzo[f]chromene-3-carboxylic acid; 5, 4-(2-hydroxy-naphthalen-1-yl)-2-oxo-but-3-enoic acid; 6, 2-hydroxy-naphthalene-1-carbaldehyde; 7, 2-hydroxy-1-naphthoic acid; 8, 5,6-benzocoumarin; 9, cis-9,10-dihydroxy-9,10-dihydrophenanthrene; 10, 9,10-dihydroxyphenanthrene; 11, 2,2’-diphenic acid; 12, cis-3,4-dihydroxy-3,4-dihydrophenanthrene; 13, 3,4-dihydroxyphenanthrene; 14, 2-hydroxy-2H-benzo[h]chromene-2-carboxylic acid; 15, 4-(1-hydroxynaphthalen-2-yl)-2-oxo-but-3-enoic acid; 16, 1-hydroxy-naphthalene-2-carbaldehyde; 17, 1-hydroxy-2-naphthoic acid; 18, 7,8-benzocoumarin; 19, 1-(2-carboxy-vinyl)-naphthalene-2-carboxylic acid; 20, 2-(2-carboxy-vinyl)-naphthalene-1-carboxylic acid; 21, naphthalene-1,2-dicarboxylic acid; 22, naphthalene-1,2-diol; 23, 2-hydroxybenzalpyruvic acid; 24, salicylic aldehyde; 25, salicylic acid; 26, gentisic acid; 27, coumarin; 28, 2-carboxycinnamic acid; 29, 2-formylbenzoic acid; 30, phthalic acid; 31, 3,4-dihydroxyphthalic acid; 32, protocatechuic acid; 33, trans-2,3-dioxo-5-(2’-hydroxyphenyl)-pent-4-enoic acid.
Recently, Seo et al. [57] elucidated that the ortho-cleavage product, 2-(2-carboxy-vinyl)-naphthalene-1-carboxylic acid, is degraded to naphthalene-1,2-diol through naphthalene-1,2-dicarboxylic acid and 1-hydroxy-2-naphthoic acid. Pagnout et al. [62] isolated and characterized a gene cluster involved in phenanthrene degradation by 3,4-phenanthrene dioxygenation and meta-cleavage. It is possible that 1,2-dioxygenation of phenanthrene forms cis-1,2-dihydroxy-1,2-dihydrophenanthrene, which undergoes enzymatic dehydrogenation to 1,2-dihydroxyphenanthrene (Scheme 3). This diol is also subsequently catabolized to naphthalene-1,2-diol through both ortho- and meta-cleavages. In general, phenanthrene-1,2- and 3,4-diols mainly undergo meta-cleavage due to the rapid accumulation of 5,6- and 7,8-benzocoumarin [57]. Naphthalene-1,2-diol converged from 1-hydroxy-2-naphthoic acid and 2-hydroxy-1-naphthoic acid is further degraded in a phthalic acid pathway through ortho-cleavage and a salicylic acid pathway through meta-cleavage [55, 57, 58]. Mallick et al. [63] reported that a novel meta-cleavage of 2-hydroxy-1-naphthoic acid to form trans-2,3-dioxo-5-(2’-hydroxyphenyl)-pent-4-enoic acid in Staphylococcus sp. PN/Y. Interestingly, phenanthrene degradation starts from 9,10-dioxygenase to yield phenanthrene cis-9,10-dihydrodiol that is further catabolized to 2,2’-diphenic acid via 9,10-dihydroxyphenanthrene [49, 57, 61].
3.4. Fluoranthene
Fluoranthene, a four-ring PAH, is one of the principal PAHs in the environment. Bacterial transformation of fluoranthene has been reported (Table 1) [40, 51, 64–73]. Mycobacterium has been extensively studied and is a well-known genus to mineralize high molecular weight PAHs such as fluoranthene, pyrene, and benzo[a]pyrene [4, 70, 74–79]. Also, strains in the genera Burkholderia, Pasteurella, Rhodococcus, Sphingomonas, and Stenotrophomonas have been isolated to degrade fluoranthene, using it as a sole carbon and energy sources [64, 66, 71, 72, 80, 81].
Bacterial degradation of fluoranthene is generally initiated by 1,2- or 7,8-dioxygenation to form cis-1,2- or cis-7,8-fluoranthene dihydrodiol, respectively (Scheme 4). These two dihydrodiols are dehydrogenated to 1,2- or 7,8-dihydroxyfluoranthene, respectively. 7,8-Dihydroxy-fluoranthene is further transformed via meta-cleavage to 1-acenaphthenone and 3-hydroxymethyl-3H-benzo[de]-chromen-2-one through 2-hydroxyl-4-(2-oxo-2H-acenaphthylen-1-ylidene)-but-2-enoic acid, 2-hydroxylmethyl-2H-acenaphthylen-1-one, and 2-oxo-acenaphthene-1-carboxylic acid. Kelley et al. [67] reported that the dehydrogenation of a transient cis-7,8-fluoranthene dihydrodiol to form 7,8-dihydroxyfluoranthene with O-methylation at the 7-position would form 7-methoxy-8-hydroxy-fluoranthene. However, Lee et al. [68] suggested that there are four possible initial dioxygenation at 1,2-, 2,3-, 7,8-, and 8,9-positions of fluoranthene and four possible dimethoxyfluoranthenes in Mycobacterium sp. JS14. 1,2-Dihydroxy-fluoranthene is also further degraded by meta-cleavage to 9-fluorenone through 9-fluorenone-1-(carboxy-2-hydroxy-1-propenol) and 9-fluorenone-1-carboxylic acid which can be converted by protonation to 9-fluorenol-1-carboxy-3-propenyl-2-one and 9-fluorenol-1-carboxylic acid, respectively. The conversion of 9-fluorenone to 9-fluorenol is also possible.
Scheme 4.
Proposed catabolic pathways of fluoranthene by bacteria [3, 67–70, 85, 147].
Compound designations: 1, fluoranthene; 2, cis-7,8-fluoranthene dihydrodiol; 3, 7,8-dihydroxy-fluoranthene; 4, 7,8-dimethoxyfluoranthene; 5, 2-hydroxy-4-(2-oxo-2H-acenaphthylen-1-ylidene)-but-2-enoic acid; 6, 2-oxo-acenaphthene-1-carboxylic acid; 7, 2-hydroxylmethyl-2H-acenaphthylen-1-one; 8, 1-acenaphthenone; 9, 3-hydroxymethyl-3H-benzo[de] chromen-2-one; 10, 2-hydroxymethyl-acenaphthylen-1-ol; 11, cis-1,2-fluoranthene dihydrodiol; 12, 1,2-dihydroxy-fluoranthene; 13, 1,2-dimethoxyfluoranthene; 14, 9-fluorenone-1-(carboxy-2-hydroxy-1-propenol); 15, 9-fluorenol-1-carboxy-3-propenyl-2-one; 16, 9-fluorenone-1-carboxylic acid; 17, 9-fluorenol-1-carboxylic acid; 18, 9-fluorenone; 19, 9-fluorenol; 20, 2’,3’-dihydroxybiphenyl-2,3-dicarboxylic acid; 21, benzene-1,2,3-tricarboxylic acid; 22, cis-8,9-fluoranthene dihydrodiol; 23, 8,9-dihydroxy-fluoranthene; 24, 8,9-dimethoxyfluoranthene; 25, naphthalene-1,8-dicarboxylic acid; 26, cis-2,3-fluoranthene dihydrodiol; 27, 2,3-dihydroxy-fluoranthene; 28, 2,3-dimethoxyfluoranthene; 29, 9-carboxymethylene-9H-fluorene-1-carboxylic acid; 30, cis-1,9a-dihydroxy-1-hydrofluorene-9-one-8-carboxylic acid; 31, 4-hydroxybenzochromene-6-one-7-carboxylic acid.
Rehmann et al. [69] reported 2,3-dioxygenation of fluoranthene and found five metabolites, namely cis-2,3-fluoranthene dihydrodiol, 9-carboxymethylene-9H-fluorene-1-carboxylic acid, cis-1,9a-dihydroxy-1-hydrofluorene-9-one-8-carboxylic acid, 4-hydroxybenzochromene-6-one-7-carboxylic acid, and benzene-1,2,3-tricarboxylic acid. cis-2,3-Fluoranthene dihydrodiol is catabolized by ortho-cleavage to benzene-1,2,3-tricarboxylic acid via 9-carboxymethylene-9H-fluorene-1-carboxylic acid, cis-1,9a-dihydroxy-1-hydrofluorene-9-one-8-carboxylic acid, and 4-hydroxybenzo-chromene-6-one-7-carboxylic acid. Recently, Lee et al. [68] proposed 8,9-dioxygenation of fluoranthene from the detection of 8,9-dimethoxyfluoranthene. Also, they detected 25 proteins related to fluoranthene catabolism in Mycobacterium sp. JS14 using 1-D SDS PAGE, 2-D SDS PAGE, and nano-LC/MS/MS.
3.5. Pyrene
Pyrene possessing four benzene rings is a byproduct of gasification processes and other incomplete combustion processes. Many bacterial isolates capable of degrading pyrene have been studied (Table 1). Mycobacterium as Gram-positive species has been most widely studied for degrading pyrene by using it as a sole carbon and energy source [4, 34, 35, 51, 61, 75, 78, 79, 83–85]. Mycobacterium spp. are known to have high cell surface hydrophobicity and adhere to the emulsified solvent droplets [83]. Other pyrene degrading strains isolated include Rhodococcus sp. [86], Bacillus cereus [87], Burkholderia cepacia [80], Cycloclasticus sp. P1 [88], Pseudomonas fluorescens [64], Pseudomonas stutzeri [87], Sphingomonas sp. VKM B-2434 [26], Sphingomonas paucimobilis [89], and Stenotrophomonas maltophilia [64, 66, 90].
