Abstract
The prefrontal cortices mediate cognitive functions that critically depend on local dopamine levels. In male rats, many prefrontal tasks where performance is disrupted by changes in dopamine signaling are also impaired by gonadectomy, a manipulation that increases cortical dopamine concentration, prefrontal dopamine axon density and possibly extracellular prefrontal dopamine levels as well. Because these actions could be responsible for the impairing effects of gonadectomy on prefrontal function, the question of how they might arise comes to the fore. Accordingly, the present studies asked whether dopamine levels might be increased via a hormone sensitivity of transporter mediated dopamine uptake. Specifically, 3HWIN 35,428 and 3Hnisoxetine, ligands selective for the dopamine (DAT)- and norepinephrine transporter (NET) respectively, were used in in vitro binding assays to ask whether gonadectomy altered transporter affinity (Kd) and/or binding site number (Bmax) in prefrontal cortex, sensorimotor cortex and/or caudate. Assays performed on tissues dissected from sham-operated, gonadectomized and gonadectomized rats supplemented with testosterone propionate or estradiol for 4 or 28 days revealed no significant group differences or obvious trends in Kd or Bmax for DAT binding or in measures of Bmax for NET binding. However, affinity constants for 3Hnisoxetine were found to be significantly higher in sensorimotor and/or prefrontal cortex of rats gonadectomized and gonadectomized and supplemented with estradiol for 4 or 28 days but similar to control in gonadectomized rats given testosterone. Because the NET contributes substantially to extracellular prefrontal dopamine clearance, these androgen-mediated effects could influence prefrontal dopamine levels and might thus be relevant for observed effects of gonadectomy on dopamine-dependent prefrontal behaviors. A hormone sensitivity of the NET could also have bearing on the prefrontal dopamine dysfunction seen in disorders like schizophrenia that disproportionately affect males, whose severity correlates with abnormal testosterone levels, and for which the NET is among suspected sites of pathology.
Keywords: prefrontal cortex, estrogen, androgen, working memory, schizophrenia, ADHD
The prefrontal cortices mediate highest order executive and mnemonic functions including working memory and behavioral flexibility (Goldman-Rakic, 1990, Dalley et al., 2004). It is well known that these complex operations depend on local prefrontal dopamine (DA) levels being maintained within certain limits. In rats, for example, chemical lesions of prefrontal DA afferents, cortical infusions of selective DA D1 receptor agonists and antagonists and pharmacological and stress-induced increases in prefrontal DA turnover have all been shown to produce significant deficits in prefrontal-dependent behaviors and tasks including open field activity, acquisition of T-maze delayed alternation, behavioral flexibility, and novel object recognition (Tassin et al., 1978, Kessler and Markowitsch, 1981, Kalsbeek et al., 1989, Stam et al., 1989, Murphy et al., 1996, Verma and Moghaddam, 1996, Zahrt et al., 1997, Morrow et al., 2000). More recent studies, however, have shown that performance in each of these tasks is also impaired by gonadectomy in adult male rats (Adler et al., 1999, Kritzer et al., 2001, Kritzer et al., 2007, Aubele et al., 2008). Because this is a manipulation that has also been shown to increase cortical DA axon density (Kritzer et al., 1999, Kritzer, 2000) and concentration (Battaner et al., 1987) it is possible that the effects of gonadectomy on cognitive and mnemonic information processing are related to its dysregulation of prefrontal DA systems. Further, more recent evidence of sex differences in extracellular prefrontal DA levels (Shansky et al., 2006) combine with preliminary in vivo microdialysis data from this lab (Aubele et al., 2008) showing that GDX increases basal prefrontal DA overflow to suggest more specifically that the behaviorally deleterious consequences of GDX could be related to stimulation of supranormal extracellular levels of DA in the prefrontal cortex. Although this could come about by several means, this study focused on the possibility that prefrontal DA levels might be affected via hormone regulation/gonadectomy-induced dysregulation of transporter-mediated DA uptake, a process that along with catabolic enzymes and other mechanisms is involved in setting prefrontal DA tone.
In the prefrontal cortex, transporter-mediated DA uptake uniquely involves both the dopamine (DAT) and norepinephrine transporters (NET) whose low local levels (Sesack et al., 1998a, Sesack et al., 1998b, Miner et al., 2003) facilitate the unusually long-lived extracellular DA levels/lifetimes that are hallmark of the frontal cortex and essential for its function (Carboni et al., 1990, Elsworth et al., 1993, Cass and Gerhardt, 1995, Yamamoto and Novotney, 1998, Moron et al., 2002, Valentini et al., 2004, Carboni et al., 2006). As key controllers of the functionally pivotal parameter of prefrontal DA level, it may not be surprising that both the DAT and the NET have also been implicated in disorders including ADHD and schizophrenia (Cook et al., 1995, Gill et al., 1997, Laakso et al., 2001, DeLisi et al., 2002, Hsiao et al., 2003, Madras et al., 2005, Rybakowski et al., 2006) where prefrontal deficits and prefrontal hyper- and hypodopaminergia, respectively, are prominent parts of the clinical scenario (Shaywitz et al., 1977, Davis et al., 1991). That in both disorders the DA-dependent prefrontal processes at risk are not only disproportionately so males (Goodman and Stevenson, 1989, Seeman and Lang, 1990, Leviton et al., 1993) but are linked to anomalous adult, adolescent and/or intrauterine levels of testosterone (Shirayama et al., 2002, Goyal et al., 2004, Taherianfard and Shariaty, 2004, Akhondzadeh et al., 2006, de Bruin et al., 2006) gives further impetus to focus investigation of the hormone sensitivity of the DAT and NET specifically on males. To begin this line of inquiry, this study used the selective ligands 3H WIN 35,428 and 3H nisoxetine and in vitro homogenate binding assays to examine the effects of gonadectomy and hormone replacement in adult male rats on the affinity and maximal numbers of ligand binding sites of the DAT and NET, respectively, in the medial prefrontal cortex, the sensorimotor cortex and in the caudate nucleus. For purposes of comparison, these assays were conducted on tissue samples micro-dissected from separate groups of rats that were sham-operated, gonadectomized and gonadectomized and supplemented with testosterone propionate or estradiol for 4 or 28 days according to methods used in previous studies that have identified highly selective and differentially estrogen- and androgen-sensitive effects of long and short term gonadectomy on the anatomical organization, physiology and function of mesoprefrontal DA systems in adult male rats (Adler et al., 1999, Kritzer et al., 1999, Kritzer, 2000, Kritzer, 2003, Kritzer et al., 2007). Some of this work has appeared in abstract form (Aubele et al., 2008).
EXPERIMENTAL PROCEDURES
Animal Subjects
Adult male Sprague-Dawley rats (Taconic Farms, Germantown, NY) were used. All animals were housed with food and water freely available under a 12-hour light/dark cycle and were gonadectomized, with and without replacement with 17- β-estradiol or testosterone propionate (below), or sham operated 4 or 28 days prior to euthanasia. Pilot studies in gonadally intact animals determined that due to the small sizes of the prefrontal samples that could be discretely dissected from individual animals and the low numbers of cortical transporter sites, the DAT assays required tissue pooled from 8 rats per group and the NET assays required tissue pooled from 5 rats per group. All procedures involving animals are approved by the Institutional Animal Care and Use Committee of Stony Brook University, and minimize their use and discomfort.
Surgical Procedures
Gonadectomy and sham surgeries were performed under aseptic conditions using ketamine (.09ml/100g) and xylazine (.05ml/100g) for anesthesia. For both operations, the sac of the scrotum and underlying tunica were incised; for gonadectomies, the vas deferens was also bilaterally ligated and the testis were removed. For gonadectomized, hormone-replaced rats, slow-release pellets (below) were implanted within the scrotal sac. Incisions were closed using sterile surgical staples; for rats with longer survival times these were removed after 10 days.
