Abstract
There is a need to evaluate oral glucose-lowering agents not only for their value in achieving glycemic control but also for their impact on cardiac risk factor modification. This article reviews the evidence base for the two thiazolinediones currently available, pioglitazone and rosiglitazone. These drugs exert their effects through actions affecting metabolic control, lipid profiles, and the vascular wall. They have been shown to be as efficacious in establishing glycemic control, in both monotherapy and combination therapy regimens, as more traditional oral agents, and may be able to sustain that control in the long term. Both thiazolidinediones have demonstrated favorable effects on markers of cardiovascular disease. Evidence from the large PROactive outcomes study suggests that pioglitazone may exert protective effects in patients with type 2 diabetes and macrovascular disease. Thiazolidinediones are generally well tolerated but they can cause weight gain, induce fluid retention, and may contribute to bone loss in postmenopausal women. The place of thiazolidinediones in the management of type 2 diabetes is well established. The potential for additional benefits in reducing macrovascular risk encourages further long-term study of these agents.
Keywords: cardiovascular disease, pioglitazone, PPAR-gamma, rosiglitazone, thiazolidinediones, type 2 diabetes
Introduction
The thiazolidinediones (TZDs, or glitazones) class, which currently includes rosiglitazone and pioglitazone, are effective and frequently prescribed treatments for type 2 diabetes that complement existing treatment approaches and form an important part of treatment algorithms. In the decade since their introduction, the prevalence of obesity, diabetes, and the metabolic syndrome has increased exponentially.1–3 Diabetes is also closely associated with cardiovascular disease – myocardial infarction and stroke are the major causes of premature death in people with diabetes, and type 2 diabetes is considered an independent risk equivalent for developing another vascular event.4 The increasing prevalence of diabetes will therefore be closely followed by increases in cardiovascular-related morbidity and mortality. However, diabetes and cardiovascular disease develop only over time, providing a window of opportunity for interventions to prevent both diseases and/or delay their progression. As the use of glucose-lowering agents continues to increase and new agents appear, there is a growing need to evaluate products not only on the basis of their use in achieving glycemic control, but also in the context of their effect on global cardio-metabolic risk factor modification.
The TZDs are a unique class of oral glucose-lowering agents that work primarily by activating the nuclear transcription factor peroxisome proliferator-activated receptor gamma (PPAR-γ), thereby turning on and off specific genes for the regulation of glucose, lipids and protein metabolism. There is now considerable research to suggest, that beyond reducing insulin resistance and providing durable glycemic control, the TZDs exert a number of pleiotropic effects that may play an important role in the treatment of type 2 diabetes mellitus.
Documented evidence for the benefits of pioglitazone on cardiovascular outcomes in patients with type 2 diabetes has been provided by the results of the Prospective Pioglit-Azone Clinical Trial In Macrovascular Events (PROactive).5 However, there has been recent debate about the possible differences between the two TZDs in terms of cardiovascular disease outcome.6 In this context, the complex, nonoverlapping mechanisms of action and impact on metabolic parameters such as lipid profiles of pioglitazone and rosiglitazone may be relevant, making it unwise to extrapolate these results to other drugs in the class.
It therefore seems timely to review TZDs and their place in the management of type 2 diabetes. This review will focus on what is known about TZDs as a class and the current clinical evidence base regarding the efficacy and safety of individual agents. We consider the contemporary literature on TZDs, highlighting these agents’ multiple metabolic effects and summarizing the data relating to their clinical effectiveness in the management of type 2 diabetes (in terms of both glucose control and clinical outcomes) when used as monotherapy or in combination with other glucose-lowering agents.
Type 2 diabetes – complex pathogenesis
Type 2 diabetes is a complex disorder. Hyperglycemia is the core metabolic defect and combines with a range of metabolic risk factors to impart high risk for cardiovascular events. Insulin resistance and β-cell dysfunction both play important roles in the development and progression of type 2 diabetes.7,8 Evidence has shown, that while insulin resistance lays the groundwork for glucose intolerance, the progression to type 2 diabetes does not occur until a degree of β-cell dysfunction has taken place, allowing blood glucose levels to rise.9,10 Both defects remain closely linked with the progression of the disease – declining β-cell function is associated with deteriorating glycemic control11,12 and insulin resistance is associated with numerous risk factors for cardiovascular disease.13–15
TZDs – rationale for a role in the management of diabetes
TZDs bind to the ligand-activated transcription factor PPAR-γ.16 Members of the PPAR family (PPAR-α, -γ and -δ) play a pivotal role in the regulation of lipid metabolism and homeostasis and are important indirect as well as direct regulators of cellular insulin sensitivity. However, PPAR subtypes appear to have highly specialized functions when acting on endogenous genes.17,18 Thus, PPAR-α primarily activates genes encoding proteins involved in fatty acid oxidation. PPAR-δ is ubiquitously expressed in various tissues and is one of the key regulators of energy homeostasis in skeletal muscle. PPAR-γ is expressed predominantly in adipose tissue and skeletal muscle and is involved in the regulation of adipocyte proliferation and differentiation, as well as lipid storage.19 This is achieved by an increase in the number of insulin-sensitive small adipocytes, which leads to a transfer of fat distribution from visceral to subcutaneous depots. The effect of PPAR-γ activation is to enhance the action of insulin in insulin-sensitive tissue by increasing glucose uptake in skeletal muscle and adipose tissue and decreasing hepatic glucose production.