Heitkamp et al. [76] found the three products of ring oxidation, pyrene-cis-4,5-dihydrodiol, pyrene-trans-4,5-dihydrodiol, and pyrenol, and four products of ring fission (Scheme 5), 4-hydroxyperinaphthenone, 4-phenanthroic acid, phthalic acid, and cinnamic acid by multiple analyses, including UV, infrared, mass spectrometry, NMR, and GC. The formation of pyrene-cis-4,5-dihydrodiol by dioxygenase and pyrene-trans-4,5-dihydrodiol by monooxygenase suggested multiple initial oxidative attacks on pyrene. Pyrene-1,2-diol derived from the dioxygenation at pyrene 1,2-C positions is metabolized to 4-hydroxyperinaphthenone via cis-2-hydroxy-3-(perinaphthenone-9-yl)-propenic acid and 2-hydroxy-2H-1-oxa-pyrene-2-carboxylic acid (Scheme 5). Kim and Freeman [61] found 1,2-dimethoxypyrene as a subproduct of pyrene-1,2-diol. Pyrene-4,5-diol is degraded by ortho-cleavage to phenanthrene-4,5-dicarboxylic acid, which is further metabolized through phenanthrene-3,4-diol pathway and 6,6’-dihydroxy-2,2’-biphenyl-dicarboxylic acid pathway. Meta-cleavage of pyrene-4,5-diol lead to 5-hydroxy-5H-4-oxa-pyrene-5-carboxylic acid via 2-hydroxy-2-(phenanthrene-5-one-4-enyl)-acetic acid (Scheme 5). A novel metabolite, 6,6’-dihydroxy-2,2’-biphenyl-dicarboxylic acid, was identified by Vila et al. [85] from the degradation of pyrene by Mycobacterium sp. strain AP1. Liang et al. [91] reported pyrene-4,5-dione formation and identified almost all the enzymes required during the initial steps of pyrene degradation in Mycobacterium sp. KMS. Kim et al. [92] identified 27 enzymes necessary for constructing a complete pathway for pyrene degradation from both genomic and proteomic data.
Scheme 5.
Proposed catabolic pathways of pyrene by bacteria [3, 9, 61, 76, 78, 82, 85, 86, 91, 139].
Compound designations: 1, pyrene; 2, pyrene-cis-1,2-dihydrodiol; 3, pyrene-1,2-diol; 4, 2-hydroxy-3-(perinaphthenone-9-yl)-propenic acid; 5, 2-hydroxy-2H-1-oxa-pyrene-2-carboxylic acid; 6, 4-hydroxyperinaphthenone; 7, 1,2-dimethoxypyrene; 8, pyrene-trans-4,5-dihydrodiol; 9, pyrene-cis-4,5-dihydrodiol; 10, pyrene-4,5-diol; 11, phenanthrene-4,5-dicarboxylic acid; 12, 4-carboxyphenanthrene-5-ol; 13, 4-carboxy-5-hydroxy-phenanthrene-9,10-dihydrodiol; 14, 4-carboxyphenanthrene-5,9,10-triol; 15, 2,6,6’-tricarboxy-2’-hydroxybiphenyl; 16, 2,2’-dicarboxy-6,6’-dihydroxybiphenyl; 17, phthalic acid; 18, 4-phenantroic acid; 19, 3,4-dihydroxy-3,4-dihydro-phenanthrene-4-carboxylic acid; 20, phenanthrene-3,4-diol; 21, 4-phenanthroic acid methyl ester; 22, 4-hydroxyphenanthrene; 23, 7,8-benzocoumarin; 24, 2-hydroxy-2-(phenanthrene-5-one-4-enyl)-acetic acid; 25, 5-hydroxy-5H-4-oxa-pyrene-5-carboxylic acid; 26, pyrene-4,5-dione; 27, 4-oxa-pyrene-5-one.
3.6. Benzo[a]pyrene
Benzo[a]pyrene, a five aromatic ring PAH, is one of the most potent carcinogenic PAHs. Benzo[a]pyrene has low water solubility (0.0023 mg/L) and a high octanol/water partition coefficient (LogKow, 6.06), which is related to high recalcitrance to microbial degradation. It also has relatively low abundance in environmental samples which limits its catabolism by bacterial assemblages. The concentration of benzo[a]pyrene as well as other PAHs in contaminated soils in industrial sites can vary depending on the industrial activities associated with the site. To date, there is only limited information regarding the bacterial degradation of PAHs with five or more rings. There are several bacterial species capable of benzo[a]pyrene degradation including Mycobacterium sp. [74, 79, 93], Sphingomonas paucimobilis [94], and Stenotrophomonas maltophilia [95] (Table 1). Juhasz et al. [66] found that S. maltophilia VUN 10,003 can degrade benzo[a]pyrene by 22% for 14 days incubation. A S. paucimobilis EPA 505 grown on fluoranthene degraded 33% of benzo[a]pyrene [94].
Gibson et al. [96] first identified cis-7,8 and 9,10-benzo[a]pyrene-dihydrodiol formed in Beijerinckia sp. strain B1, except ring cleavage metabolites (Scheme 6). Schneider et al. [79] reported that Mycobacterium sp. strain RJGII-135 is capable of transforming benzo[a]pyrene to initial ring oxidation and ring cleavage metabolites. They found one ring oxidation metabolite, benzo[a]pyrene cis-7,8-dihydrodiol, and three ring cleavage metabolites, 4,5-chrysene-dicarboxylic acid, cis-4-(8-hydroxypyrene-7-yl)-2-oxobut-3-enoic acid or cis-4-(7-hydroxypyrene-8-yl)-2-oxobut-3-enoic acid, and 7,8-dihydro-pyrene-7-carboxylic acid or 7,8-dihydro-pyrene-8-carboxylic acid (Scheme 6). These metabolites indicate that the initial enzymatic attack occurs at the 4,5-, 7,8-, and 9,10-positions of benzo[a]pyrene.
Scheme 6.
Proposed catabolic pathways of benzo[a]pyrene by bacteria [79, 99, 100].
Compound designations: 1, benzo[a]pyrene; 2, benzo[a]pyrene-11,12-epoxide; 3, benzo[a]pyrene trans-11,12-dihydrodiol; 4, benzo[a]pyrene cis-11,12-dihydrodiol; 5, 11,12-dihydroxybenzo[a]pyrene; 6, hydroxymethoxybenzo[a]pyrene; 7, dimethoxybenzo[a]pyrene; 8, benzo[a]pyrene cis-4,5-dihydrodiol; 9, 4,5-dihydroxybenzo[a]pyrene; 10, 4-formylchrysene-5-carboxylic acid; 11, 4,5-chrysene-dicarboxylic acid; 12, chrysene-4 or 5-carboxylic acid; 13, benzo[a]pyrene cis-7,8-dihydrodiol; 14, 7,8-dihydroxybenzo[a]pyrene; 15, cis-4-(7-hydroxypyrene-8-yl)-2-oxobut-3-enoic acid; 16, pyrene-7-hydroxy-8-carboxylic acid; 17, 7,8-dihydro-pyrene-8-carboxylic acid; 18, benzo[a]pyrene cis-9,10-dihydrodiol; 19, 9,10-dihydroxybenzo[a]pyrene; 20, cis-4-(8-hydroxypyrene-7-yl)-2-oxobut-3-enoic acid; 21, pyrene-8-hydroxy-7-carboxylic acid; 22, 7,8-dihydro-pyrene-7-carboxylic acid; 23, 10-oxabenzo[def]chrysene-9-one.
The genes encoding the α-subunit of the PAH-ring hydroxylating dioxygenases involved in the initial step of metabolism of PAH in bacteria were recently quantified and characterized by Cebron et al. [97] and Lozada et al. [98]. Benzo[a]pyrene cis-7,8-dihydrodiol is further catabolized via meta-cleavage to 7,8-dihydro-pyrene-8-carboxylic acid through cis-4-(7-hydroxypyrene-8-yl)-2-oxobut-3-enoic acid. Benzo[a]pyrene cis-9,10-dihydrodiol is degraded via meta-cleavage to 7,8-dihydro-pyrene-7-carboxylic acid through cis-4-(8-hydroxypyrene-7-yl)-2-oxobut-3-enoic acid. In addition, 10-oxabenzo[def]-chrysene-9-one formed by dehydration of benzo[a]pyrene cis-9,10-dihydrodiol to give cis-4-(8-hydroxypyrene-7-yl)-2-oxobut-3-enoic acid with subsequent meta-cleavage and aromatic ring closure [99]. 4,5-Chrysene-dicarboxylic acid is transformed from 4-formylchrysene-5-carboxylic acid formed by the ortho-cleavage of 4,5-dihydroxy benzo[a]pyrene. Moody et al. [99] reported new degradation pathways of benzo[a]pyrene in Mycobacterium vanbaalehii PYR-1. The new pathways are initiated by dioxygenase and monooxygenase at the 11,12-positions of benzo[a]pyrene molecule to form benzo[a]pyrene cis-11,12-dihydrodiol and benzo[a]pyrene trans-11,12-dihydrodiol, respectively. Benzo[a]pyrene cis-11,12-dihydrodiol is further catabolized to dimethoxybenzo[a]pyrene via hydroxymethoxybenzo[a]pyrene. The formation of benzo[a]pyrene trans-11,12-dihydrodiol is due to cytochrome P-450 acting to form benzo[a]pyrene 11,12-epoxide with subsequent hydrolysis by epoxide hydrolase. Recently, Rentz et al. [100] identified two novel ring cleavage metabolites, pyrene-8-hydroxy-7-carboxylic acid and pyrene-7-hydroxy-8-carboxylic acid, formed by Sphingomonas yanoikuyae JAR02.
4. Aerobic Bacterial Catabolism of Aromatic Heterocycles
Various types of heterocycles containing oxygen, nitrogen, and sulfur are found in the environment, originating from anthropogenic or natural sources. Dibenzofurans, dibenzodioxins, and dibenzothiophene are among the most important environmental pollutants and are well reviewed [2, 101, 102]. Therefore, only aerobic bacterial degradation of non-halogenated heterocyclic aromatics is briefly discussed in this review to make a general relation with PAH degradation.