Hormone Treatments
Subsets of gonadectomized animals were implanted with slow release pellets that contained either testosterone propionate or 17-β-estradiol (Innovative Research of America, Toledo, OH). The testosterone propionate-containing pellets used have been used in previous studies in this laboratory and others (Kritzer, 2000, Turvin et al., 2007) and have been shown to release between 0.5–2ng of TP per milliliter of blood per day; likewise, the estradiol-containing pellets used were also identical to those used previously (Kritzer, 2000, Turvin et al., 2007) and known to release between 10–40pg of E per milliliter of blood per day.
Euthanasia
Four or twenty eight days after gonadectomy or sham surgery, rats were weighed and euthanized by rapid decapitation. Immediately afterward the brains were removed. For the NET assays, brains were rapidly frozen in powdered dry ice and stored at −80 deg. C until assays were performed. For DAT assays, brain regions of interest (medial prefrontal cortex, sensorimotor cortex and caudate nuclei, see Fig. 1) were dissected out over ice and used immediately. To maximize consistency, all dissections were performed by the same person (MFK). The bulbospongiosus muscles (BSM) were also dissected out and weighed in all of the subjects at the time of euthanasia.
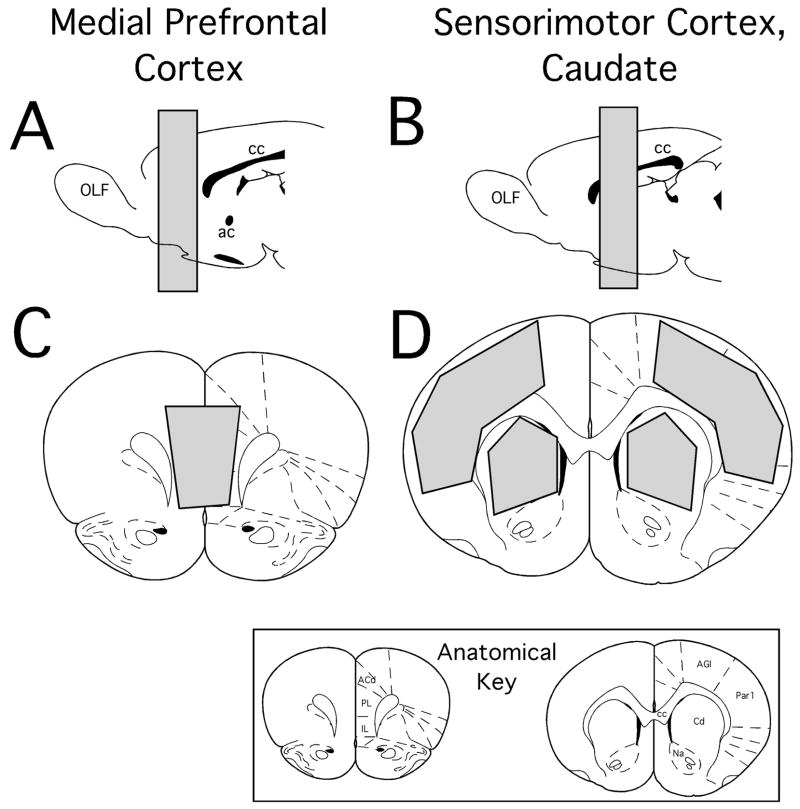
Figure 1.
Schematic diagrams showing the anterior/poster (A, B) and coronal (C,D) locations of brain regions marked in grey that were micro-dissected out for the use in homogenate binding assays. These regions of interest were pregenual portions of the medial prefrontal cortex (A, C) and portions of the sensorimotor cortex and caudate nucleus taken at levels around the anterior commisure (B, D). The templates showing these locations are modified from the atlas of Paxinos and Warson (1998). Anatomical abbreviations: ACd, anterior cingulate cortex; ac, anterior commisure; AGl, primary motor cortex; Cd, caudate nucleus; cc, corpus callosum; IL, infralimbic cortex; Na, nucleus accumbens; OLF, olfactory bulb; Par1, primary somatosensory cortex; PL, prelimbic cortex.
NET: 3H Nisoxetine Binding Assay
a. Tissue preparation
Frozen brains were partially thawed and bilateral samples of medial prefrontal cortex, sensorimotor cortex and caudate nucleus were dissected out over ice (Fig. 1). Tissues from 5 rats per group were pooled and homogenized using a motor-driven Ultra Turrax homogenizer (IKA Works, Inc, Wilmington, NC) for 10 sec, in 10 ml of ice-cold 50 mM phosphate buffer, pH 7.4. The homogenate was then centrifuged at 20,000g for 10 min at 4°C and the supernatant was discarded. The pellet was then re-suspended and re-centrifuged as above in 10 ml of ice-cold 50 mM phosphate buffer, pH 7.4 and recollected. After this process was repeated, the final, washed pellet was re-suspended at a concentration of 60mg wet weight/ml in incubation buffer (50mM Tris Hcl, pH 7.4, 120 mM NaCl2, 5 mM KCl).
b. Assay conditions
Uptake sites were analyzed by full saturation isotherms according to methods adapted from Tejani-Butt (1992) and optimized in pilot studies using tissue from gonadally intact sham-operated control animals and initially using 8 concentrations of 3H nisoxetine (Perkin Elmer, Boston, MA, 87.2 Ci/mmol) ranging from 0.25–40 nmol. Because specific binding plateaued at ligand concentrations of less than 10 nmol, subsequent saturation assays used an 8-ligand concentration range of 0.25–14 nmol. Each assay point was run in triplicate with replicates measuring total binding consisting of 100μl tissue homogenate, 50μl radioligand, and 350 μl cold incubation buffer and those measuring non-specific binding also including 1μM tomoxetine (Sigma Chemical Co, St. Louis, MO). Each saturation isotherm was repeated a total three times for each brain region using tissue from three different sets of animal subjects.
Assays were conducted at 4deg C, and were terminated after 4 hours by rapid filtration using a 48-well cell harvester (Brandel Inc, Gaithersburg. MD) and Whatmann GF/B filter mats followed by 3 rapid rinses with ice-cold incubation buffer. The filters were then dissolved in 10 ml of scintillation fluid (EcoLite, MP Biomedicals, Irvine, CA) and radioactivity was counted in a Beckmann-Coulter Liquid scintillation counter (Beckman Instruments, Inc. Fullerton, CA).
DAT: 3H WIN35,428 Binding Assay
a. Tissue preparation
Immediately upon brain removal, bilateral samples of medial prefrontal cortex, sensorimotor cortex and caudate nuclei were dissected out over ice (Fig. 1). Tissues were pooled from 8 animal subjects and were homogenized and centrifuged as per the NET assay above. The final pellet was re-suspended at a concentration of 100mg wet weight/ml in ice-cold incubation buffer consisting of 15mM HEPES, 127mM NaCl, 5 mM KCl, 1.2 mM Mg2SO4, 2.5mM CaCl2, 1.3 mM NaH2PO4 and 10 mM D-glucose- pH 7.4.
b. Assay Conditions
Full saturation isotherm assays were performed according to methods adapted from Izenwasser et al., (1993) and optimized in pilot studies using tissue from gonadally intact, sham-operated control rats and 8 concentrations of 3H WIN35,428 (Perkin Elmer, 85.9Ci/mmol) that ranged from 0.25–40 nmol. These analyses showed that specific binding plateaued at ligand concentrations of 10 nmol or less. Subsequent assays thus used an 8-ligand concentration range of 0.25–14 nmol. Each assay point was run in triplicate with replicates measuring total binding consisting of 350μl incubation buffer, 50μl tissue homogenate, and 100μl ligand; non-specific binding was determined from replicated that additionally included 1μM nomifensine. All assays were conducted at 4deg C for 1.5 hours and were terminated by rapid filtration and rinsing with the filters subsequently dissolved in 10 ml of scintillation fluid (EcoLite) and radioactivity counted as per the NET assay above. Each saturation isotherm assay was repeated a total of three times using tissue from three independent groups of animal subjects.