While improvements in metabolic control and lipid profiles have important effects on cardiovascular disease in patients with diabetes, PPAR-γ agonists also have a range of independent actions on the vascular wall, which impacts on atherogenesis.20,21 PPAR-γ is expressed in vascular and inflammatory cells, where it interacts with several processes involved in the development and progression of atherosclerosis, particularly with respect to macrophage foam cell formation and the transcriptional regulation of genes mediating the inflammatory response. In preclinical studies, activation of PPAR-γ by TZDs acts on a number of pathogenic pathways implicated in the development of atherosclerosis, including inflammation, oxidative stress, metalloproteinase activity, advanced glycation end product accumulation and activation of the renin–angiotensin system.22 These actions manifest as reduced lipid deposition in vessels.23
TZDs may also enhance β-cell function, which has potential implications for maintaining long-term glycemic control in type 2 diabetes.
Altering the natural history of type 2 diabetes – TZDs and durable glycemic control
Type 2 diabetes is a progressive condition characterized by a combination of two fundamental defects: insulin resistance and impaired β-cell function. Insulin resistance in the liver, muscle, and adipose tissue leads to decreased glucose uptake in peripheral tissues, increased hepatic glucose production, and increased lipolysis. Once established, insulin resistance remains fairly constant throughout the natural course of type 2 diabetes; whereas declining β-cell function appears to be the critical factor in the disease’s progression. Early in the natural history of this disease, before the emergence of marked hyperglycemia, increased insulin secretion partially compensates for insulin resistance. Eventually, however, as β-cell function deteriorates, insulin secretion can no longer overcome the metabolic burden posed by insulin resistance, and hyperglycemia results. The presence of dual pathophysiological defects suggests that optimal glucose-lowering therapy for patients with type 2 diabetes should address both sources of metabolic dysregulation.
The primary goal of patient management in diabetes is to achieve and maintain glycemic control. Initially, mono-therapy with oral glucose-lowering agents may be effective, but because diabetes is a progressive disease there is a continual need to reassess and intensify therapy (either through dose increases or additional therapies) in order to maintain glycemic control over the longer term.24,25
Monotherapy
A number of trials have reported the use of TZDs as mono-therapy and have shown significant reductions in the level of glycated hemoglobin (HbA1c) compared with placebo. In randomized, double-blind, placebo-controlled studies with pioglitazone, mean HbA1c reductions ranged from 0.8% (at a 30 mg dose) to 1.6% (with 45 mg).26–28 Similar studies using rosiglitazone showed mean HbA1c reductions ranging from 0.9% (2 mg twice daily) to 1.5% (4 mg twice daily).29,30 A meta-analysis of 23 randomized, placebo-controlled trials comparing monotherapy with pioglitazone or rosiglitazone for 12 to 26 weeks found that each drug similarly reduced HbA1c levels more than placebo, by 1.0% to 1.5%.31
The TZDs have also been shown to be at least as effective as traditional oral glucose-lowering agents (metformin and sulfonylureas) in achieving and maintaining good glycemic control. A comparison of pioglitazone (45 mg) and gliclazide demonstrated an HbA1c reduction of 1.4% for both drugs after 52 weeks of treatment.32 In another study, the effects of pioglitazone and gliclazide were compared for 2 years in 567 patients.33 In patients who had received pioglitazone, the target HbA1c was reached more often (47.8%) than in the patients who had received gliclazide (37.0%).
A comparison of 45 drug-naïve patients randomized to treatment with rosiglitazone (4 mg twice daily), metformin, or placebo showed that both treatments significantly reduced HbA1c after 26 weeks in comparison with placebo.34 Several randomized studies have compared pioglitazone and metformin and showed a comparable reduction in HbA1c.35–39
While a number of studies comparing TZDs with sulfonylureas or metformin have demonstrated similar decreases in HbA1c after 1 year of treatment, the TZDs appear better able to sustain glycemic control in the long term. The A Diabetes Outcome Progression Trial (ADOPT) investigated the durability of the antihyperglycemic effects of rosiglitazone, metformin, and glyburide (or glibencamide [UK]) in 4360 drug-naive patients.40 The 4-year trial found that in the long-term rosiglitazone-treated patients experienced significantly greater durability in terms of reduction of both HbA1c and fasting plasma glucose levels; albeit, the absolute differences in glycemic control between the rosiglitazone and metformin groups were small.
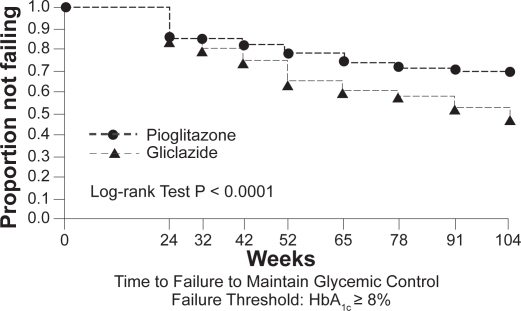
Pioglitazone has also demonstrated sustained glycemic control. Data from a 2-year extension study, in which patients were initially randomized to pioglitazone or gliclazide, have shown that the proportion of patients in the pioglitazone group who maintained HbA1c < 8% at any time during the second year of treatment was higher than that in the gliclazide group (Figure 1).41,33 This evidence for durability of effect on blood glucose control with the TZDs has been recognized in the recently updated NICE guidelines.42 Declining β-cell function is the predominant reason for deterioration in glucose tolerance. Both rosiglitazone and pioglitazone have been shown to slow the rate of loss of β-cell function and improve insulin sensitivity to a greater extent than other currently used oral agents.5,40 These findings are consistent with a greater durability of glycemic control with the TZDs.
Figure 1.
Kaplan–Meier curves showing the proportion of patients in pioglitazone and gliclazide treatment groups not failing (HbA1c <8.0%) at various time points over 2 years. Copyright © 2005 American Diabetes Association. From Diabetes Care®, Vol. 28, 2005;544–550. Reprinted with permission from The American Diabetes Association.