Many bacterial species have been reported to decompose dibenzofurans and carbazole, a structural analogue with nitrogen instead of oxygen [103–108], although some white rot fungi are well known to decompose halogenated dibenzofurans. Guo et al. [106] isolated a stable carbazole-degrading microbial consortium consisting of Chryseobacterium sp. NCY and Achromobacter sp. NCW. As for the degradation of unsubstituted PAHs, bacterial catabolism of dibenzofurans starts at insertion of two oxygen atoms catalyzed by dioxygenases. Although many bacterial PAH dioxygenases are evolutionarily related to phenylpropionate dioxygenase, striking diversities have been reported in catalytic activity, mechanisms, regulations, and substrates of these important enzymes. In short, initial reactions of dibenzofuran and carbazole can be classified into angular and lateral dioxygenation, which may be catalyzed by different enzymes (Scheme 7). These enzymes were found in Gram-positive and negative bacteria [109–112]. Some bacterial dioxygenases from Pseudomonas sp. CA10 and Sphingomonas wittichii RW1, for example, catalyze predominantly angular insertion of oxygen [109, 112] while the commonly known naphthalene dioxygenase from Pseudomonas sp. NCIB 9816-4 catalyzes only lateral dioxygenation [113]. According to the metabolite analyses accompanied by biochemical studies, angular and lateral dioxygenases were considered from different origins.
Scheme 7.
Simplified bacterial catabolic pathways of dibenzofuran (X= O) and carbazole (X = S).
However, recent studies with cloned dioxygenase from Norcardioides aromaticivorans IC177 revealed that some dioxygenase can catalyze both reactions [110]. It is well known that PAH dioxygenases can catalyze various reactions, including reduction, mono- and di-oxygenation [113]. In addition to the multiple reactions with specific dioxygenases, current genomic or proteomic research with several PAH degrading bacteria (e.g., Burkholderia spp. and Mycobacterium spp.) revealed that multiple dioxygenases, probably playing different roles in PAH degradation, exist in a single bacterium [91, 114, 115]. For example, metabolite profiling of culture supernatant of Janibacter sp. YY1 treated with dibenzofuran showed that it may have both angular and lateral dioxygenases [116]. Multiple catabolic pathways can produce various metabolites with structural diversities, which probably are metabolized by different types of enzymes. For example, Nojiri et al. [107] reported that Terrabacter sp. DBF63 can metabolize fluorene and dibenzofuran by the same dioxygenase but the metabolites, namely phthalate and salicyclate from fluorene and dibenzofuran, respectively, are further catabolized by different enzymes, of which the related genes are located in different areas of chromosomes.
Dioxygenases involving in dibenzo-p-dioxins degradation catalyze predominantly an angular dioxygenation to produce trihydroxy diphenyl ethers (Scheme 8). PCDDs are well known pollutants with extreme toxicity, especially tetra to hexachloro analogues. Some bacterial species can catabolize up to hexachlorodibenzo-p-dioxins [112, 117]. In comparison with dibenzofuran catabolism, chlorophthalates or salicylates and chlorophenols are common metabolites of PCDDs [112]. Chlorophenols are well known to be toxic to various organisms, including their degrading bacteria. Toxic effects of chlorometabolites are also reported from the catabolism of PCBs [118]. Although it is not clear how the bacteria can cope with the toxicity of chlorophenols during dioxin metabolism, glutathione or other sulfur-containing primary metabolites may be involved in its detoxification [118].
Scheme 8.
Bacterial catabolic pathways of dibenzo-p-dioxin.
Dibenzothiophene and its higher molecular weight analogues are commonly found in higher molecular weight fractions of petroleum, but are not pyrogenic and biogenic PAH components. Although the chemical profiles of sulfur heterocycles are well documented in petroleum products, studies of their biodegradation in petroleum contaminated natural environments are very limited. Because of the economic importance of desulfurization of unprocessed petroleum, detailed research was performed with various bacterial species [119–121]. Catabolism of dibenzothiophene is catalyzed by distinct enzymes in two pathways (Scheme 9). The catabolic branch of initial sulfur oxidation is also called 4S pathway, through which rapid desulfurization can be obtained. Flavin-containing monooxygenases, noted as DszA and DszB (or SoxA and SoxB), are widely distributed in bacteria and catalyze a consecutive addition of single oxygen atom. Consecutive desulfurization is achieved by desulfinase. These monooxygenases require FAD as a cofactor, accompanied by a specific flavin reductase [122]. FAD containing monooxygenases are very common in all biota and catalyze various detoxification steps. For example, Sutherland et al. [123] reported a FAD containing monooxygenase of high sequence homology with dibenzothiophene desulfurization enzymes. In general, bacterial species with high-desulfurization activities have all of the enzymes and broad substrate range [120, 121]. However, some bacterial species are defective in some components, which results in accumulation of intermediates. In comparison with desulfurization pathway, the enzymes for lateral dioxygenation and consecutive reactions are very different (Kodama pathway, Scheme 9). Many bacterial species have been reported to metabolize dibenzothiphene through Kodama pathway [105, 108, 113]. Although no detailed research has been done, the structural similarities of the metabolites suggest that common PAH dioxygenase and enzymes in successive steps may be involved in Kodama pathway. According to Resnick and Gibson [113], naphthalene dioxygenase from Pseudomonas sp. NCIB 9816-4 can produce dibenzothiophene dihydrodiol, a lateral ring dioxygenation product, which supports the broad action of PAH dioxygenase on sulfur heterocycles.
Scheme 9.
Bacterial catabolic pathways of dibenzothiophene.
Among the nitrogen heterocycles, catabolism of carbazole is well documented. However, other heterocycles, including benzoquinolines and phenanthridine are not well studied. In comparison with the other heterocycles, nitrogen heterocycles are well known metal chelators and can easily deactivate various metalloenzymes. Seo et al. [108] reported carbazole catabolism in Arthrobacter sp. P1-1. Catabolism of benzoquinoline in Mycobacterium gilvum LB307T suggests that at least some bacteria can overcome the toxic effect of nitrogen heterocycles [124].
5. Bacterial Catabolism of Alky PAHs
Alkyl- and nitro-PAHs are among common substituted PAHs and have substantial toxicities. Substitution on aromatic rings produces several problems in their degradation. The ability of PAH dioxygenase to remove the substitutions is currently the subject of debate. An alkyl branch is not a good leaving group and probably requires additional steps to be removed. Their presence may inhibit proper orientation and accessibility of the PAHs into dioxygenases.
In general, methyl-/ethyl-naphthalenes and phenanthrenes are prevalent contaminants in the environment and, however, limited numbers of studies have been done in relation to bacterial degradation [28, 125, 126]. Catabolism of alkyl PAHs in aerobic bacteria suggests a very diversity of enzymes involved [125]. These include oxidation of methyl group to alcohol, aldehyde, or carboxylic acid, decarboxylation, demethylation, and dioxygenation. However, production of alkyl salicylate or alkylphthalate suggests that the reaction may prefer non-substituted PAH systems. Until recently, research with anaerobic bacteria has been limited to halogenated pollutants such as PCBs, PBDEs, PCDDs/Fs and halogenated solvents [127, 128]. However, PAHs and their alkyl derivatives can be transformed by various anaerobes through novel catabolic pathways (Scheme 10) [129–131, 156]. In comparison with aerobic bacteria initiating dioxygenation, anaerobic bacteria usually insert carboxyl groups from carbon dioxide or succinic acids. Part of the ring is reduced to tetralin type metabolites [130]. The presence of ring cleavage products (e.g., derivatives of cyclohexane) suggests that oxygen molecule or oxygen equivalents may be required in these consecutive catabolic steps (Scheme 10). Metabolism of alkylbenzenes, alkanes, and other hydrocarbons by anaerobic bacteria was well reviewed by Spormann and Widdel [157].
Scheme 10.
Catabolism of naphthalene, methylnaphthalene, and tetralin by anaerobic bacteria [129–131].
Relatively speaking, studies with nitro-PAHs are much more limited [2, 132]. It is noteworthy that a large amount of nitro-PAHs (e.g., 1-nitropyrene) are produced from automobile exhaustion. It is well known that nitropyrene can be transformed into mutagenic metabolites, including amino-, nitroso-, and hydroxyamin-pyrene. Rafii et al. [132] reported that 1-nitropyrene can be transformed into mutagenic 1-aminopyrene by Mycobacterium sp. High genotoxic effects of various nitro-PAHs call for more detailed research on their biodegradation.
6. Protoemics and Metabolomics in Understanding of Bacterial Degradation of PAHs
Proteomics and metabolomics have been recently employed in studies of environmental microbiology and have shown their high impact on the field of biodegradation and bioremediation [158, 159]. Proteomics is an effective technique to identify proteins and their functions involved in the biodegradation of aromatics while metabolomics can be used to profile degradation products of PAHs and primary metabolites in response to PAH exposures. Intent of a brief discussion of proteomics and metabolomics here is to bring attention to these emerging fields rather than offering a comprehensive review and a long list of references.