Protein Quantification
Protein content was measured according to the method of Bradford (1976) using a Bio Rad Protein Assay Kit (BioRad Life Sciences, Hercules, CA). Samples of tissue homogenates used in binding assays were diluted 8-fold and measured at 595 nm in a Nanodrop 1000 Spectrophotometer (Nanodrop Technologies, Inc. Wilmington, DE). Sample protein concentrations were calculated in relation to standard curves generated using 0.2–1.4 mg/ml of bovine gamma globulin (provided with the kits); these values were used to normalize all binding data prior to analysis (below).
Data Analysis
Specific binding, equilibrium dissociation constants (Kd), and maximum number of binding sites (Bmax) were all calculated in three separate assays from triplicate measures of total and non-specific binding data (normalized for protein, above) determined at each of eight ligand concentrations for each animal group and tissue. These values were obtained using a commercial nonlinear regression analysis/iterative curve fitting routine and always included comparisons of fit for one- versus two-site binding models (Prism, GraphPad Software Inc, San Diego, CA). Affinity and Bmax values obtained from the three separate assays were evaluated using descriptive statistics and compared per tissue across animals and animal groups using the non-parametric Kruskal- Wallis test to identify significant main effects of hormone treatment and the Mann-Whitney U test (with Bonferroni correction) to identify significant between group differences. Bulbospongiosus muscle weights were assessed within and across groups using parametric analyses of variance and allowed post-hoc testing that used the Fisher’s PLSD. For all statistical assessments, a p< 0.05 level was accepted as significant (Stat View, 4.5).
RESULTS
Efficacy of Hormone Treatments
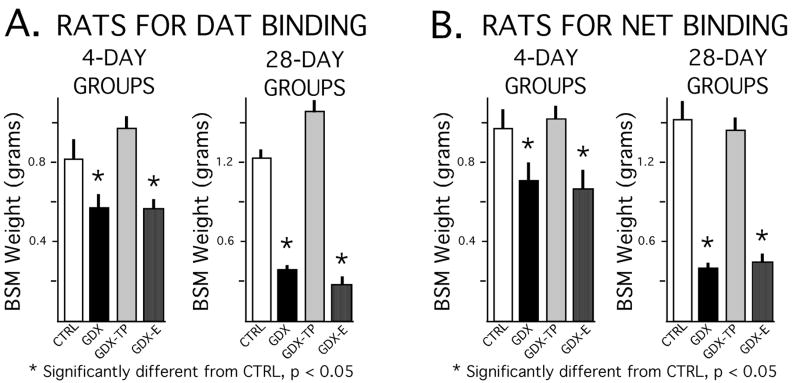
The dissected weights of the androgen-sensitive bulbospongiosus muscles showed expected differences between the GDX and GDX-E groups on the one hand and the control and GDX-TP groups on the other (Fig. 2). Although absolute differences in the lower mean muscle weights of the GDX and GDX-E groups compared to the higher weights of the sham-operated control and GDX-TP animals (Fig, 2A) were greater for the 28-day compared to the 4-day treatment groups (Fig, 2B), analyses of variance (ANOVA) identified significant main effects of hormone treatment on muscle mass at both time points [animals used for DAT assays: 4 day F(3,28) = 15.28, p < 0.0001; 28 day, F(3,28) = 174.51, p < 0.0001; animals used for NET assays: 4 day F (3, 16) = 6.13, p < 0.0056; 28 day, F(3, 16) = 49.97, p < 0.0001]. Allowed post hoc analyses further identified significant differences in muscle mass between the GDX and GDX-E groups compared to the GDX-TP rats and sham-operated controls, but no significant differences in muscle mass between the GDX and GDX-E groups or between the control and GDX-TP cohorts (Fig. 2).
Figure 2.
Bar graphs showing means weights of the bulbospongiousus muscles (BSM) in grams, ± standard error of the mean, for animal subjects used for dopamine transporter (DAT) binding assays (A) and norepinephrine transporter (NET) binding assays (B). Data from animals that were sham-operated (CRTL), gonadectomized (GDX) and gonadectomized and supplemented with testosterone propionate (GDX-TP) or estradiol (GDX-E) for 4 or 28 days are shown separately. As expected, there were significant effects of hormone treatment on the weights of these androgen-sensitive muscles for both time points, and BSM weights in both the 4- and 28-day GDX and GDX-E groups were significantly less (*) than those in the corresponding CTRL and GDX-TP cohorts.
DAT Binding
All data from the 3H WIN 35,428 saturation assays were analyzed using both one and two-site binding models (Prism GraphPad Software). For all tissues and all animal groups, comparisons of convergence and goodness of fit with respect to these two models revealed concordance/congruence only with the one-site model and data that either did not converge or that showed an extremely poor goodness of fit (r2 values of 0.1 or less) for the two-site model. Graphical representations of the saturation data and their Scatchard transformations were also consistent with binding at single sites (e.g., Fig. 3).
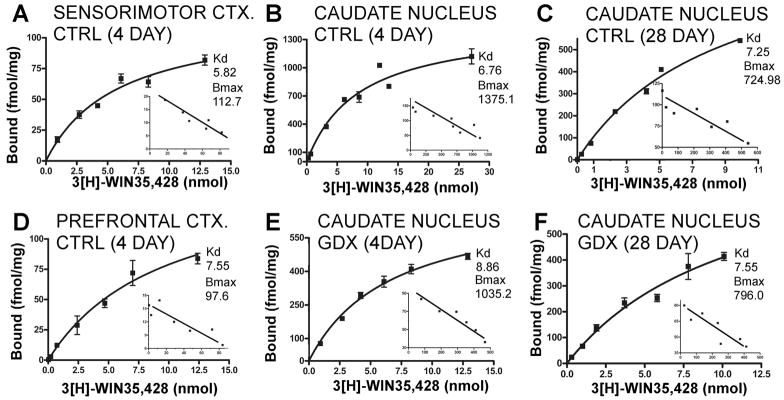
Figure 3.
Representative saturation isotherms with Scatchard transformations of the data shown as insets for 3H-WIN 35,428 binding. Each graph represents data from a single assay. For the saturation data, points are means of triplicate measures obtained of ligand bound in nmol/mg protein (Y-axis) at each ligand concentration (X-axis) and error bars are standard deviations of these means; the inserted Scatchard transformations plot corresponding measures of bound to bound/free ligand. Data from sensorimotor cortex (A), caudate nucleus (B) and prefrontal cortex (D) dissected from 4-day sham-operated controls (CTRL) are shown. For comparison, binding data for the caudate nucleus dissected from 28-day sham operated controls (C) and from 4- and 28 day gonadectomized animals (E, F) are shown. For each panel, calculated maximal numbers of binding sites (Bmax) and binding affinity constants (Kd) are also shown. These graphs illustrate that there can be variance in the data regarding measures of Bmax, e.g. compare 4 and 28-day controls, and linear regressions in the Scatchard plots that yield single straight lines indicative of binding to a single site.
In both the 4- and 28-day control groups, Kd values for ligand binding sites were between 6 and 8 nmol for all three tissues evaluated (Figs. 3, 4A, C). These values were similar to those previously reported for ligand binding affinity in fresh rat brain homongenates (Katz et al., 2000). The corresponding Kd values that were obtained in the gonadectomized and hormone-supplemented groups were all found to be similar to control values. Statistical analyses further confirmed that there were no significant effects of hormone treatment (Kruskal Wallis) or significant group differences (Mann-Whitney U) aong any of the ligand binding affinity values measured in the either cortical or subcortical tissues examined (Figs 4A, C).