Combination therapy with oral agents
Type 2 diabetes is a chronic disease with a progressive deterioration in glycemic control due to the continuing loss of β-cell function. Monotherapy for type 2 diabetes may therefore not be sufficient to maintain glycemic control over time. Subsequent therapeutic decisions are made principally on the basis of the HbA1c value – a goal of <6.5% is consistent with the most recent recommendations from NICE and the IDF.42,43
Early, aggressive control of glucose levels with combination therapy may be able to slow the decline in glycemic control, compared with monotherapy,44 and reduce the complications of diabetes. To meet the goal of achieving and maintaining glucose levels close to the nondiabetic range, current guidelines and treatment algorithms emphasize initial therapy with lifestyle intervention and metformin and then rapid addition of medications and transition to new regimens when target glycemic goals are not achieved or sustained.42,43,45
When selecting a therapeutic regimen, it is important to consider whether agents address the underlying pathophysiology. The sulfonylureas, metformin, and TZDs act at different sites in the body to improve insulin secretion or to improve insulin action. The sulfonylureas act on the β-cells in the pancreas to stimulate insulin secretion. Metformin is an insulin sensitizer with effects on the liver and muscle. It decreases hepatic glucose production by inhibiting both gluconeogenesis and glycogenolysis, and it also increases the uptake and utilization of glucose by muscle tissue. The TZDs also improve insulin sensitivity by increasing glucose uptake in adipose, liver, and skeletal muscle tissue.
The advantages of combination therapy are that drugs with complementary modes of action can target both the underlying insulin resistance and β-cell dysfunction. For example, although both the TZDs and metformin effectively increase sensitivity to insulin, they have different target organs – metformin exerting most of its glycemic effect by decreasing hepatic glucose production and the TZDs by enhancing insulin sensitivity primarily in muscle and adipose tissue. As a result the two agents have additive effects, and the addition of a TZD to metformin can lower HbA1c by up to 0.8%.46,47
The combination of a sulfonylurea and a TZD is also logical as these agents exert opposing effects on β-cell function. The sulfonylureas focus on stimulating β-cells to secrete more insulin. Over time, studies have shown that chronic exposure to a sulfonylurea can lead to acceleration of β-cell apoptosis, exhaustion, or desensitization.48 The TZDs may attenuate this effect. Although the exact manner in which TZDs achieve this is not entirely understood, possible mechanisms suggested by results from animal studies include direct or indirect reductions in lipotoxicity, prevention of decreases in β-cell mass via an effect on reducing apoptosis and reduced secretory demand, as well as a possible contribution from a reduction in glucotoxicity.49
To determine whether TZD-induced improvement in glycemic control is associated with improved β-cell function, 53 patients with type 2 diabetes were randomized to receive a TZD or placebo for 4 months.50 The study examined insulin secretion during an oral glucose tolerance test while simultaneously taking into account changes in insulin sensitivity. Following 4 months of pioglitazone or rosiglitazone treatment, β-cell glucose sensitivity, ie, the ability of the β-cell to respond to a given change in plasma glucose concentration, improved by ∼2- to 2.8-fold and remained unchanged in the placebo-treated groups.
Further evidence of the positive effects of pioglitazone and rosiglitazone therapy on β-cell function is available from a number of randomized, controlled trials using these agents as monotherapy or in combination with metformin or a sulfonylurea. Rosiglitazone has been shown to restore normal insulin secretion in individuals with impaired glucose tolerance51 and pioglitazone to reduce the development of diabetes in women of Latin American descent with a history of gestational diabetes by improving insulin sensitivity and preventing the progressive deterioration of β-cell function. 52 In the large, randomized ACT NOW study, pioglitazone (up to 45 mg/day) prevented the progression to diabetes in patients with impaired glucose tolerance by 81% compared with placebo at an average 2.6 years of follow-up.53 The rate of progression to diabetes (fasting plasma glucose ≥7.0 mmol/L or higher during follow-up) was 1.5% per year for pioglitazone compared with 6.8% per year for placebo (hazard ratio 0.19, p < 0.00001). Patients treated with pioglitazone were also more likely to return to normal glucose tolerance (42% versus 28% with placebo, p < 0.001). These benefits appeared to be due to a greater improvement in β-cell function with pioglitazone (as demonstrated using a variety of measures). In the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial, rosiglitazone 8 mg administered daily for 3 years significantly reduced progression to type 2 diabetes (by 60%) and allowed reversion to normoglycemia among a large proportion of adults with impaired fasting glucose, impaired glucose tolerance, or both.54 The ADOPT trial tested the hypothesis that rosiglitazone preserves β-cell function better than other drugs used as first-line therapy for type 2 diabetes, thus delaying or preventing deterioration in glycemic control. The results showed that initial treatment with rosiglitazone slowed the progression to monotherapy failure more effectively than either metformin or glyburide.40 In PROactive, patients randomized to pioglitazone had a reduced need to start taking insulin compared with those on placebo.5
A wealth of short- and long-term studies and literature reviews attest to the fact that the combined use of TZDs with agents such as metformin or sulfonylureas provides better glycemic control compared with further intensifying the metformin or sulfonylurea monotherapy.32,36,38,44,47,48,55–64 A TZD–metformin combination has a powerful effect on reducing insulin resistance and is effective in the early stages of type 2 diabetes when more endogenous insulin is still available. This combination is also associated with minimal hypoglycemia and less weight gain. A sulfonylurea–TZD combination offers the added benefit of lowered insulin resistance and potential improvement in β-cell function. The combination of pioglitazone or rosiglitazone with metformin or with a sulfonylurea has been shown to be an effective alternative to combined metformin and sulfonylurea.38,63,65–68
The observation that early introduction of oral combination therapy is more effective in achieving glycemic control than increasing doses of metformin or sulfonylurea monotherapy has prompted the introduction of fixed-dose single-tablet combinations of TZDs with metformin or a sulfonylurea.