A good number of genomic sequences or expressed sequence tags (ESTs) of bacteria are currently available. These include several PAH degrading bacteria in the genus of Mycobacterium, Acinetobacter, Arthrobacter, and Burkholderia. Detailed transcriptomes in Burkholderia xenovorans LB400 during PCB catabolism were analyzed by microarray techniques [114, 133–135]. The results revealed that large changes at genomic and proteomic levels occurred during the PAH catabolism, compared with the catabolism of natural carbon sources. These include up-regulation of the genes related to a) PAH catabolism, b) removal of reactive oxygen species, c) primary carbon metabolism, including one-carbon metabolism, d) energy production, e.g., ATP synthesis, e) various transporters, and f) synthesis of energy reservoir, e.g., polyphosphate. Many of these phenomena have been demonstrated by specific studies. For example, polyphosphate accumulation during PCB catabolism was confirmed by electron microscopy [136]. The accumulation of polyphosphate and polyphosphate kinase (Ppk) under stressed conditions are common in all biota. Up-regulation of superoxide dismutase or catalase/peroxidase is also common phenomena during PAHs or toxic chemical catabolism. The activation of one-carbon metabolism is another common phenomenon in bacteria during xenobiotic metabolism [137]. The exact mechanism of the gene activation is not clear. The up-regulation of one-carbon metabolism may be induced by several factors, including limitation of primary metabolite pools, introduction of one carbon unit, e.g., formate in PAH metabolism, and the loss of concerted regulation of general metabolism. Another common phenomenon is the changes of fatty acid content or composition in bacteria [138]. However, transcriptome analysis usually did not show a significant change in fatty acid metabolism-related genes. Discrepancies between genomic and proteomic data also occur. In general, proteomic analyses in relation to aromatics catabolism are very limited. However, a few proteomic studies of PAH catabolism in Mycobacterium spp. have been reported [61, 68, 91, 115, 139]. For example, Lee et al. [68] studied fluoranthene catabolism and associated proteins in Mycobacterium sp. JS14. An increased expression of 25 proteins related to fluoranthene catabolism is found with 1D-PAGE and nano liquid chromatography tandem MS/MS. Detection of fluoranthene catabolism associated proteins coincides well with its multiple degradation pathways that are mapped via metabolites identified. Kim et al. [115] reported the protein profiles of Mycobacterium vanbaalenii PYR-1 grown in the presence of pyrene, pyrene-4,5-quinoline, phenanthrene, anthracene, and fluoranthene after two dimensional electrophoresis (2DE) separation of proteins and mass spectrometry analysis. They found PAH-induced proteins (catalase-peroxidase, putative monooxygenase, dioxygenase small subunit, small subunit of naphthalene-inducible dioxygenase, and aldehyde dehydrogenase), carbohydrate metabolism related proteins (enolase, 6-phosphogluconate dehydrogenase, indole-3-glycerol phosphate synthase, and fumarase), DNA translation proteins, heat shock proteins, and energy production protein (ATP synthase). Proteomics studies showed that several Mycobacterium species have multiple dioxygenases and related enzymes, which may be involved in different substrates [61, 68, 91]. Those studies also revealed that many of stress related proteins, including enzymes related to reactive oxygen removal and transcriptional regulators are commonly up-regulated during PAH metabolism.
The workflow of proteomics has been extended from qualitative analyses after 1DE or 2DE to quantitative studies. Systematic quantitation of proteomes has become common by using methods such as isotope-coded affinity tag (ICAT), isobaric tags for relative and absolute quantitation (iTRAQ), and stable isotope labeling by essential amino acid culture (SILAC). The labeled proteins or peptides by those methods are analyzed by tandem MS [140, 141]. Recently, Kim et al. [142] conducted the proteome analysis of Pseudomonas putida KT2440 induced with monocyclic aromatic compounds using 2-DE/MS and cleavable isotope-coded affinity tag (ICAT) to determine the changes of proteins involved in aromatic degradation pathways. After translation, attachment of functional groups such as carbohydrates, lipids, phosphates or sulphur to a protein is referred to as post-translational modification (PTM). PTM extends protein functions and makes proteomes further complicated. It is noteworthy that in tandem mass spectrometry, a combination of collision induced dissociation (CID) and electron transfer dissociation (ETD) is becoming very useful in PTM elucidation. ETD cleaves randomly the peptide backbone while modifications are left intact. CID produces fragment ions for structural determination as well as sensitive and specific detection of a particular molecule.
Among the various system-wide evaluation of bacterial PAH degradation, almost no research has been reported in relation to metabolomics. Keum et al. [159] studied comparative metabolic responses of Sinorhizobium sp. C4 during degradation of phenanthrene. Comprehensive profiling of metabolites profiles, including polar metabolites, fatty acids and polyhydroxyalkanoates was performed through untargeted metabolome analyses. Large metabolome differences of strain C4 were observed between the cultures with phenanthrene and natural carbon sources. The changes include TCA cycle, pyruvate metabolism, cofactor biosynthesis, fatty acid compositions, and polyhydroxyalkanoate biosynthesis, which indicate metabolic adaptation in response to using phenanthrene as a substrate. Although the analyses of transcriptomes and proteomes can provide significant information indicating metabolic networks, a large number of metabolites interact with each other and thus metabolomes can show much more complex networks than transcriptomes and proteomes. As proteomics is advancing rapidly in environmental microbiology, metabolomics will also become a powerful tool. In conjunction with functional genomics, proteomics and metabolomics are becoming indispensable in elucidation of mechanisms of biodegradation and biotransformation of organic pollutants in the environment, particularly for the situations of multiple chemicals and microbial consortia.
Acknowledgments
This review was supported in part by US EPA award no. 989512-01-1 and USDA-TSTAR grants (34135–9576, 34135–11295, and 34135–12724), and NRL award N00173–05–2–C003.
References and Notes
- 1.Alexander M. Biodegradation and Bioremediation. 2nd Ed. Academic Press: San Diego, USA; 1999. pp. 325–327. [Google Scholar]
- 2.Klein J. Biotechnology. Environmental Processes II – Soil Decontamination. 2nd Ed. volume 11b. Wiley-VCH: Weinhein; 2000. [Google Scholar]
- 3.Cerniglia CE. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 4.Cheung PY, Kinkle BK. Mycobacterium diversity and pyrene mineralization in petroleum-contaminated soils. Appl. Environ. Microbiol. 2001;67:2222–2229. doi: 10.1128/AEM.67.5.2222-2229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzie CA, Potochi BB, Santodonato J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992;26:1278–1284. [Google Scholar]
- 6.Pitter P, Chudoba J. Biodegradation of Organic Substances in the Aquatic Environment. CRC Press: Boca Raton, FL; 1990. [Google Scholar]
- 7.Capotori G, Digianvincenzo P, Cesti P, Bernardi A, Guglielmetti G. Pyrene and benzo[a]pyrene metabolism by an Aspergillus terreus strain isolated from a polycyclic aromatic hydrocarbons polluted soil. Biodegradation. 2004;15:79–85. doi: 10.1023/b:biod.0000015612.10481.e6. [DOI] [PubMed] [Google Scholar]
- 8.Ahn YH, Sanseverino J, Sayler GS. Analyses of polycyclic aromatic hydrocarbon-degrading bacteria isolated from contaminated soils. Biodegradation. 1999;10:149–157. doi: 10.1023/a:1008369905161. [DOI] [PubMed] [Google Scholar]
- 9.Kanaly RA, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J. Bacteriol. 2000;182:2059–2067. doi: 10.1128/jb.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TJ, Lee EY, Kim YJ, Cho KS, Ryu HW. Degradation of polyaromatic hydrocarbons by Burkholderia cepacia 2A–12. World J. Microbiol. Biotechnol. 2003;19:411–417. [Google Scholar]
- 11.Juhasz AL, Naidu R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: a review of the microbial degradation of benzo[a]pyrene. Int. Biodeter. Biodegr. 2000;45:57–88. [Google Scholar]
- 12.Wilson SC, Jones KC. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): A review. Environ. Pollut. 1993;81:229–249. doi: 10.1016/0269-7491(93)90206-4. [DOI] [PubMed] [Google Scholar]
- 13.Freeman DJ, Cattell DWS. Wood burning as a source of atmospheric polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 1990;24:1581–1585. [Google Scholar]
- 14.Nishioka M, Chang HC, Lee ML. Structural characteristics of polycyclic aromatic hydrocarbons in coal tars and combustion products. Environ. Sci. Technol. 1986;20:1023–1027. doi: 10.1021/es00152a010. [DOI] [PubMed] [Google Scholar]
- 15.Max Nestler FH. Characterization of wood-preserving coal-tar creosote by gas-liquid chromatography. Anal. Chem. 1974;46:46–53. [Google Scholar]
- 16.Tsuda H, Hagiwara A, Shibata M, Ito N. Carcinogenic effect of carbazole in the liver of a (C57BL/6NxC3H/HeN) F1 mice. J. Nat. Cancer Ins. 1982;69:1389–1393. [PubMed] [Google Scholar]
- 17.Gottstein D, Gross D. Phytoalexins from woody plants. Trees. 1992;6:55–68. [Google Scholar]
- 18.Olie K, Vermeulen PL, Hutzinger O. Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some municipal incinerators in the Netherlands. Chemosphere. 1977;6:455–459. [Google Scholar]
- 19.Yang Z, Xia C, Zhang Q, Chen J, Liang X. Catalytic detoxification of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans in fly ash. Waste Manage. 2007;27:588–592. doi: 10.1016/j.wasman.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Boylan DB, Tripp BW. Determination of hydrocarbons in sea water extracts of crude oil and crude oil fractions. Nature. 1971;230:44–47. doi: 10.1038/230044a0. [DOI] [PubMed] [Google Scholar]
- 21.Alexander R, Kagi RI, Rowland SJ, Sheppard PN, Chirila TV. The effect of thermal maturity on distribution of dimethylnaphthalenes and trimethylnaphthalenes in some ancient sediments and petroleums. Geochim. Cosmochim. Acta. 1985;49:385–395. [Google Scholar]
- 22.Wirtz RA, Turrentine JD, Fox RC. Area repellents for mosquitoes (Diptera: culicidae): identification of the active ingredients in a petroleum oil fraction. J. Med. Entomol. 1981;18:126–128. doi: 10.1093/jmedent/18.2.126. [DOI] [PubMed] [Google Scholar]
- 23.DiGiovanni J. Multi stage carcinogenesis in mouse skin. Pharmacol. Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 24.Goyal AK, Zylstra GJ. Genetics of naphthalene and phenanthrene degradation by Comamonas testosteroni. J. Ind. Microbiol. Biotechnol. 1997;19:401–407. doi: 10.1038/sj.jim.2900476. [DOI] [PubMed] [Google Scholar]
- 25.Auger RL, Jacobson AM, Domach MM. Effect of nonionic surfactant addition on bacterial metabolism of naphthalene: Assessment of toxicity and overflow metabolism potential. J. Hazard Mater. 1995;43:263–272. [Google Scholar]
- 26.Baboshin M, Akimov V, Baskunov B, Born TL, Khan SU, Golovleva L. Conversion of polycyclic aromatic hydrocarbons by Sphingomonas sp. VKM B-2434. Biodegradation. 2008;19:567–576. doi: 10.1007/s10532-007-9162-2. [DOI] [PubMed] [Google Scholar]
- 27.Denome SA, Stanley DC, Olson ES, Young KD. Metabolism of dibenzothiophene and naphthalene in Pseudomonas Strains: Complete DNA sequence of an upper naphthalene catabolic pathway. J. Bacteriol. 1993;175:6890–6901. doi: 10.1128/jb.175.21.6890-6901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta TK, Selifonov SA, Gunsalus IC. Oxidation of methyl-substituted naphthalenes: Pathways in a versatile Sphingomonas paucimobilis strain. Appl. Environ. Microbiol. 1998;64:1884–1889. doi: 10.1128/aem.64.5.1884-1889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuenmayor SL, Wild M, Boyes AL, Williams PA. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. Strain U2. J. Bacteriol. 1998;180:2522–2530. doi: 10.1128/jb.180.9.2522-2530.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedlund BP, Geiselbrecht AD, Staley JT. Marinobacter strain NCE312 has a Pseudomonas-like naphthalene dioxygenase. FEMS Microbiol. Lett. 2001;201:47–51. doi: 10.1111/j.1574-6968.2001.tb10731.x. [DOI] [PubMed] [Google Scholar]
- 31.Jouanneau Y, Micoud J, Meyer C. Purification and characterization of a three-component salicylate 1-hydroxylase from Sphingomonas sp. Strain CHY-1. Appl. Environ. Microbiol. 2007;73:7515–7521. doi: 10.1128/AEM.01519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang H, Hwang SY, Kim YM, Kim E, Kim YS, Kim SK. Degradation of phenanthrene and naphthalene by a Burkholderia species strain. Can. J. Microbiol. 2003;49:139–144. doi: 10.1139/w03-009. [DOI] [PubMed] [Google Scholar]
- 33.Kiyohara H, Torigoe S, Kaida N, Asaki T, Iida T, Hayashi H, Takizawa N. Cloning and characterization of a chromosomal gene cluster, pah, that encodes the upper pathway for phenanthrene and naphthalene utilization by Pseudomonas putida OUS82. J. Bacteriol. 1994;176:2439–2443. doi: 10.1128/jb.176.8.2439-2443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo JS.Bacterial proteomes and metabolism of aromatic compounds. Ph.D. dissertation, University of Hawaii at Manoa: Honolulu, Hawaii, USA, 2006.