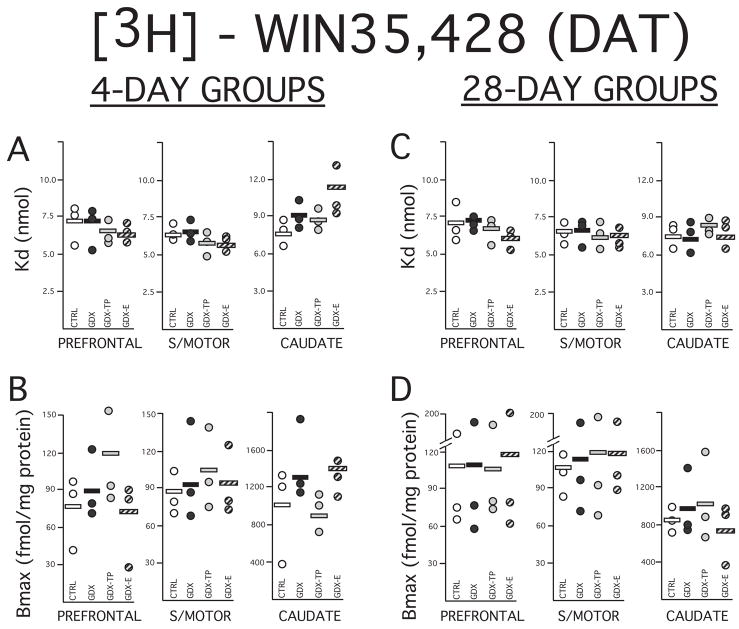
Figure 4.
Scatter plots showing data (circles) and means mean (horizontal black bars) for dissociation constants (Kd) in nmol (A, C) and maximal numbers of binding sites (Bmax) in fmol/mg protein (B,D) calculated for the dopamine transporter (DAT) using selective ligand 3H WIN35,428. The Kd and Bmax data obtained from homogenate assays performed on prefrontal cortex, sensorimotor cortex (S/MOTOR) and caudate nucleus tissue dissected from rats that were sham-operated (CRTL, open circles), gonadectomized (GDX, black circles) and gonadectomized and supplemented with testosterone propionate (GDX-TP, light gray circles) or estradiol (GDX-E, dark gray circles) for 4 (left) or 28 days (right) are shown separately. The paucity of the DAT in the cerebral cortex is reflected in Bmax values from both cortical samples that are lower than those obtained in the caudate nucleus. Overall the Bmax measures were also more variable across assays than measures of Kd. There was, however, considerable overlap in both Bmax and Kd values across groups and neither significant main effects of hormone treatment nor significant group differences for either set of values among the 4 or 28 day treatment groups.
Compared to Kd values, the maximal numbers of binding sites (Bmax) that were calculated for the two control groups showed more assay-to-assay variability, particularly within the especially DAT-sparse cortical tissues (Fig. 4). Visual inspection of the data, however, revealed that variance was driven largely by single outliers in the data (Figs. 4B,D) and that, as expected, maximal numbers of binding sites in both the 4- and 28-day controls were on average roughly 10 times higher in the caudate nucleus (~ 800–1000 fmol/mg protein) than in the prefrontal (~70–100 fmol/mg protein) or sensorimotor cortex (~90–120 fmol/mg protein, Fig. 3, Figs. 4B, D). Within-group variance was also seen in Bmax values for the gonadectomized and hormone-supplemented groups that, like the controls, usually involved single outliers (Figs. 4B,D). Nonetheless, from tissue-to-tissue Bmax values from all of the experimental groups as well as the controls were largely overlapping and showed no obvious group differences. Quantitative analyses supported these observations and showed that tissue-specific measures of Bmax had similar group means, especially among gonadectomized rats and controls groups and that there were neither significant main effects of hormone treatment (Kruskal Wallis) or group differences (Man-Whitney U) in this parameter for any of the cortical or subcortical tissues assessed (Figs. 4B, D).
NET Binding
Binding data for 3H nisoxetine were analyzed using both single- and two-site models (Prism GraphPad Software). For every tissue and animal group convergence, goodness of fit (r2 values) and graphical representations of the saturation isotherms and their Scatchard transformations (Fig. 5) were all consistent with binding to single sites.
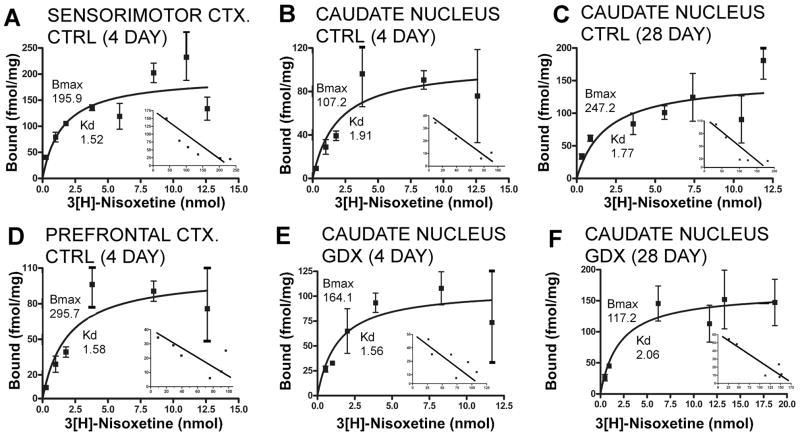
Figure 5.
Representative saturation isotherms with Scatchard transformations of the data shown as insets for 3H-nisoxetine binding. Each graph represents data from a single assay. For the saturation data, points are means of triplicate measures obtained of ligand bound in nmol/mg protein (Y-axis) at each ligand concentration (X-axis) and error bars are standard deviations of these means; the inserted Scatchard transformations plot corresponding measures of bound to bound/free ligand. Data from sensorimotor cortex (A), caudate nucleus (B) and prefrontal cortex (D) dissected from 4-day sham-operated controls (CTRL) are shown. For comparison, binding data for the caudate nucleus dissected from 28-day sham operated controls (C) and from 4- and 28 day gonadectomized animals (E, F) are shown. For each panel, calculated maximal numbers of binding sites (Bmax) and binding affinity constants (Kd) are also shown. These graphs illustrate that there can be variance in the data regarding measures of Bmax, e.g. compare 4 and 28-day controls, and linear regressions in the Scatchard plots that yield single straight lines indicative of binding to a single site.