The clinical evidence – impact of TZDs on cardiovascular risk factors and outcomes
Diabetic dyslipidemia, characterized by increased concentrations of triglycerides, reduced concentrations of high-density lipoprotein cholesterol (HDL-C), and increased concentrations of small dense low-density lipoprotein (LDL) particles is a major risk factor for cardiovascular disease. The TZDs appear to improve this atherogenic diabetes lipid profile, and a host of clinical studies have demonstrated improved lipid profiles with pioglitazone and to a lesser extent with rosiglitazone.26,28,39,66,69–72 A large meta-analysis evaluated the effects of rosiglitazone and pioglitazone therapies on diabetic dyslipidemia. Both TZDs significantly raised HDL-C levels.31 Compared with placebo, pioglitazone further improved the lipid profile, significantly lowering triglyceride levels and having a neutral effect on LDL-C and total cholesterol levels. In contrast, rosiglitazone was found to increase LDL-C and total cholesterol levels and to demonstrate a neutral effect on triglyceride levels. The results of the meta-analysis suggest that pioglitazone produces a more favorable lipid profile. More recent head-to-head comparisons of the two agents have confirmed these findings.73,74 In the study of Goldberg et al a total of 802 subjects were randomized to blinded treatment with maximal dose of either pioglitazone or rosiglitazone to determine the effect of these agents on fasting lipids in the setting of no other glucose or lipid-lowering therapy.73 The observed changes in lipid concentrations are shown in Table 1. A significant difference in favor of pioglitazone over rosiglitazone was noted for HDL-C, triglycerides, LDL particle size, and LDL particle concentration.73 Furthermore, in an open-label study, patients with type 2 diabetes demonstrated marked improvements in lipid profiles along with stable glycemic control after treatment conversion from rosiglitazone to pioglitazone while maintaining stable statin therapy.72 In addition, pioglitazone but not rosiglitazone therapy significantly increased LDL-C particle size to large, less-atherogenic particles.39,72,75–77
Table 1.
In the study of Goldberg et al a total of 802 subjects were randomized to blinded treatment with maximal dose of either pioglitazone or rosiglitazone to determine the effect of these agents on fasting lipids in the setting of no other glucose or lipid-lowering therapy. The observed changes in lipid concentrations from baseline are shown73
| Outcome measure | Pioglitazone (n = 363) | Rosiglitazone (n = 356) |
|---|---|---|
| Triglycerides, mmol/L | −0.59 ± 0.09 | 0.15 ± 0.09 |
| HDL-C, mmol/L | 0.13 ± 0.01 | 0.06 ± 0.01 |
| Non-HDL, mmol/L | 0.09 ± 0.05 | 0.67 ± 0.05 |
| LDL, mmol/L | 0.32 ± 0.04 | 0.55 ± 0.04 |
| TC, mmol/L | 0.23 ± 0.05 | 0.73 ± 0.05 |
| TC-to-HDL ratio | −0.3 ± 0.1 | 0.7 ± 0.1 |
| Apolipoprotein B, g/L | 0.00 ± 0.01 | 0.11 ± 0.01 |
Notes: Data are means ± SE; p < 0.001 for all outcomes measures listed here.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
The TZDs have demonstrated favorable effects on other surrogate markers of cardiovascular disease. Adipose tissue produces substantial amounts of plasminogen activator inhibitor type-1 (PAI-1) – this when increased is an established cardiovascular risk factor. Both rosiglitazone and pioglitazone have been shown to reduce levels of PAI-1.78,79 Similarly, both agents have demonstrated significantly greater anti-inflammatory and antiatherogenic effects, compared with control agents, including reductions in C-reactive protein and matrix metalloproteinase-979,80 and increases in adiponectin levels.80,81 Carotid intima-media thickness (CIMT) is a marker of coronary atheroscelerosis and independently predicts subsequent cardiovascular events. Two studies of pioglitazone versus glimepiride have demonstrated beneficial effects on CIMT. In a 24-week study, a significant reduction in CIMT was observed with pioglitazone,80 while the CHI-CAGO (Carotid intima-media tHICkness in Atherosclerosis using pioGlitazOne) trial demonstrated that pioglitazone significantly slowed progression of CIMT compared with glimepiride over an 18-month period.82
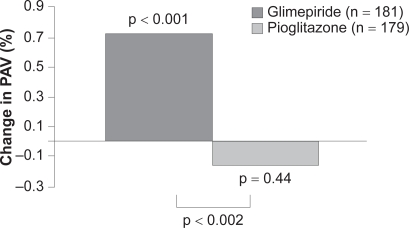
In addition to favorable effects on surrogate markers of cardiovascular disease, recently published data from the PERISCOPE trial demonstrate a significant effect of pioglitazone on atheroma volume.83 In patients with type 2 diabetes undergoing angiography for clinical indications, baseline intravascular ultrasound (IVUS) was performed to determine atheroma volume. A total of 543 patients were then randomized to pioglitazone 15 to 45 mg or glimepiride 1 to 4 mg titrated to maximally tolerated dose by 16 weeks. After 18 months, IVUS of the originally examined coronary artery was performed in 360 participants and revealed that pioglitazone had prevented the progression of coronary atherosclerosis compared with glimepiride (Figure 2).
Figure 2.
In patients with type 2 diabetes and coronary artery disease, treatment with pioglitazone resulted in a significantly lower rate of progression of coronary atherosclerosis compared with glimepiride. Developed from data of Nissen et al 2008.83
Abbreviation: PAV, percent atheroma volume.