- 35.Seo JS, Keum YS, Harada RM, Li QX. Isolation and characterization of bacteria capable of degrading polycyclic aromatic hydrocarbons (PAHs) and organophosphorus pesticides from PAHs-contaminated soil in Hilo, Hawaii. J. Agric. Food. Chem. 2007;55:5383–5389. doi: 10.1021/jf0637630. [DOI] [PubMed] [Google Scholar]
- 36.Story SP, Parker SH, Hayasaka SS, Riley MB, Kline EL. Convergent and divergent points in catabolic pathways involved in utilization of fluoranthene, naphthalene, anthracene, and phenanthrene by Sphingomonas paucimobilis var. EPA505. J. Ind. Microbiol. Biotechnol. 2001;26:369–382. doi: 10.1038/sj.jim.7000149. [DOI] [PubMed] [Google Scholar]
- 37.Simon MJ, Osslund TD, Saunders R, Ensley BD, Suggs S, Harcourt A, Suen WC, Cruder DL, Gibson DT, Zylstra GJ. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816–4. Gene. 1993;127:31–37. doi: 10.1016/0378-1119(93)90613-8. [DOI] [PubMed] [Google Scholar]
- 38.Goyal AK, Zylstra GJ. Molecular cloning of novel genes for polycyclic aromatic hydrocarbon degradation from Comamonas testosteroni GZ39. Appl. Environ. Microbiol. 1996;62:230–236. doi: 10.1128/aem.62.1.230-236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parales RE, Parales JV, Gibson DT. Aspartate 205 in the catalytic domain of naphthalene dioxygenase is essential for activity. J. Bacteriol. 1999;181:1831–1837. doi: 10.1128/jb.181.6.1831-1837.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boldrin B, Tiehm A, Fritzsche C. Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl. Environ. Microbiol. 1993;59:1927–1930. doi: 10.1128/aem.59.6.1927-1930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casellas M, Grifoll M, Bayona JM, Solanas AM. New metabolites in the degradation of fluorene by Arthrobacter sp. strain F101. Appl. Environ. Microbiol. 1997;63:819–826. doi: 10.1128/aem.63.3.819-826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grifoll M, Selifonov SA, Chapman PJ. Transformation of substituted fluorenes and fluorene analogs by Pseudomonas sp. strain F274. Appl. Environ. Microbiol. 1995;61:3490–3493. doi: 10.1128/aem.61.9.3490-3493.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grifoll M, Selifonov SA, Gatlin CV, Chapman PJ. Actions of a versatile fluorene-degrading bacterial isolate on polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 1995;61:3711–3723. doi: 10.1128/aem.61.10.3711-3723.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trenz SP, Engesser KH, Fischer P, Knackmuss HJ. Degradation of fluorene by Brevibacterium sp. strain DPO 1361: a novel C-C bond cleavage mechanism via 1, 10-dihydro-1, 10-dihydroxyfluorene-9-one. J. Bacteriol. 1994;176:789–795. doi: 10.1128/jb.176.3.789-795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wattiau P, Bastiaens L, vanHerwijnen R, Daal L, Parsons JR, Renard ME, Springael D, Cornelis GR. Fluorene degradation by Sphingomonas sp. LB126 proceeds through protocatechuic acid: a genetic analysis. Res. Microbiol. 2001;152:861–872. doi: 10.1016/s0923-2508(01)01269-4. [DOI] [PubMed] [Google Scholar]
- 46.Monna L, Omori T, Kodama T. Microbial degradation of dibenzofuran, fluorene, and dibenzo-p-dioxin by Staphylococcus auriculans DBF63. Appl. Environ. Microbiol. 1993;59:285–289. doi: 10.1128/aem.59.1.285-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grifoll M, Selifonov SA, Chapman PJ. Evidence for a novel pathway in the degradation of fluorine by Pseudomonas sp. strain F274. Appl. Environ. Microbiol. 1994;60:2438–2449. doi: 10.1128/aem.60.7.2438-2449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habe H, Chung JS, Kato H, Ayabe Y, Kasuga K, Yoshida T, Nojiri H, Yamane H, Omori T. Characterization of the upper pathway genes for fluorene metabolism in Terrabacter sp. strain DBF63. J. Bacteriol. 2004;186:5938–5944. doi: 10.1128/JB.186.17.5938-5944.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moody JD, Freeman JP, Doerge DR, Cerniglia CE. Degradation of phenanthrene and anthracene by cell suspensions of Mycobacterium sp. Strain PYR-1. Appl. Environ. Microbiol. 2001;67:1476–1483. doi: 10.1128/AEM.67.4.1476-1483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balashova NV, Kosheleva IA, Golovchenko NP, Boronin AM. Phenanthrene metabolism by Pseudomonas and Burkholderia strains. Process Biochem. 1999;35:291–296. [Google Scholar]
- 51.Churchill SA, Harper JP, Churchill PF. Isolation and characterization of a Mycobacterium species capable of degrading three- and four-ring aromatic and aliphatic hydrocarbons. Appl. Environ. Microbiol. 1999;65:549–552. doi: 10.1128/aem.65.2.549-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerin WF, Jones GE. Mineralization of phenanthrene by a Mycobacterium sp. Appl. Environ. Microbiol. 1988;54:937–944. doi: 10.1128/aem.54.4.937-944.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moser R, Stahl U. Insights into the genetic diversity of initial dioxygenases from PAH-degrading bacteria. Appl. Microbiol. Biotechnol. 2001;55:609–618. doi: 10.1007/s002530000489. [DOI] [PubMed] [Google Scholar]
- 54.Pinyakong O, Habe H, Yoshida T, Nojiri H, Omori T. Identification of three novel salicylate 1-hydroxylases involved in the phenanthrene degradation of Sphingomonas sp. strain P2. Biochem. Biophys. Res. Commun. 2003;301:350–357. doi: 10.1016/s0006-291x(02)03036-x. [DOI] [PubMed] [Google Scholar]
- 55.Prabhu Y, Phale PS. Biodegradation of phenanthrene by Pseudomonas sp. strain PP2: novel metabolic pathway, role of biosurfactant and cell surface hydrophobicity in hydrocarbon assimilation. Appl. Microbiol. Biotechnol. 2003;61:342–351. doi: 10.1007/s00253-002-1218-y. [DOI] [PubMed] [Google Scholar]
- 56.Samanta SK, Chakraborti AK, Jain RK. Degradation of phenanthrene by different bacteria: evidence for novel transformation sequences involving the formation of 1-naphthol. Appl. Microbiol. Biotechnol. 1999;53:98–107. doi: 10.1007/s002530051621. [DOI] [PubMed] [Google Scholar]
- 57.Seo JS, Keum YS, Hu Y, Lee SE, Li QX. Phenanthrene degradation in Arthrobacter sp. P1-1: Initial 1,2-, 3,4- and 9,10-dioxygenation, and meta- and ortho-cleavages of naphthalene-1,2-diol after its formation from naphthalene-1,2-dicarboxylic acid and hydroxyl naphthoic acids. Chemosphere. 2006;65:2388–2394. doi: 10.1016/j.chemosphere.2006.04.067. [DOI] [PubMed] [Google Scholar]
- 58.Seo JS, Keum YS, Hu Y, Lee SE, Li QX. Degradation of phenanthrene by Burkholderia sp. C3: Initial 1,2- and 3,4-dioxygenation and meta- and ortho-cleavages of naphthalene-1,2-diol. Biodegradation. 2007;18:123–131. doi: 10.1007/s10532-006-9048-8. [DOI] [PubMed] [Google Scholar]
- 59.Wong JWC, Lai KM, Wan CK, Ma KK, Fang M. Isolation and optimization of PAH-degradative bacteria from contaminated for PAHs bioremediation. Water Air Soil Poll. 2002;139:1–13. [Google Scholar]
- 60.Samanta SK, Singh OV, Jain RK. Polycyclic aromatic hydrocarbons: environmental pollution and bioremediation. Trends Biotechnol. 2002;20:243–248. doi: 10.1016/s0167-7799(02)01943-1. [DOI] [PubMed] [Google Scholar]
- 61.Kim YH, Freeman JP. Effects of pH on the degradation of phenanthrene and pyrene by Mycobacterium vanbaalenii PYR-1. Appl. Microbiol. Biotechnol. 2005;67:275–285. doi: 10.1007/s00253-004-1796-y. [DOI] [PubMed] [Google Scholar]
- 62.Pagnout C, Frache G, Poupin P, Maunit B, Muller JF, Ferard JF. Isolation and characterization of a gene cluster in PAH degradation in Mycobacterium sp. strain SNP11: Expression in Mycobacterium smegmatis mc2155. Res. Microbiol. 2007;158:175–186. doi: 10.1016/j.resmic.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Mallick S, Chatterjee S, Dutta TK. A novel degradation pathway in the assimilation of phenanthrene by Staphylococcus sp. strain PN/Y via meta-cleavage of 2-hydroxy-1-naphthoic acid: formation of trans-2,3-dioxo-5-(2’-hydroxyphenyl)-pent-4-enoic acid. Microbiology. 2007;153:2104–2115. doi: 10.1099/mic.0.2006/004218-0. [DOI] [PubMed] [Google Scholar]
- 64.Boonchan S, Britz ML, Stanley GA. Surfactant-enhanced biodegradation of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia. Biotechnol. Bioeng. 1998;59:482–494. doi: 10.1002/(sici)1097-0290(19980820)59:4<482::aid-bit11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 65.Dean-Ross D, Moody J, Cerniglia CE. Utilization of mixtures of polycyclic aromatic hydrocarbons by bacteria isolated from contaminated sediment. FEMS Microbiol. Ecol. 2002;41:1–7. doi: 10.1111/j.1574-6941.2002.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 66.Juhasz AL, Stanley GA, Britz ML. Microbial degradation and detoxification of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia strain VUN 10,003. Lett. Appl. Microbiol. 2000;30:396–401. doi: 10.1046/j.1472-765x.2000.00733.x. [DOI] [PubMed] [Google Scholar]