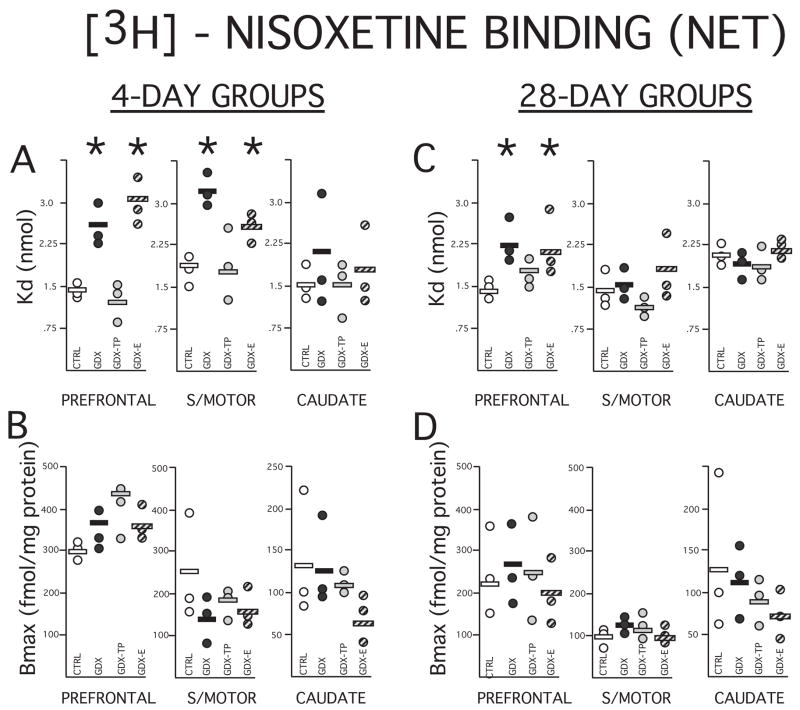
In both the 4- and 28-day sham-operated control groups, Kd values calculated for the three brain regions assessed were between 1.5 and 2 nmol (Figs. 5, 6 A,C). These values are comparable to those previously reported for rat brain homogenates (Tejani-Butt, 1992, Vathy et al., 1997, Shang et al., 1999). Across all of the 4 and 28-day treated groups, values of ligand binding affinity for the caudate nucleus were similar to corresponding controls. However, for cortical tissues, there were noticeable group differences in Kd value (Figs. 6A, C). Specifically, among the 4-day treatment groups, Kd values obtained from both the prefrontal and sensorimotor cortical tissue were consistently higher than control in the GDX and GDX-E but not the GDX-TP groups, and among the 28 day treatment groups, Kd values in the prefrontal cortex alone were higher than control in the GDX and GDX-E but not the GDX-TP rats (Figs. 6A, C). Statistical analyses confirmed that there were also significant main effects of hormone treatment on cortical Kd after 4 days of treatment for prefrontal and sensorimotor cortex (Kruskal -Wallis: sensorimotor cortex, H=7.82, p < 0.049; prefrontal cortex, H=8.95, p < 0.030), near significant main effects of hormone treatment on Kd for prefrontal cortex (Kruskal-Wallis, H=7.21, p< 0.065) and significant differences in the cortical Kd values of GDX and GDX-E compared to GDX-TP rats and controls in every case (Mann-Whitney, 4 day prefrontal cortex, 4 day sensorimotor cortex, 28 day prefrontal cortex: all U=0, p <0.0495, Figs. 6A, C).
Figure 6.
Scatter plots showing data (circles) and means (horizontal black lines) for dissociation constants (Kd) in nmol (A, C) and maximal numbers of binding sites (Bmax) in fmol/mg protein (B, D) calculated for the norepinephrine transporter (NET) using selective ligand 3H nisoxetine. The Kd and Bmax data collected from homogenate assays performed on the prefrontal cortex, sensorimotor cortex (S/MOTOR) and caudate nucleus tissue dissected from rats that were sham-operated (CRTL, open circles), gonadectomized (GDX, black circles) and gonadectomized and supplemented with testosterone propionate (GDX-TP, light gray circles) or estradiol (GDX-E, dark gray circles) for 4 (left) or 28 days (right) are shown separately. As for the DAT binding, measures of the fairly sparse maximal numbers of NET binding sites (Bmax) were more variable than Kd values, and in all tissues were largely overlapping and not significantly different across any groups. Among the less variable measures of Kd, there were no significant main effects of hormone treatment or group differences seen for values from the caudate nucleus. However, among the 4-day treatment groups, Kd values for the prefrontal and sensorimotor cortex of the GDX and GDX-E groups were significantly higher (*) than those of the control and GDX-TP groups, and at 28 days, Kd values for the prefrontal of the GDX and GDX-E groups were significantly higher than those of the control and GDX-TP cohorts.
Similar to that seen for DAT assays, Bmax values for NET binding were more variable than measures of Kd in all tissues and in both control and experimental groups (Fig. 6). Nonetheless, as expected maximal numbers of binding sites in the controls were higher in prefrontal cortex (~135–300 fm/mg protein) than in sensorimotor cortex (~150–250 fmol/mg protein) or caudate nucleus (~100–135 fmol/mg protein, Figs. 5, 6B, D) and showed values that on average were comparable to those previously reported for rat brain homogenates (Tejani-Butt, 1992, Vathy et al., 1997, Shang et al., 1999). Visual inspection of the data further showed that for the control and experimental groups alike variance in the data was typically accounted for by single outliers in the data, that for the most part the tissue-specific values that were obtained across these groups were largely overlapping and that there were no obvious group trends in these data (Figs. 6B, D). Quantitative analyses were consistent with these observations, showing that corresponding tissue specific measures of Bmax had roughly similar group means and that there were no significant effects of hormone treatment (Kruskal-Wallis) or group differences (Mann-Whitney U) among these measures for any treatment group or tissue (Figs. 6B, D).
DISCUSSION
Information from microdialysis (Finlay and Zigmond, 1997, Watanabe et al., 1997, Feenstra et al., 2000), voltammetry, electrophysiology (Lavin et al., 2005) and computational models (Compte et al., 2000, Durstewitz et al., 2000, Durstewitz and Seamans, 2002) all indicate that DA’s contributions to prefrontal cortical function include a long-term biasing of local cortical networks. Such protracted actions are consistent with DA’s uniquely extended extracellular lifetime in the prefrontal cortex (Garris et al., 1993, Garris and Wightman, 1994, Cass and Gerhardt, 1995) which is in part a consequence of the cortical paucity of the co-utilized DAT and NET (Carboni et al., 1990, Elsworth et al., 1993, Sesack et al., 1998a, Sesack et al., 1998b, Yamamoto and Novotney, 1998, Moron et al., 2002, Miner et al., 2003, Valentini et al., 2004, Carboni et al., 2006). The present studies paired methods of in vitro binding with classical hormone manipulation paradigms to examine the gonadal hormone sensitivity of the maximal numbers and affinities of ligand binding sites for these two transporters in selected cortical and subcortical DA-enriched regions of adult male rats. Previous findings in this same animal model demonstrating selective androgen-driven stimulatory effects of gonadectomy on cortical DA concentration DA innervation density (Battaner et al., 1987, Kritzer et al., 1999, Kritzer, 2000, Kritzer, 2003, Kritzer et al., 2007) and, according to preliminary data, on extracellular prefrontal DA levels as well (Aubele et al., 2008) suggest that the adverse behavioral effects of gonadectomy may be related to its ability to abnormally increase prefrontal cortical DA tone. Although this could also be achieved via actions on DA release, metabolism or catabolism, this study explored the possibility that gonadectomy modulates prefrontal DA levels by diminishing the capacity and/or efficiency of DA transport in an androgen-sensitive, estrogen-insensitive manner. The results that were obtained from the present binding studies are in keeping with this scenario. Specifically, while no obvious or statistically significant effects of long or short tem gonadectomy on DAT binding were found, both 4- and 28-day gonadectomy did significantly increase Kd values for 3H nisoxetine binding in cortical but not subcortical sites in an androgen-sensitive, estrogen-insensitive manner. Further, the regional and temporal selectivity of these effects were found to mirror those previously described for the effects of 4 and 28 day gonadectomy on DA axon density in that they impacted both sensorimotor and prefrontal cortex in the 4 day groups, and were selective for prefrontal cortex by 28 days of treatment (Kritzer et al., 1999, Kritzer, 2000). Although studies such as those indicating that the effects of mutations on the DAT can independently influence Kd for synthetic versus endogenous ligands, e.g, DA (Schmitt et al., 2008) indicate that caution is needed in interpreting both the positive and negative findings presented here insofar as DA itself may be concerned, given that DA clearance in the prefrontal cortex is known to utilize the NET to a significant degree, the present findings suggest that impact on the NET may part of the emerging story of androgen modulation of the function and physiology of mesocortical/mesoprefrontal DA systems in males. This possibility is considered further below, following separate comparisons of the present findings to those from previous studies of gonadal hormone regulation of DAT and NET binding.