Improved glycemic control is linked to better clinical outcomes in diabetes. In addition, the TZDs have beneficial effects on a number of cardiovascular risk markers. However, few studies have compared outcomes for glucose-lowering medications beyond their glucose-lowering efficacy.
PROactive is the only large treatment trial to date designed a priori to examine cardiovascular endpoints in TZD-treated patients. In this trial, a total of 5238 patients with type 2 diabetes and macrovascular disease were randomized to receive either pioglitazone (15 to 45 mg daily) or placebo while continuing existing therapy with glucose-lowering agents, lipid-lowering medications, and antihypertensives.5 The primary endpoint, which was a composite of all-cause mortality, nonfatal myocardial infarction (including silent infarction), stroke, acute coronary syndrome, endovascular or surgical intervention in the coronary or leg arteries, and amputation above the ankle, did not reach statistical significance. This composite endpoint was challenging, however; it included procedural endpoints and was designed to demonstrate benefit in multiple vascular beds – cardiac, cerebral, and peripheral. Pioglitazone was associated with a statistically significant 16% reduction in the occurrence of the predetermined principal secondary endpoint (composite of all-cause mortality, nonfatal myocardial infarction, and stroke). This endpoint is identical or similar to primary composite endpoints used in many other major cardiovascular outcome studies.
In a prespecified subgroup analysis, the PROactive investigators looked at stroke endpoints in patients with (n = 984) and without (n = 4254) a prior history of stroke.85 Recurrent stroke was reduced by 47% in patients with a prior history of stroke who received pioglitazone compared with those administered placebo (hazard ratio 0.53; 95% CI 0.34–0.85, p = 0.008). Similarly, in the subgroup of patients who had a previous myocardial infarction (n = 2445), pioglitazone had a statistically significant beneficial effect on the prespecified endpoints of fatal and nonfatal myocardial infarction (28% risk reduction; p = 0.045) and acute coronary syndrome (37% risk reduction; p = 0.035).86 As in other TZD trials, pioglitazone was associated with greater weight gain and with increased rates of edema and heart failure compared with placebo-treated patients, although mortality due to heart failure did not differ between the groups.86 Less robust data in the form of a meta-analysis of noncardiovascular endpoint trials, which included 16,390 patients in 19 studies, provide further support for pioglitazone’s cardiovascular safety.87
The evidence base for rosiglitazone is less extensive, with no completed cardiovascular outcomes trials. Contrary to findings with pioglitazone, a recent meta-analysis of noncardiovascular endpoint trials has suggested that rosiglitazone may be associated with an increased risk of cardiovascular events in patients with type 2 diabetes.88 The findings warrant further investigation, however. In the individual large published trials included in the study (specifically DREAM and ADOPT), there were no increases in the rates of myocardial ischemia or cardiovascular death. The findings have also not been confirmed by randomized prospective trials (including the interim analysis of the RECORD trial).58 Rosiglitazone was also widely prescribed in two large cardiovascular outcomes trials comparing intensive and standard glucose-lowering targets in type 2 diabetes that have been published since the meta-analysis.89,90 While these trials were assessing a treatment strategy and not any specific drug, no evidence that rosiglitazone is associated with excess cardiovascular events was found.
Other large cardiovascular outcomes trials with rosiglitazone, such as BARI 2D, will provide further information on the cardiovascular safety profile of rosiglitazone. However, based on available data, the US Food and Drug Administration has concluded that the use of rosiglitazone for the treatment of type 2 diabetes may be associated with a greater risk of myocardial ischemic events than placebo, metformin, or sulfonylureas. The agency has added label warnings to the prescribing information until the results of long-term cardiovascular outcome trials for rosiglitazone become available.
Safety aspects of TZDs
Weight gain is a class effect of the TZDs either as mono-therapy or in combination with other glucose-lowering agents. Most studies report an average weight gain of 3 to 4 kg over the first 6 months of TZD treatment, in line with the weight gain observed with sulfonylureas and insulin. Weight gain associated with TZDs may vary greatly depending on the individual and on the treatment regimen employed. In particular, weight gain is more pronounced when TZDs are combined with sulfonylureas or insulin.70,91
The most important side effects of the TZDs are fluid retention (usually manifest as peripheral edema) and an increase in subcutaneous fat, which both contribute to weight gain. The individual contributions of excess fluid and subcutaneous fat to TZD-associated weight gain have not been confirmed, although one study suggests that fluid accounts for as much as 75% of body weight increase.92 Other studies, however, have estimated fat to have the greater contribution.93 The likelihood of edema increases when TZDs are used in combination with insulin – patients using this combination should be monitored carefully. TZD-induced fluid retention may cause or aggravate diabetic macular edema by increasing plasma volume and vascular permeability.94,95 In addition, as edema can be associated with new or worsened heart failure, these agents should be used with caution in patients with edema or a history of heart failure. Four recent large-scale outcomes studies have shown an increased risk of non-fatal heart failure versus comparator drugs or placebo.5,40,54,58 In the US, initiation of rosiglitazone and pioglitazone is contraindicated in patients with established New York Heart Association Class III or IV heart failure and both TZDs carry a box warning for congestive heart failure, which is entirely separate from the recent concerns over the increased myocardial ischemia risk associated with rosiglitazone. In Europe, heart failure at any stage is a contraindication to the use of TZDs.