- 67.Kelley I, Freeman JP, Evans FE, Cerniglia CE. Identification of metabolites from the degradation of fluoranthene by Mycobacterium sp. Strain PYR-1. Appl. Environ. Microbiol. 1993;59:800–806. doi: 10.1128/aem.59.3.800-806.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SE, Seo JS, Keum YS, Lee KJ, Li QX. Fluoranthene metabolism and associated proteins in Mycobacterium sp. JS14. Proteomics. 2007;7:2059–2069. doi: 10.1002/pmic.200600489. [DOI] [PubMed] [Google Scholar]
- 69.Rehmann K, Hertkorn N, Kettrup AA. Fluoranthene metabolism in Mycobacterium sp. Strain KR20: Identity of pathway intermediates during degradation and growth. Microbiology. 2001;147:2783–2794. doi: 10.1099/00221287-147-10-2783. [DOI] [PubMed] [Google Scholar]
- 70.Sepic E, Bricelj M, Leskovsek H. Degradation of fluoranthene by Pasteurella sp. IFA and Mycobacterium sp. PYR-1: Isolation and identification of metabolites. J. Appl. Microbiol. 1998;85:746–754. doi: 10.1111/j.1365-2672.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- 71.van Herwijnen R, Sande BF, Wielen FEM, Springael D, Govers HAJ, Parsons JR. Influence of phenanthrene and fluoranthene on the degradation of fluorene and glucose by Sphingomonas sp. strain LB126 in chemostat cultures. FEMS Microbiol. Ecol. 2003;46:105–111. doi: 10.1016/S0168-6496(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 72.van Herwijnen R, Wattiau P, Bastiaens L, Daal L, Jonker L, Springael D. Elucidation of the metabolic pathway of fluorene and cometabolic pathways of phenanthrene, fluoranthene, anthracene and dibenzothiophene by Sphingomonas sp. LB126. Res. Microbiol. 2003;154:199–206. doi: 10.1016/S0923-2508(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 73.Weissenfels WD, Beyer M, Klein J. Degradation of phenanthrene, fluorene, and fluoranthene by pure bacterial cultures. Appl. Microbiol. Biotechnol. 1990;32:479–484. doi: 10.1007/BF00903787. [DOI] [PubMed] [Google Scholar]
- 74.Heitkamp MA, Cerniglia CE. Polycyclic aromatic hydrocarbon degradation by a Mycobacterium sp. in microcosm containing sediment and water from a pristine ecosystem. Appl. Environ. Microbiol. 1989;55:1968–1973. doi: 10.1128/aem.55.8.1968-1973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heitkamp MA, Franklin W, Cerniglia CE. Microbial metabolism of polycyclic aromatic hydrocarbons: Isolation and characterization of a pyrene-degrading bacterium. Appl. Environ. Microbiol. 1988;54:2549–2555. doi: 10.1128/aem.54.10.2549-2555.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heitkamp MA, Freeman JP, Miller DW, Cerniglia CE. Pyrene degradation by a Mycobacterium sp.: Identification of ring oxidation and ring fission products. Appl. Environ. Microbiol. 1988;54:2556–2565. doi: 10.1128/aem.54.10.2556-2565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramirez N, Cutright T, Ju LK. Pyrene biodegradation in aqueous solutions and soil slurries by Mycobacterium PYR-1 and enriched consortium. Chemosphere. 2001;44:1079–1086. doi: 10.1016/s0045-6535(00)00475-6. [DOI] [PubMed] [Google Scholar]
- 78.Rehmann K, Noll HP, Steinberg CEW, Kettrup AA. Pyrene degradation by Mycobacterium sp. Strain KR2. Chemosphere. 1998;36:2977–2992. doi: 10.1016/s0045-6535(97)10240-5. [DOI] [PubMed] [Google Scholar]
- 79.Schneider J, Grosser R, Jayasimhulu K, Xue W, Warshawsky D. Degradation of pyrene, benz[a]anthracene, and benzo[a]pyrene by Mycobacterium sp. Strain RJGII-135, isolated from a former coal gasification site. Appl. Environ. Microbiol. 1996;62:13–19. doi: 10.1128/aem.62.1.13-19.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Juhasz AL, Britz ML, Stanley GA. Degradation of fluoranthene, pyrene, benz[a]anthracene and dibenz[a,h]anthracene by Burkholderia cepacia. J. Appl. Microbiol. 1997;83:189–198. [Google Scholar]
- 81.Sepic E, Bricelj M, Leskovsek H. Toxicity of fluoranthene and its biodegradation metabolites to aquatic organisms. Chemosphere. 2003;52:1125–1133. doi: 10.1016/S0045-6535(03)00321-7. [DOI] [PubMed] [Google Scholar]
- 82.Dean-Ross D, Cerniglia CE. Degradation of pyrene by Mycobacterium flavescens. Appl. Microbiol. Biotechnol. 1996;46:307–312. doi: 10.1007/s002530050822. [DOI] [PubMed] [Google Scholar]
- 83.Jimenez IY, Bartha R. Solvent-augmented mineralization of pyrene by a Mycobacterium sp. Appl. Environ. Microbiol. 1996;62:2311–2316. doi: 10.1128/aem.62.7.2311-2316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller CD, Hall K, Liang Y-N, Nieman K, Sorensen D, Issa B, Anderson AJ, Sims RC. Isolation and characterization of polycyclic aromatic hydrocarbon-degrading Mycobacterium isolates from soil. Microb. Ecol. 2004;48:230–238. doi: 10.1007/s00248-003-1044-5. [DOI] [PubMed] [Google Scholar]
- 85.Vila J, Lopez Z, Sabate J, Minguillon C, Solanas AM, Grifoll M. Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. Strain Ap1: Actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2001;67:5497–5505. doi: 10.1128/AEM.67.12.5497-5505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walter U, Beyer M, Klein J, Rehm HJ. Degradation of pyrene by Rhodococcus sp. UW1. Appl. Microbiol. Biotechnol. 1991;34:671–676. [Google Scholar]
- 87.Kazunga C, Aitken MD. Products from the incomplete metabolism of pyrene by polycyclic aromatic hydrocarbon-degrading bacteria. Appl. Environ. Microbiol. 2000;66:1917–1922. doi: 10.1128/aem.66.5.1917-1922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang B, Lai Q, Cui Z, Tan T, Shao Z. A pyrene-degrading consortium from deep-sea sediment of the west pacific and its key member Cycloclasticus sp. P1. Environ. Microbiol. 2008;10:1948–1963. doi: 10.1111/j.1462-2920.2008.01611.x. [DOI] [PubMed] [Google Scholar]
- 89.Kastner M, Breur-Jammali M, Mahro B. Impact of inoculation protocols, salinity, and pH on the degradation of polycyclic aromatic hydrocarbons (PAHs) and survival of PAH-degrading bacteria introduced into soil. Appl. Environ. Microbiol. 1998;64:359–362. doi: 10.1128/aem.64.1.359-362.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boonchan S, Britz ML, Stanley GA. Degradation and mineralization of high-molecular-weight polycyclic aromatic hydrocarbons by defined fungal-bacterial cocultures. Appl. Environ. Microbiol. 2000;66:1007–1019. doi: 10.1128/aem.66.3.1007-1019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang Y, Gardner DR, Miller CD, Chen D, Anderson AJ, Weimer BC, Sims RC. Study of biochemical pathways and enzymes involved in pyrene degradation by Micobacterium sp. strain KMS. Appl. Environ. Microbiol. 2006;72:7821–7828. doi: 10.1128/AEM.01274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim SJ, Kweon O, Jones RC, Freeman JP, Edmondson RD, Cerniglia CE. Complete and integrated pyrene degradation pathway in Mycobacterium vanbaalenii PYR-1 based on systems biology. J. Bacteriol. 2007;189:464–472. doi: 10.1128/JB.01310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grosser RJ, Warshawsky D, Vestal JR. Indigenous and enhanced mineralization of pyrene, benzo[a]pyrene, and carbazole in soils. Appl. Environ. Microbiol. 1991;57:3462–3469. doi: 10.1128/aem.57.12.3462-3469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye D, Siddiqi MA, Maccubbin AE, Kumar S, Sikka HC. Degradation of polynuclear aromatic hydrocarbons by Sphingomonas paucimobilis. Environ. Sci. Technol. 1996;30:136–142. [Google Scholar]
- 95.Juhasz AL, Stanley GA, Britz ML. Metabolite repression inhibits degradation of benzo[a]pyrene and dibenz[a,h]anthracene by Stenotrophomonas maltophilia VUN 10,003. J. Ind. Microbiol. Biotechnol. 2002;28:88–96. doi: 10.1038/sj/jim/7000216. [DOI] [PubMed] [Google Scholar]