Gonadal Hormone-Sensitivity of the DAT: Comparison to Previous Studies
Unlike the mesocortical system, both the mesolimbic and mesostriatal DA systems rely heavily on high-affinity transmitter reuptake via the DAT to control extracellular DA levels (Garris and Wightman, 1994). The fact that drugs of abuse such as cocaine and methamphetamine block DA reuptake via the DAT (Ritz et al., 1987, Moghaddam and Bunney, 1989, Kuhar et al., 1991, Giros et al., 1996) coupled with the sex differences that have been identified in humans and animal models in addictive behaviors, other responses associated with these drugs (Becker et al., 2001, Chen and Kandel, 2002, Festa and Quinones-Jenab, 2004) and in DAT function (Morissette et al., 1990, Morissette and Di Paolo, 1993a, Morissette and Di Paolo, 1993b, Rivest et al., 1995, Lavalaye et al., 2000, Walker et al., 2000, Mozley et al., 2001, Staley et al., 2001, Bhatt and Dluzen, 2005) have focused significant attention on the hormone sensitivity of this transporter moiety. However, to date the typically greater sensitivity/susceptibility of females to cocaine administration (Kosten et al., 1993, Chen and Kandel, 2002, Chin et al., 2002, Chen et al., 2003) and the protective effects of estrogen on nigrostriatal DA systems in, for example, Parkinson’s disease (Horstink et al., 2003, Haaxma et al., 2007) and conditions of DA-selective neurotoxin challenge (Dluzen and Horstink, 2003) has led to a body of work that is concentrated mainly on hormone sensitivity of the DAT in female subjects and in subcortical, primarily striatal structures. This literature describes complex and in some cases contradictory effects of estrogen on DAT binding site number (Morissette et al., 1990, Morissette and Di Paolo, 1993a, Morissette and Di Paolo, 1993b, Attali et al., 1997, Bosse et al., 1997, Le Saux and Di Paolo, 2006, McArthur et al., 2007) and/or affinity (Disshon et al., 1998) and also identifies sex differences favoring females in striatal DAT density and activity (Morissette and Di Paolo, 1993b, Rivest et al., 1995, Walker et al., 2000, Bhatt and Dluzen, 2005). However in only two of these studies were examinations of the effects of hormone manipulations in males included. As in the present study, neither found significant differences in Bmax or Kd values for the striatal DAT in intact compared to gonadectomized or hormone-replaced adult male rats (Attali et al., 1997, Chen et al., 2003). To our knowledge, there have also been no prior studies of sex differences or gonadal hormone impact on DAT binding in the cerebral cortex. The present analyses of 3H WIN35,428 binding in sensorimotor and prefrontal cortex, which may thus be a first, revealed no significant effects of long-or short-term gonadectomy or hormone replacement on cortical DAT binding site number or affinity. Although our data cannot exclude the possibility for effects of gonadectomy on DA’s affinity for the DAT nor of subtle effects on Bmax, that our negative findings for striatal WIN 35,428 binding were corroborated in two studies in males, one of which used a different ligand (3H-BTCP) and the other which used DA itself, makes these possibilities less likely. This in turn lends support to conclusions of essentially negative findings in the cerebral cortex as well. Thus, it appears that hormone modulation of prefrontal DA levels in adult male rats may not be a consequence of gonadal steroid modulation of the DAT. However, as discussed below, because the prefrontal cortex engages transporter species in addition to the DAT to mediate extracellular DA clearance, the process of receptor-mediated DA transport remains a potentially relevant target of hormone stimulation.
Gonadal Hormone-Sensitivity of the NET: Comparison to Previous Studies
Studies using knockout mice and/or pharmacological transporter blockade have shown that in the prefrontal cortex, extracellular DA clearance is controlled to an equal if not greater extent by the NET than the DAT (Carboni et al., 1990, Yamamoto and Novotney, 1998, Moron et al., 2002, Valentini et al., 2004, Carboni et al., 2006). In terms of contributing to the effects of gonadectomy on cortical DA level, the NET may also be the more likely candidate than the DAT, as previous studies have also shown that this site may be hormone-sensitive. For example, maximal numbers of frontal cortical 3H-nisoexteine binding sites have been shown to be significantly lower in male than in female rats (Vathy et al., 1997), and Kd and Bmax values for 3H-nisoexteine binding have been shown to be significantly higher in the cortex of adult male rats that have been gonadectomized for two weeks compared to intact controls (Shang et al., 1999). Although in this study no obvious effects on maximal numbers of NET binding sites were found, consistent results were obtained regarding the ability of gonadectomy to significantly raise cortical Kd values for 3H nisotexine that confirmed and extended previous findings in several ways. First, the effects of long and short-term gonadectomy were found to be selective for cortical compared to subcortical sites. Further, differential effects of long versus short term gonadectomy were found in which Kd values were elevated in both sensorimotor and prefrontal cortex following 4 days of treatment and in the prefrontal cortex alone 28 days after gonadectomy. And finally, the effects of gonadectomy were shown to be attenuated by supplementing gonadectomized rats with testosterone propionate but not estradiol. These latter findings provide a previously lacking definitive link between gonadal steroids and NET regulation, and also identify androgens in particular as active species in males. Although not directly examined in these studies, the protracted time courses over which the effects of gonadectomy on cortical NET sites took place makes it likely that underlying mechanisms of hormone regulation are, at least in part, genomic in nature and mediated by intracellular androgen receptors. This scenario fits well with anatomical data showing that in the locus coeruleus, which supplies the cerebral cortex with virtually all of its NET-bearing axons (Miner et al., 2003), 50–85% of constituent norepinephrine (NE) neurons concentrate the androgen-selective ligand 3H dihydrotestosterone (Heritage et al., 1980, Heritage et al., 1981). While much of the work exploring the underpinnings of gonadal hormone influence over the function and physiology of mesocortical DA systems has logically focused on midbrain DA neurons, these data sum to suggest that the cortically projecting NE neurons of the locus coeruleus should be similarly investigated.