Although the underlying mechanisms of TZD-induced edema remain unclear, in vitro and animal data suggest that PPAR-γ agonists stimulate sodium reabsorption in the distal nephron by upregulating the expression and the translocation of the collecting duct epithelial sodium channel. Preliminary evidence suggests that diuretic agents, such as spironolactone and hydrochlorothiazide, which interfere with the signaling of PPAR-γ in the renal distal collecting duct, may be an effective means of reversing TZD-induced fluid retention.96
Both TZDs have been associated with reductions in markers of bone formation and reductions in bone mineral density.97–99 Preliminary analyses of the ADOPT trial revealed a small but significant number of leg and forearm fractures in postmenopausal women with rosiglitazone.40 A similar finding has been reported for pioglitazone in an analysis carried out using the manufacturer’s clinical trial database.100 An observational study has also reported increased bone loss with TZD use in 160 older diabetic men,101 although the study did not have sufficient power to control for potential confounders such as HbA1c level, use of other medications, or diabetic complications. With both agents, the majority of fractures observed were in the upper arm (humerus), hand, or foot. These sites of fracture are different from those associated with postmenopausal osteoporosis (eg, hip or spine). None of the studies were designed to study the effect of TZDs on bone and therefore multiple known risk factors for fractures cannot be excluded as confounding variables. However, as it is known that PPAR-γ activation may influence bone metabolism (for a review see Lau and Harper 2007102), future research should include a randomized, controlled trial in which fracture incidence and type of fracture are prospective outcome measures. Manufacturers have advised practitioners to consider the risk of fracture when initiating or treating female patients with type 2 diabetes using TZD-containing products.
The first available medication in the TZD class, troglitazone, was withdrawn from the market due to severe liver toxicity. Pioglitazone and rosiglitazone have not been associated with severe liver toxicity either as monotherapy or with oral antidiabetic agent or insulin combinations; however, it is recommended that liver enzymes are checked before initiating therapy in all patients and are monitored periodically thereafter based on clinical judgment. Both TZDs are contraindicated for use in patients with hepatic impairment.
Conclusions
A wealth of clinical data attest to the efficacy of pioglitazone and rosiglitazone mono- and combination therapies in achieving and sustaining glycemic control, both in patients with newly diagnosed disease and in those with more advanced disease who are not well controlled on other therapies. Conventional glucose-lowering agents such as sulfonylureas or metformin are often unable to maintain durable glycemic control when used as monotherapy. As agents that can preserve β-cell function and reduce insulin resistance either as monotherapy or in combination, the TZDs address fundamental mechanisms in the development and progression of type 2 diabetes, and complement existing treatments. Current data also hold the promise that early therapy with TZDs may decrease cardiovascular risk independently of glycemic control. Pioglitazone and (to a lesser extent) rosiglitazone have demonstrated favorable effects on surrogate markers of cardiovascular disease such as lipid profiles, inflammatory markers, and CIMT, and recently published data for pioglitazone also demonstrate a significant reduction in atheroma volume.83 Documented evidence for a benefit on cardiovascular outcomes has been demonstrated only with pioglitazone,5 but a number of trials are being conducted to address the effect of TZDs on cardiovascular outcomes – specifically, prevention of macrovascular complications. The UKPDS showed that the lower the HbA1c level the lower the risk for long-term complications.103 Therefore attaining and maintaining HbA1c treatment goals is critical in the management of type 2 diabetes.
Although generally well tolerated, TZDs can cause weight gain and induce fluid retention that occasionally leads to a diagnosis of heart failure (in susceptible individuals) and may contribute to bone loss in a small number of postmenopausal women. Concerns over heart failure risks associated with TZDs in general are entirely separate from the concerns over the increased myocardial ischemia risk associated with rosiglitazone. To date, increased risk of cardiac ischemia has not been reported with pioglitazone. While the ADA and EASD urge greater caution in the use of TZDs, particularly in patients with heart failure, in the latest update to their diabetes treatment guidelines, pioglitazone remains a possible choice for a second-line agent in patients who do not achieve HbAlc levels below 7% with lifestyle modification and metformin.104 Overall, the place of TZDs in the management of type 2 diabetes is well established and the potential for additional benefits on macrovascular risk beyond glucose-lowering efficacy continue to encourage further study of the long-term effects of these agents.
Footnotes
Disclosures
Professor Barnett has received research funding and honoraria for advisory work and lectures from relevant companies, including Takeda, GSK, Servier, MSD and Novartis.
References
- 1.Department of Health Forecasting obesity to 2010 Department of Health; August2006. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsStatistics/DH_4138630 (accessed 17 October 2008). [Google Scholar]
- 2.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14:S1–S85. [PubMed] [Google Scholar]
- 3.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the US. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 6.Bloomgarden ZT. The Avandia debate. Diabetes Care. 2007;30:2401–2408. doi: 10.2337/dc07-zb09. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SE. The importance of the β-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108(Suppl 6a):2S–8S. doi: 10.1016/s0002-9343(00)00336-3. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA. 1988 Lilly Lecture The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1987;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 10.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of β-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–S220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group UKPDS 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 13.Nesto RW. The relation of insulin resistance syndromes to risk of cardiovascular disease. Rev Cardiovasc Med. 2003;4(Suppl 6):S11–18. [PubMed] [Google Scholar]