- 96.Gibson DT, Mahadevan V, Jerina DM, Yogi H, Yeh HJ. Oxidation of the carcinogens benzo[a]pyrene and benzo[a]anthracene to dihydrodiols by a bacterium. Science. 1975;189:295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- 97.Cebron A, Norini M-P, Beguiristain T, Leyval C. Real-Time PCR quantification of PAH-ring hydroxylating dioxygenase (PAH-RHDα) genes from Gram positive and Gram negative bacteria in soil and sediment samples. J. Microbiol. Methods. 2008;73:148–159. doi: 10.1016/j.mimet.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 98.Lozada N, Mercadal JPR, Guerrero LD, Marzio WDD, Ferrero MA, Dionisi HM. Novel aromatic ring-hydroxylating dioxygenase genes from coastal marine sediments of Patagonia. BMC Microbiol. 2008;8:50. doi: 10.1186/1471-2180-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moody JD, Freeman JP, Fu PP, Cerniglia CE. Degradation of benzo[a]pyrene by Mycobacterium vanbaalenii PYR-1. Appl. Environ. Microbiol. 2004;70:340–345. doi: 10.1128/AEM.70.1.340-345.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rentz JA, Alvarez PJJ, Schnoor JL. Benzo[a]pyrene degradation by Sphingomonas yanoikuyae JAR02. Environ. Pollut. 2008;151:669–677. doi: 10.1016/j.envpol.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 101.Bressler DC, Fedorak PM. Bacterial metabolism of fluorene, dibenzofuran, dibenzothiophene, and carbazole. Can. J. Microbiol. 2000;46:397–409. [PubMed] [Google Scholar]
- 102.Nojiri H, Omori T. Molecular bases of aerobic bacterial degradation of dioxins: involvement of angular dioxygenation. Biosci. Biotechnol. Biochem. 2002;66:2001–2016. doi: 10.1271/bbb.66.2001. [DOI] [PubMed] [Google Scholar]
- 103.Becher D, Specht M, Hammer E, Francke W, Schauer F. Cometabolic degradation of dibenzofuran by biphenyl-cultivated Ralstonia sp. strain SBUG 290. Appl. Environ. Microbiol. 2000;66:4528–4531. doi: 10.1128/aem.66.10.4528-4531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fortnagel P, Harms H, Wittich R-M, Krohn S, Meyer H, Sinnwell V, Wilkes H, Francke W. Metabolism of dibenzofuran by Pseudomonas sp. strain HH69 and the mixed culture HH27. Appl. Environ. Microbiol. 1990;56:1148–1156. doi: 10.1128/aem.56.4.1148-1156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gai Z, Yu B, Li L, Wang Y, Ma C, Feng J, Deng Z, Xu P. Cometabolic degradation of dibenzofuran and dibenzothiophene by a newly isolated carbazole-degrading Sphingomonas sp. strain. Appl. Environ. Microbiol. 2007;73:2832–2838. doi: 10.1128/AEM.02704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo W, Li D, Tao Y, Gao P, Hu J. Isolation and description of a stable carbazole-degrading microbial consortium consisting of Chryseobacterium sp. NCY and Achromobacter sp. NCW. Curr. Microbiol. 2008;57:251–257. doi: 10.1007/s00284-008-9185-x. [DOI] [PubMed] [Google Scholar]
- 107.Nojiri H, Kamakura M, Urata M, Tanaka T, Chung JS, Takemura T, Yoshida T, Habe H, Omori T. Dioxin catabolic genes are dispersed on the Terrabacter sp. DBF63 genome. Biochem. Biophys. Res. Commun. 2002;296:233–240. doi: 10.1016/s0006-291x(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 108.Seo JS, Keum YS, Cho IK, Li QX. Degradation of dibenzothiophene and carbazole by Arthrobacter sp. P1-1. Int. Biodeter. Biodeg. 2006;58:36–43. [Google Scholar]
- 109.Habe H, Chung JS, Lee JH, Kasuga K, Yoshida T, Nojiri H, Omori T. Degradation of chlorinated dibenzofurans and dibenzo-p-dioxins by two types of bacteria having angular dioxygenases with different features. Appl. Environ. Microbiol. 2001;67:3610–3617. doi: 10.1128/AEM.67.8.3610-3617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inoue K, Habe H, Yamane H, Nojiri H. Characterization of novel carbazole catabolism genes from Gram-positive carbazole degrader Nocardioides aromaticivorans IC177. Appl. Environ. Microbiol. 2006;72:3321–3329. doi: 10.1128/AEM.72.5.3321-3329.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nam JW, Nojiri H, Noguchi H, Uchimura H, Yoshida T, Habe H, Yamane H, Omori T. Purification and characterization of carbazole 1,9a-dioxygenase, a three-component dioxygenase system of Pseudomonas resinovorans strain CA10. Appl. Environ. Microbiol. 2002;68:5882–5890. doi: 10.1128/AEM.68.12.5882-5890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nam IH, Kim YM, Schmidt S, Chang YS. Biotransformation of 1,2,3-tri- and 1,2,3,4,7,8-hexachlorodibenzo-p- dioxin by Sphingomonas wittichii strain RW1. Appl. Environ. Microbiol. 2006;72:112–116. doi: 10.1128/AEM.72.1.112-116.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Resnick SM, Gibson DT. Regio- and stereospecific oxidation of fluorene, dibenzofuran, and dibenzothiophene by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816–4. Appl. Environ. Microbiol. 1996;62:4073–4080. doi: 10.1128/aem.62.11.4073-4080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chain PS, Denef VJ, Konstantinidis KT, Vergez LM, Aqullo L, Reves VL, Hauser L, Cordova M, Gomez L, Gonzalez M, Land M, Lao V, Larimer F, LiPuma JJ, Mahenthiralingam E, Malfatti SA, Marx CJ, Parnell JJ, Ramette A, Richardson P, Seeger M, Smith D, Spilker T, Sul WJ, Tsoi TV, Ulrich LE, Zhulin IB, Tiedje JM. Burkholderia xenovorans LB400 harbors a multiple replicon, 9.73-Mbp genome shaped for versatility. Proc. Natl. Acad. Sci. USA. 2006;103:15380–15287. doi: 10.1073/pnas.0606924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim SJ, Jones RC, Cha CJ, Kweon O, Edmondson RD, Cerniglia CE. Identification of proteins induced by polycyclic aromatic hydrocarbon in Mycobacterium vanbaalenii PYR-1 using two-dimensional polyacrylamide gel electrophoresis and de novo sequencing methods. Proteomics. 2004;4:3899–3908. doi: 10.1002/pmic.200400872. [DOI] [PubMed] [Google Scholar]
- 116.Yamazoe A, Yagi O, Oyaizu H. Degradation of polycyclic aromatic hydrocarbons by a newly isolated dibenzofuran-utilizing Janibacter sp. strain YY-1. Appl. Microbiol. Biotechnol. 2004;65:211–218. doi: 10.1007/s00253-003-1541-y. [DOI] [PubMed] [Google Scholar]
- 117.Habe H, Ide K, Yotsumoto M, Tsuji H, Yoshida T, Nojiri H, Omori T. Degradation characteristics of a dibenzofuran-degrader Terrabacter sp. strain DBF63 toward chlorinated dioxins in soil. Chemosphere. 2002;48:201–207. doi: 10.1016/s0045-6535(02)00064-4. [DOI] [PubMed] [Google Scholar]
- 118.Fortin PD, Horsman GP, Yang HM, Eltis LD. A glutathione S-transferase catalyzes the dehalogenation of inhibitory metabolites of polychlorinated biphenyls. J. Bacteriol. 2006;188:4424–4430. doi: 10.1128/JB.01849-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bressler DC, Fedorak PM. Purification, stability, and mineralization of 3-hydroxy-2- formylbenzothiophene, a metabolite of dibenzothiophene. Appl. Environ. Microbiol. 2001;67:821–826. doi: 10.1128/AEM.67.2.821-826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Folsom BR, Schieche DR, DiGrazia PM, Werner J, Palmer S. Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-19. Appl. Environ. Microbiol. 1999;65:4967–4972. doi: 10.1128/aem.65.11.4967-4972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kirimura K, Furuya T, Sato R, Ishii Y, Kino K, Usami S. Biodesulfurization of naphthothiophene and benzothiophene through selective cleavage of carbon-sulfur bonds by Rhodococcus sp. strain WU-K2R. Appl. Environ. Microbiol. 2002;68:3867–3872. doi: 10.1128/AEM.68.8.3867-3872.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsubara T, Ohshiro T, Nishina Y, Izumi Y. Purification, characterization, and overexpression of flavin reductase involved in dibenzothiophene desulfurization by Rhodococcus erythropolis D-1. Appl. Environ. Microbiol. 2001;67:1179–1184. doi: 10.1128/AEM.67.3.1179-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sutherland TD, Horne I, Russell RJ, Oakeshott JG. Gene cloning and molecular characterization of a two-enzyme system catalyzing the oxidative detoxification of ß-endosulfan. Appl. Environ. Microbiol. 2002;68:6237–6245. doi: 10.1128/AEM.68.12.6237-6245.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Willumsen PA, Nielsen JK, Karlson U. Degradation of phenanthrene-analogue azaarenes by Mycobacterium gilvum strain LB307T under aerobic conditions. Appl. Microbiol. Biotechnol. 2001;56:539–544. doi: 10.1007/s002530100640. [DOI] [PubMed] [Google Scholar]
- 125.Mahajan MC, Phale PS, Vaidyanathan CS. Evidence for the involvement of multiple pathways in the biodegradation of 1- and 2-methylnaphthalene by Pseudomonas putida CSV86. Arch. Microbiol. 1994;161:425–433. doi: 10.1007/BF00288954. [DOI] [PubMed] [Google Scholar]