Gonadal Hormone Regulation of Cortical NET Binding: Possible Roles in Regulating Prefrontal Cortical DA Function and Tone
Although currently more extensively studied in females (Gibbs and Gabor, 2003, Luine et al., 2003, Korol, 2004), there is growing evidence from clinical studies and animal models that gonadal and especially androgen hormones also influence DA-dependent prefrontal function in males (Christiansen and Knussmann, 1987, Gouchie and Kimura, 1991, Ceccarelli et al., 2001, Kritzer et al., 2001, Daniel et al., 2003, Janowsky, 2006, Thilers et al., 2006, Aubele et al., 2008). While there are likely to be multiple physiological endpoints that are relevant to these actions, recent evidence showing that the effects of gonadectomy on DA-dependent prefrontal constructs of spatial working memory and behavioral flexibility are correlated with gonadectomy-induced increases in prefrontal DA innervation density (Kritzer et al., 2007) combines with independent evidence that gonadectomy also increases cortical DA concentrations (Battaner et al., 1987) and preliminary data showing that gonadectomy increases prefrontal DA overflow in vivo (Aubele et al., 2008) to suggest that actions of gonadal steroids on prefrontal DA levels may be especially important. A number of parallels have emerged in this study between the effects of gonadectomy and hormone replacement on cortical 3H nisoxetine binding and on previously identified physiological and functional measures of the mesoprefrontal DA system in adult male rats that suggest that influence over this transporter site could be a means by which the functionally important endpoint of prefrontal cortical DA level is hormonally regulated. First, like the effects of gonadectomy on behavioral and biochemical DA measures, those exerted on cortical 3H nisoxetine binding were androgen sensitive and estrogen insensitive, which is somewhat uncommon even for the male brain where actions of testosterone often requires its enzymatic conversion to estrogenic metabolites (Simpson et al., 1994, Lephart, 1996). Further, exactly mirroring the regionally differential effects of long and short-term gonadectomy on cortical DA axon density (Kritzer et al., 1999, Kritzer, 2000), 4-days after gonadectomy effects on NET binding affinity were observed in both sensorimotor and prefrontal cortex, and 28 days after gonadectomy effects on Kd were found in the prefrontal cortex alone. That gonadectomy has also been shown to increase cortical concentrations of DA but not NE (Battaner et al., 1987) and alter cortical axon densities of DAergic but not NEergic fibers (Kritzer, 2003) may also be congruent with the two-fold decrease in NET ligand binding affinity that this manipulation brings, which to the extent that these data may reflect the situation for native ligands, could arguably have greater and perhaps even selective adverse impact on the uptake of DA compared to its preferred substrate, NE. Finally, it may be noteworthy that gonadectomy and androgen replacement coincidently increase prefrontal DA levels/innervation and lower cortical NET binding affinity. Because these actions run counter to the reciprocal, homeostatic relationships that normally drive compensatory changes in transporter efficiently and/or capacity in response to changes in substrate levels, they may be most parsimoniously explained by principal effects of gonadectomy on the NET and resultant changes in cortical DA levels. Although questions remain that can only be empirically addressed about transporter function, and while it remains unclear what dictates the temporal and regional selectivity of the effects that have been observed, the data collected do suggest a specific and novel working hypothesis; that androgen-sensitive, gonadectomy-induced decreases in the affinity of cortical NET sites in adult male rats play a mechanistic role in elevating cortical and especially prefrontal cortical DA levels and in turn lead to the deficits in the DA-dependent prefrontal cortical functions that have been repeatedly seen in this animal model. In further exploring this previously unsuspected route of gonadal hormone and more specifically androgen influence over DA levels and DA dependent prefrontal cortical functions in males it will be especially important to make direct assessments of transporter mediated DA uptake. Such continued investigation along these lines should provide insights into neurobiological basis for the sex differences and hormone-sensitivity that are well known to characterize the development (Diamond, 1985, Clark and Goldman-Rakic, 1989, Bachevalier and Hagger, 1991, Overman, 2004) and adult functioning (Einon, 1980, Tees et al., 1981, van Haaren et al., 1990) of normal prefrontal cortical operations in humans and animal models. In view of recent evidence that also links the NET to the pathophysiology in schizophrenia and ADHD (Stober et al., 1996, Rybakowski et al., 2006, Kim et al., 2008, Retz et al., 2008) it is also possible that investigation of the hormone sensitivity of the NET and its role in modulating cortical DA biochemistry and behavior may also help explain the greater vulnerability of the mesocortical DA systems and DA-dependent prefrontal processes at risk in these disorders in males (Goodman and Stevenson, 1989, Seeman and Lang, 1990, Leviton et al., 1993).
Acknowledgments
This work supported by RO1- NS41966 to MFK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, Abbasi SH. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84:405–410. doi: 10.1016/j.schres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Attali G, Weizman A, Gil-Ad I, Rehavi M. Opposite modulatory effects of ovarian hormones on rat brain dopamine and serotonin transporters. Brain Res. 1997;756:153–159. doi: 10.1016/s0006-8993(97)00136-4. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54:244–252. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Meyers B, Liu A, Kritzer MF. Gonadectomy in adult male rats increases extracellular dopamine levels in the prefrontal cortex: Possible causative role for effects on the norepinephrin transporter, but not the dopamine transporter or the enzyme catechol-o-methyltransferase. Abstract, Soc. for Neuroscience Annual Meeting.2008. [Google Scholar]
- Bachevalier J, Hagger C. Sex differences in the development of learning abilities in primates. Psychoneuroendocrinology. 1991;16:177–188. doi: 10.1016/0306-4530(91)90077-7. [DOI] [PubMed] [Google Scholar]
- Battaner E, Rodriguez del Castillo A, Guerra M, Mas M. Gonadal influences on spinal cord and brain monoamines in male rats. Brain Res. 1987;425:391–394. doi: 10.1016/0006-8993(87)90528-2. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Bhatt SD, Dluzen DE. Dopamine transporter function differences between male and female CD-1 mice. Brain Res. 2005;1035:188–195. doi: 10.1016/j.brainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Bosse R, Rivest R, Di Paolo T. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Brain Res Mol Brain Res. 1997;46:343–346. doi: 10.1016/s0169-328x(97)00082-x. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Vacca C, Di Chiara G. Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J Neurochem. 2006;96:473–481. doi: 10.1111/j.1471-4159.2005.03556.x. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: comparison with dorsal striatum and nucleus accumbens. J Neurochem. 1995;65:201–207. doi: 10.1046/j.1471-4159.1995.65010201.x. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Scaramuzzino A, Aloisi AM. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav Brain Res. 2001;123:65–76. doi: 10.1016/s0166-4328(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Chen R, Osterhaus G, McKerchar T, Fowler SC. The role of exogenous testosterone in cocaine-induced behavioral sensitization and plasmalemmal or vesicular dopamine uptake in castrated rats. Neurosci Lett. 2003;351:161–164. doi: 10.1016/j.neulet.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quinones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945:123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Clark AS, Goldman-Rakic PS. Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behav Neurosci. 1989;103:1287–1295. doi: 10.1037//0735-7044.103.6.1287. [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder-not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48:962–965. doi: 10.1017/S0012162206002118. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Shaw SH, Crow TJ, Shields G, Smith AB, Larach VW, Wellman N, Loftus J, Nanthakumar B, Razi K, Stewart J, Comazzi M, Vita A, Heffner T, Sherrington R. A genome-wide scan for linkage to chromosomal regions in 382 sibling pairs with schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:803–812. doi: 10.1176/appi.ajp.159.5.803. [DOI] [PubMed] [Google Scholar]
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Dev. 1985;56:868–883. [PubMed] [Google Scholar]
- Disshon KA, Boja JW, Dluzen DE. Inhibition of striatal dopamine transporter activity by 17beta-estradiol. Eur J Pharmacol. 1998;345:207–211. doi: 10.1016/s0014-2999(98)00008-9. [DOI] [PubMed] [Google Scholar]
- Dluzen D, Horstink M. Estrogen as neuroprotectant of nigrostriatal dopaminergic system: laboratory and clinical studies. Endocrine. 2003;21:67–75. doi: 10.1385/endo:21:1:67. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15:561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Einon D. Spatial memory and response strategies in rats: age, sex and rearing differences in performance. Q J Exp Psychol. 1980;32:473–489. doi: 10.1080/14640748008401840. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Berger P, Roth RH. Cocaine-sensitive and -insensitive dopamine uptake in prefrontal cortex, nucleus accumbens and striatum. Neurochem Int. 1993;23:61–69. doi: 10.1016/0197-0186(93)90144-t. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, Mastenbroek S. Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience. 2000;100:741–748. doi: 10.1016/s0306-4522(00)00319-5. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ. The effects of stress on central dopaminergic neurons: possible clinical implications. Neurochem Res. 1997;22:1387–1394. doi: 10.1023/a:1022075324164. [DOI] [PubMed] [Google Scholar]