- 14.Smiley T, Oh P, Shane LG. The relationship of insulin resistance measured by reliable indexes to coronary artery disease risk factors and outcomes–a systematic review. Can J Cardiol. 2001;17:797–805. [PubMed] [Google Scholar]
- 15.Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 16.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPAR-γ. Ann Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 17.Ravnskjær K, Børgesen M, Rubi B, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) potentiates, whereas PPARgamma attenuates, glucose-stimulated insulin secretion in pancreatic beta-cells. Endocrinology. 2005;146:3266–3276. doi: 10.1210/en.2004-1430. [DOI] [PubMed] [Google Scholar]
- 18.Smith SA.Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism Biochem Soc Trans 200230(Pt 6):1086–1090. [DOI] [PubMed] [Google Scholar]
- 19.Olefsky JM, Saltiel AR. PPAR gamma and the treatment of insulin resistance. Trends Endocrinol Metab. 2000;11:362–368. doi: 10.1016/s1043-2760(00)00306-4. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca VA. Rationale for the use of insulin sensitizers to prevent cardiovascular events in type 2 diabetes mellitus. Am J Med. 2007;120(9 Suppl 2):S18–S25. doi: 10.1016/j.amjmed.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Leiter LA. Can thiazolidinediones delay disease progression in type 2 diabetes? Curr Med Res Opin. 2006;22:1193–1201. doi: 10.1185/030079906X112507. [DOI] [PubMed] [Google Scholar]
- 22.Calkin AC, Thomas MC.PPAR agonists and cardiovascular disease in diabetes PPAR Res 2008. 245410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel CB, De Lemos JA, Wyne KL, McGuire DK. Thiazolidinediones and risk for atherosclerosis: pleiotropic effects of PPARγ agonism. Diabetes Vasc Dis Res. 2006;3:65–71. doi: 10.3132/dvdr.2006.016. [DOI] [PubMed] [Google Scholar]
- 24.Turner RC, Cull CA, Frighi V, Holman R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 25.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. doi: 10.2337/diacare.28.5.995. [DOI] [PubMed] [Google Scholar]
- 26.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 27.Scherbaum WA, Goke B. Metabolic efficacy and safety of once-daily pioglitazone monotherapy in patients with type 2 diabetes: a double-blind, placebo-controlled study. Horm Metab Res. 2002;34:589–595. doi: 10.1055/s-2002-35421. [DOI] [PubMed] [Google Scholar]
- 28.Herz M, Johns D, Reviriego J, et al. A randomized, double-blind, placebo-controlled, clinical trial of the effects of pioglitazone on glycemic control and dyslipidemia in oral antihyperglycemic medication-naive patients with type 2 diabetes mellitus. Clin Ther. 2003;25:1074–1095. doi: 10.1016/s0149-2918(03)80068-1. [DOI] [PubMed] [Google Scholar]
- 29.Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI, for the Rosiglitazone Clinical Trials Study Group Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:280–288. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 30.Phillips LS, Grunberger G, Miller E, et al. for the Rosiglitazone Clinical Trials Study Group Once- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care. 2001;24:308–315. doi: 10.2337/diacare.24.2.308. [DOI] [PubMed] [Google Scholar]
- 31.Chiquette E, Ramirez G, DeFronzo R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med. 2004;164:2097–2104. doi: 10.1001/archinte.164.19.2097. [DOI] [PubMed] [Google Scholar]
- 32.Charbonnel BH, Matthews DR, Schernthaner G, Hanefeld M, Brunetti P, for The QUARTET Study Group A long-term comparison of pioglitazone and gliclazide in patients with type 2 diabetes mellitus: a randomized, double-blind, parallel-group comparison trial. Diabet Med. 2005;22:399–405. doi: 10.1111/j.1464-5491.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 33.Tan MH, Baksi A, Krahulec B, et al. for the GLAL Study Group Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care. 2005;28:544–550. doi: 10.2337/diacare.28.3.544. [DOI] [PubMed] [Google Scholar]
- 34.Hällsten K, Virtanen KA, Lönnqvist F, et al. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes. 2002;51:3479–3485. doi: 10.2337/diabetes.51.12.3479. [DOI] [PubMed] [Google Scholar]
- 35.Pavo I, Jermendy G, Varkonyi TT, et al. Effect of pioglitazone compared with metformin on glycemic control and indicators of insulin sensitivity in recently diagnosed patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:1637–1645. doi: 10.1210/jc.2002-021786. [DOI] [PubMed] [Google Scholar]
- 36.Hanefeld M, Brunetti P, Schernthaner GH, Matthews DR, Charbonnel BH, for the QUARTET Study Group One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27:141–147. doi: 10.2337/diacare.27.1.141. [DOI] [PubMed] [Google Scholar]
- 37.Nagasaka S, Aiso Y, Yoshizawa K, Ishibashi S. Comparison of pioglitazone and metformin efficacy using homeostasis model assessment. Diabet Med. 2004;21:136–141. doi: 10.1111/j.1464-5491.2004.01083.x. [DOI] [PubMed] [Google Scholar]
- 38.Charbonnel B, Schernthaner G, Brunetti P, et al. Long-term efficacy and tolerability of add-on pioglitazone therapy to failing monotherapy compared with addition of gliclazide or metformin in patients with type 2 diabetes. Diabetologia. 2005;48:1093–1104. doi: 10.1007/s00125-005-1751-1. [DOI] [PubMed] [Google Scholar]
- 39.Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P, for the QUARTET Study Group Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89:6068–6076. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 40.Kahn SE, Haffner SM, Heise MA, et al. for the ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide mono-therapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 41.Tan MH, Johns D, Strand J, et al. for the GLAC Study Group Sustained effects of pioglitazone vs glibenclamide on insulin sensitivity, glycaemic control, and lipid profiles in patients with Type 2 diabetes. Diabet Med. 2004;21:859–866. doi: 10.1111/j.1464-5491.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 42.National Institute for Health and Clinical Excellence (NICE) Type 2 diabetes: the management of type 2 diabetes (update) Available from: http://www.nice.org.uk/CG66 (accessed 17 October 2008).