- 126.Moody JD, Fu PP, Freeman JP, Cerniglia CE. Regio- and stereoselective metabolism of 7,12-dimethylbenz[a]anthracene by Mycobacterium vanbaalenii PYR-1. Appl. Environ. Microbiol. 2003;69:3924–3931. doi: 10.1128/AEM.69.7.3924-3931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bunge M, Adrian L, Kraus A, Opel M, Lorenz WG, Andreesen JR, Görisch H, Lechner U. Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature. 2003;421:357–360. doi: 10.1038/nature01237. [DOI] [PubMed] [Google Scholar]
- 128.Chang BV, Chiu TC, Yuan SY. Dechlorination of polychlorinated biphenyl congeners by anaerobic microorganisms from river sediment. Water Environ. Res. 2006;78:764–769. doi: 10.2175/106143006x107380. [DOI] [PubMed] [Google Scholar]
- 129.Annweiler E, Materna A, Safinowski M, Kappler A, Richnow HH, Michaelis W, Meckenstock RU. Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Appl. Environ. Microbiol. 2000;66:5329–5333. doi: 10.1128/aem.66.12.5329-5333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Annweiler E, Michaelis W, Meckenstock RU. Identical ring cleavage products during anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin indicate a new metabolic pathway. Appl. Environ. Microbiol. 2002;68:852–858. doi: 10.1128/AEM.68.2.852-858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang X, Young LY. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulfidogenic consortia. Appl. Environ. Microbiol. 1997;63:4759–4764. doi: 10.1128/aem.63.12.4759-4764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rafii F, Selby AL, Newton RK, Cerniglia CE. Reduction and mutagenic activation of nitroaromatic compounds by a Mycobacterium sp. Appl. Environ. Microbiol. 1994;60:4263–4267. doi: 10.1128/aem.60.12.4263-4267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Denef VJ, Patrauchan MA, Florizone C, Park J, Tsoi TV, Verstraete W, Tiedje JM, Eltis LD. Growth substrate- and phase-specific expression of biphenyl, benzoate, and C1 metabolic pathways in Burkholderia xenovorans LB400. J. Bacteriol. 2005;187:7996–8005. doi: 10.1128/JB.187.23.7996-8005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Denef VJ, Klappenbach JA, Patrauchan MA, Florizone C, Rodrigues JLM, Tsoi V, Verstraete W, Eltis LD, Tiedje JM. Genetic and genomic insights into the role of benzoate-catabolic pathway redundancy in Burkholderia xenovorans LB400. Appl. Environ. Microbiol. 2006;72:585–595. doi: 10.1128/AEM.72.1.585-595.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parnell JJ, Park JH, Denef V, Tsoi T, Hashsham S, Quensen J, III, Tiedje JM. Coping with polychlorinated biphenyl (PCB) toxicity: Physiological and genome-wide responses of Burkholderia xenovorans LB400 to PCB-mediated stress. Appl. Environ. Microbiol. 2006;72:6607–6614. doi: 10.1128/AEM.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chávez FP, Lünsdorf H, Jerez CA. Growth of polychlorinated-biphenyl-degrading bacteria in the presence of biphenyl and chlorobiphenyls generates oxidative stress and massive accumulation of inorganic polyphosphate. Appl. Environ. Microbiol. 2004;70:3064–3072. doi: 10.1128/AEM.70.5.3064-3072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hanson RS, Hanson TE. Methanotrophic bacteria. Microbiol. Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sokolovska I, Rozenberg R, Riez C, Rouxhet PG, Agathos SN, Wattiau P. Carbon-source induced modifications in the mycolic acid content and cell wall permeability of Rhodococcus erythropolis E1. Appl. Environ. Microbiol. 2003;69:7019–7027. doi: 10.1128/AEM.69.12.7019-7027.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krivobok S, Kuony S, Meyer C, Louwagie M, Willison JC, Jouanneau Y. Identification of pyrene-induced proteins in Mycobacterium sp. strain 6PY1: Evidence for two ring-hydroxylating dioxygenase. J. Bacteriol. 2003;185:3828–3841. doi: 10.1128/JB.185.13.3828-3841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotopelabeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 141.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 142.Kim YH, Cho K, Yun SH, Kim JY, Kwon KH, Yoo JS, Kim SI. Analysis of aromatic catabolic pathways in Pseudomonas putida KT2440 using a combined approach: 2-DE/MS and cleavable isotope-coded affinity tag analysis. Proteomics. 2006;6:1301–1318. doi: 10.1002/pmic.200500329. [DOI] [PubMed] [Google Scholar]
- 143.Laurie AD, Lloyd-Jones G. Conserved and hybrid meta-cleavage operons from PAH-degrading Burkholderia RP007. Biochem. Biophys. Res. Commun. 1999;262:308–314. doi: 10.1006/bbrc.1999.1153. [DOI] [PubMed] [Google Scholar]
- 144.Fritzsche C. Degradation of pyrene at low defined oxygen concentrations by a Mycobacterium sp. Appl. Environ. Microbiol. 1994;60:1687–1689. doi: 10.1128/aem.60.5.1687-1689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Herwijnen R, Springael D, Slot P, Govers HAJ, Parsons JR. Degradation of anthracene by Mycobacterium sp. Strain LB501T proceeds via a novel pathway, through o-phthalic acid. Appl. Environ. Microbiol. 2003;69:186–190. doi: 10.1128/AEM.69.1.186-190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sepic E, Leskovsek H. Isolation and identification of fluoranthene biodegradation products. Analyst. 1999;124:1765–1769. [Google Scholar]
- 147.Caldini G, Cenci G, Manenti R, Morozzi G. The ability of an environmental isolate of Pseudomonas fluorescens to utilize chrysene and other four-ring polycyclic aromatic hydrocarbons. Appl. Microbiol. Biotechnol. 1995;44:225–229. [Google Scholar]
- 148.Romero MC, Cazau MC, Giorgieri S, Arambarri AM. Phenanthrene degradation by microorganisms isolated from a contaminated stream. Environ. Pollut. 1998;101:355–359. [Google Scholar]
- 149.Kanaly RA, Harayama S, Watanabe K. Rhodanobacter sp. Strain BPC-1 in a benzo[a]pyrene-mineralizing bacterial consortium. Appl. Environ. Microbiol. 2002;68:5826–5833. doi: 10.1128/AEM.68.12.5826-5833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pinyakong O, Habe H, Supaka N, Pinpanichkarn P, Juntongjin K, Yoshida T. Identification of novel metabolites in the degradation of phenanthrene by Sphingomonas sp. strain P2. FEMS Microbiol. Lett. 2000;191:115–121. doi: 10.1111/j.1574-6968.2000.tb09327.x. [DOI] [PubMed] [Google Scholar]
- 151.Mueller JG, Chapman PJ, Blattmann BO, Pritchard PH. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl. Environ. Microbiol. 1990;56:1079–1086. doi: 10.1128/aem.56.4.1079-1086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou NY, Al-Dulayymi J, Baird MS, Williama PA. Salicylate 5-hydroxylase from Ralstonia sp. strain U2: a monooxygenase with close relationships to and shared electron transport proteins with naphthalene dioxygenase. J. Bacteriol. 2002;184:1547–1555. doi: 10.1128/JB.184.6.1547-1555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pumphrey GM, Madsen EL. Naphthalene metabolism and growth inhibition by naphthalene in Polaromonas naphthalenivorans strain CJ2. Microbiology. 2007;153:3730–3738. doi: 10.1099/mic.0.2007/010728-0. [DOI] [PubMed] [Google Scholar]
- 154.Sota M, Yano H, Ono A, Miyazaki R, Ishii H, Genka H, Top EM, Tsuda M. Genomic and functional analysis of the IncP-9 naphthalene-catabolic plasmid NAH7 and its transposon Tn4655 suggests catabolic gene spread by a tyrosine recombinase. J. Bacteriol. 2006;188:4057–4067. doi: 10.1128/JB.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dennis JJ, Zylstra GJ. Complete sequence and genetic organization of pDTG1, the 83kilobase naphthalene degradation plasmid from Pseudomonas putida strain NCIB 9816-4. J. Mol. Biol. 2004;341:753–768. doi: 10.1016/j.jmb.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 156.Safinowski M, Meckenstock RU. Methylation is the initial reaction in anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Environ. Microbiol. 2006;8:347–352. doi: 10.1111/j.1462-2920.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- 157.Spormann AM, Widdel F. Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation. 2000;11:85–105. doi: 10.1023/a:1011122631799. [DOI] [PubMed] [Google Scholar]
- 158.Nesatyy VJ, Suter MJ-F. Proteomics for the analysis of environmental stress responses in organisms. Environ. Sci. Technol. 2007;41:6891–6900. doi: 10.1021/es070561r. [DOI] [PubMed] [Google Scholar]
- 159.Keum Y-S, Seo J-S, Li QX, Kim J-H. Comparative metabolomic analysis of Sinorhizobium sp. C4 during the degradation of phenanthrene. Appl. Microbiol. Biotechnol. 2008;80:863–872. doi: 10.1007/s00253-008-1581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]