- Garris PA, Collins LB, Jones SR, Wightman RM. Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem. 1993;61:637–647. doi: 10.1111/j.1471-4159.1993.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–643. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335; discussion 335–326. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Goodman R, Stevenson J. A twin study of hyperactivity--I. An examination of hyperactivity scores and categories derived from Rutter teacher and parent questionnaires. J Child Psychol Psychiatry. 1989;30:671–689. doi: 10.1111/j.1469-7610.1989.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Goyal RO, Sagar R, Ammini AC, Khurana ML, Alias AG. Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann N Y Acad Sci. 2004;1032:291–294. doi: 10.1196/annals.1314.042. [DOI] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- Heritage AS, Stumpf WE, Sar M, Grant LD. (3-H)-dihydrotestosterone in catecholamine neurons of rat brain stem: combined localization by autoradiography and formaldehyde-induced fluorescence. J Comp Neurol. 1981;200:289–307. doi: 10.1002/cne.902000208. [DOI] [PubMed] [Google Scholar]
- Horstink MW, Strijks E, Dluzen DE. Estrogen and Parkinson’s disease. Adv Neurol. 2003;91:107–114. [PubMed] [Google Scholar]
- Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC. Dopamine transporter change in drug-naive schizophrenia: an imaging study with 99mTc-TRODAT-1. Schizophr Res. 2003;65:39–46. doi: 10.1016/s0920-9964(03)00006-9. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10:77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, de Bruin JP, Matthijssen MA, Uylings HB. Ontogeny of open field activity in rats after neonatal lesioning of the mesocortical dopaminergic projection. Behav Brain Res. 1989;32:115–127. doi: 10.1016/s0166-4328(89)80079-8. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Terry P. Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology (Berl) 2000;148:90–98. doi: 10.1007/s002130050029. [DOI] [PubMed] [Google Scholar]
- Kessler J, Markowitsch HJ. Delayed-alternation performance after kainic acid lesions of the thalamic mediodorsal nucleus and the ventral tegmental area in the rat. Behav Brain Res. 1981;3:125–130. doi: 10.1016/0166-4328(81)90033-4. [DOI] [PubMed] [Google Scholar]
- Kim CH, Waldman ID, Blakely RD, Kim KS. Functional gene variation in the human norepinephrine transporter: association with attention deficit hyperactivity disorder. Ann N Y Acad Sci. 2008;1129:256–260. doi: 10.1196/annals.1417.023. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. J Comp Neurol. 2000;427:617–633. [PubMed] [Google Scholar]
- Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase- but not dopamine-beta-hydroxylase-, choline acetyltransferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cereb Cortex. 2003;13:282–296. doi: 10.1093/cercor/13.3.282. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Marotta J, Smirlis T. Regionally selective effects of gonadectomy on cortical catecholamine innervation in adult male rats are most disruptive to afferents in prefrontal cortex. Cereb Cortex. 1999;9:507–518. doi: 10.1093/cercor/9.5.507. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Syvalahti E, Hietala J. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res. 2001;52:115–120. doi: 10.1016/s0920-9964(00)00095-5. [DOI] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–869. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25:5013–5023. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Influence of oestrogenic compounds on monoamine transporters in rat striatum. J Neuroendocrinol. 2006;18:25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- Leviton A, Bellinger D, Allred E. The Boston Teacher Questionnaire. 3. A reassessment. J Child Neurol. 1993;8:64–72. doi: 10.1177/088307389300800109. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem. 2007;100:678–692. doi: 10.1111/j.1471-4159.2006.04226.x. [DOI] [PubMed] [Google Scholar]
- Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse. 1989;4:156–161. doi: 10.1002/syn.890040209. [DOI] [PubMed] [Google Scholar]
- Morissette M, Biron D, Di Paolo T. Effect of estradiol and progesterone on rat striatal dopamine uptake sites. Brain Res Bull. 1990;25:419–422. doi: 10.1016/0361-9230(90)90231-n. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem. 1993a;60:1876–1883. doi: 10.1111/j.1471-4159.1993.tb13415.x. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993b;58:16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Elsworth JD. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull. 2000;52:519–523. doi: 10.1016/s0361-9230(00)00290-2. [DOI] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cogn. 2004;55:134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Retz W, Rosler M, Kissling C, Wiemann S, Hunnerkopf R, Coogan A, Thome J, Freitag C. Norepinephrine transporter and catecholamine-O-methyltransferase gene variants and attention-deficit/hyperactivity disorder symptoms in adults. J Neural Transm. 2008;115:323–329. doi: 10.1007/s00702-007-0822-5. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter: gender differences and effect of chronic haloperidol. Brain Res. 1995;692:269–272. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Dmitrzak-Weglarz M, Skibinska M, Kapelski P, Hauser J. Performance on the Wisconsin Card Sorting Test in schizophrenia and genes of dopaminergic inactivation (COMT, DAT, NET) Psychiatry Res. 2006;143:13–19. doi: 10.1016/j.psychres.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith ME. Interaction of Cocaine-, Benztropine-, and Gbr12909-Like Compounds with Wildtype and Mutant Human Dopamine Transporters: Molecular Features That Differentially Determine Antagonist Binding Properties. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv Pharmacol. 1998a;42:171–174. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998b;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Boja JW, Dluzen DE. Castration differentially alters [3H]nisoxetine binding to norepinephrine uptake sites in olfactory bulb and frontal cortex of male rats. Synapse. 1999;31:250–255. doi: 10.1002/(SICI)1098-2396(19990315)31:4<250::AID-SYN2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Cohen DJ, Bowers MB., Jr CSF monoamine metabolites in children with minimal brain dysfunction: evidence for alteration of brain dopamine. A preliminary report. J Pediatr. 1977;90:67–71. doi: 10.1016/s0022-3476(77)80766-x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Suzuki Y, Higuchi T. Correlation of plasma neurosteroid levels to the severity of negative symptoms in male patients with schizophrenia. Schizophr Res. 2002;58:69–74. doi: 10.1016/s0920-9964(01)00367-x. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Stam CJ, de Bruin JP, van Haelst AM, van der Gugten J, Kalsbeek A. Influence of the mesocortical dopaminergic system on activity, food hoarding, social-agonistic behavior, and spatial delayed alternation in male rats. Behav Neurosci. 1989;103:24–35. doi: 10.1037//0735-7044.103.1.24. [DOI] [PubMed] [Google Scholar]
- Stober G, Nothen MM, Porzgen P, Bruss M, Bonisch H, Knapp M, Beckmann H, Propping P. Systematic search for variation in the human norepinephrine transporter gene: identification of five naturally occurring missense mutations and study of association with major psychiatric disorders. Am J Med Genet. 1996;67:523–532. doi: 10.1002/(SICI)1096-8628(19961122)67:6<523::AID-AJMG3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Taherianfard M, Shariaty M. Evaluation of serum steroid hormones in schizophrenic patients. Indian J Med Sci. 2004;58:3–9. [PubMed] [Google Scholar]
- Tassin JP, Stinus L, Simon H, Blanc G, Thierry AM, Le Moal M, Cardo B, Glowinski J. Relationship between the locomotor hyperactivity induced by A10 lesions and the destruction of the fronto-cortical dopaminergic innervation in the rat. Brain Res. 1978;141:267–281. doi: 10.1016/0006-8993(78)90197-x. [DOI] [PubMed] [Google Scholar]
- Tees RC, Midgley G, Nesbit JC. The effect of early visual experience on spatial maze learning in rats. Dev Psychobiol. 1981;14:425–438. doi: 10.1002/dev.420140505. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM. [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther. 1992;260:427–436. [PubMed] [Google Scholar]
- Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Turvin JC, Messer WS, Jr, Kritzer MF. On again, off again effects of gonadectomy on the acoustic startle reflex in adult male rats. Physiol Behav. 2007;90:473–482. doi: 10.1016/j.physbeh.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini V, Frau R, Di Chiara G. Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J Neurochem. 2004;88:917–927. doi: 10.1046/j.1471-4159.2003.02238.x. [DOI] [PubMed] [Google Scholar]
- van Haaren F, van Hest A, Heinsbroek RP. Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neurosci Biobehav Rev. 1990;14:23–33. doi: 10.1016/s0149-7634(05)80157-5. [DOI] [PubMed] [Google Scholar]
- Vathy I, Sokol J, Etgen AM. Gender-related differences exist in cortical [3H]nisoxetine binding and are not affected by prenatal morphine exposure. Neuroscience. 1997;76:331–334. doi: 10.1016/s0306-4522(96)00447-2. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem. 1998;71:274–280. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]