- 43.IDF Clinical Guidelines Task Force Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23:579–593. doi: 10.1111/j.1464-5491.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstock J, Rood J, Cobitz A, Biswas N, Chou H, Garber A. Initial treatment with rosiglitazone/metformin fixed-dose combination therapy compared with monotherapy with either rosiglitazone or metformin in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2006;8:650–660. doi: 10.1111/j.1463-1326.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 45.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetologia. 2006;49:1711–1721. doi: 10.1007/s00125-006-0316-2. [DOI] [PubMed] [Google Scholar]
- 46.Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus. JAMA. 2000;283:1695–1702. doi: 10.1001/jama.283.13.1695. [DOI] [PubMed] [Google Scholar]
- 47.Bailey CJ, Bagdonas A, Rubes J, et al. Rosiglitazone/metformin fixed dose combination compared with uptitrated metformin alone in type 2 diabetes mellitus: a 24 week, multicenter, randomized, double blind, parallel group study. Clin Ther. 2005;27:1548–1561. doi: 10.1016/j.clinthera.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Hanefeld M. Pioglitazone and sulfonylureas: effectively treating type 2 diabetes. Int J Clin Pract. 2007;S153:20–27. doi: 10.1111/j.1742-1241.2007.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smiley T. The role of declining beta cell function in the progression of type 2 diabetes: implications for outcomes and pharmacological management. Can J Diabetes. 2003;27:277–286. [Google Scholar]
- 50.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, Defronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292:E871–E883. doi: 10.1152/ajpendo.00551.2006. [DOI] [PubMed] [Google Scholar]
- 51.Juhl CB, Hollingdal M, Pørksen N, Prange A, Lönnqvist F, Schmitz O. Influence of rosiglitizone treatment on β-cell function in type 2 diabetes: evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab. 2003;88:3794–3800. doi: 10.1210/jc.2002-021181. [DOI] [PubMed] [Google Scholar]
- 52.Xiang A, Peters R, Kjos S, et al. Effect of pioglitizone on pancreatic β-cell function and diabetes risk in Hispanic women with gestational diabetes. Diabetes. 2006;55:517–22. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tripathy D, Banerji MA, Bray GA, et al. ACTos NOW for the Prevention of Diabetes (ACT NOW) Study [Abstract] Diabetologia. 2008;51(Suppl 1):S99. [Google Scholar]
- 54.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 55.Garber A, Klein E, Bruce S, Sankoh S, Mohideen P. Metformin–glibenclamide versus metformin plus rosiglitazone in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2006;8:156–163. doi: 10.1111/j.1463-1326.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M. Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulfonylurea plus metformin in overweight individuals with type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes. 2008;116:6–13. doi: 10.1055/s-2007-984441. [DOI] [PubMed] [Google Scholar]
- 57.Stewart MW, Cirkel DT, Furuseth K, et al. Effect of metformin plus roziglitazone compared with metformin alone on glycaemic control in well-controlled Type 2 diabetes. Diabet Med. 2006;23:1069–1078. doi: 10.1111/j.1464-5491.2006.01942.x. [DOI] [PubMed] [Google Scholar]
- 58.Home PD, Pocock SJ, Beck-Nielsen H, et al. for the RECORD Study Group Rosiglitazone evaluated for cardiovascular outcomes–an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 59.Dorkhan M, Frid A. A review of pioglitazone HCL and glimepiride in the treatment of type 2 diabetes. Vasc Health Risk Manag. 2007;3:721–731. [PMC free article] [PubMed] [Google Scholar]
- 60.Derosa G. Pioglitazone plus glimepiride: a promising alternative in metabolic control. Int J Clin Pract. 2007;S153:28–36. doi: 10.1111/j.1742-1241.2007.01362.x. [DOI] [PubMed] [Google Scholar]
- 61.Staels B. Metformin and pioglitazone: Effectively treating insulin resistance. Curr Med Res Opin. 2006;22(Suppl 2):S27–S37. doi: 10.1185/030079906X112732. [DOI] [PubMed] [Google Scholar]
- 62.Seufert J. A fixed-dose combination of pioglitazone and metformin: a promising alternative in metabolic control. Curr Med Res Opin. 2006;22(Suppl 2):S39–S48. doi: 10.1185/030079906X121002. [DOI] [PubMed] [Google Scholar]
- 63.Matthews DR, Charbonnel BH, Hanefeld M, Brunetti P, Schernthaner G. Long-term therapy with addition of pioglitazone to metformin compared with the addition of gliclazide to metformin in patients with type 2 diabetes: a randomized, comparative study. Diabetes Metab Res Rev. 2005;21:167–174. doi: 10.1002/dmrr.478. [DOI] [PubMed] [Google Scholar]
- 64.Kerenyi Z, Samer H, James R, Yan Y, Stewart M. Combination therapy with rosiglitazone and glibenclamide compared with upward titration of glibenclamide alone in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004;63:213–223. doi: 10.1016/j.diabres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Comaschi M, Demicheli A, Di Pietro C, Bellatreccia A, Mariz S, for the COM06 Study Investigators Effects of pioglitazone in combination with metformin or a sulfonylurea compared to a fixed-dose combination of metformin and glibenclamide in patients with type 2 diabetes. Diabetes Technol Ther. 2007;9:387–398. doi: 10.1089/dia.2006.0023. [DOI] [PubMed] [Google Scholar]
- 66.Betteridge DJ, Verges B. Long-term effects on lipids and lipoproteins of pioglitazone versus gliclazide addition to metformin and pioglitazone versus metformin addition to sulfonylurea in the treatment of type 2 diabetes. Diabetologia. 2005;48:2477–2481. doi: 10.1007/s00125-005-0034-1. [DOI] [PubMed] [Google Scholar]
- 67.Derosa G, Cicero AF, Gaddi A, et al. A comparison of the effects of pioglitazone and rosiglitazone combined with glimepiride on prothrombotic state in type 2 diabetic patients with the metabolic syndrome. Diabetes Res Clin Pract. 2005;69:5–13. doi: 10.1016/j.diabres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Derosa G, Gaddi AV, Piccinni MN, et al. Differential effect of glimepiride and rosiglitazone on metabolic control of type 2 diabetic patients treated with metformin: a randomized, double-blind, clinical trial. Diabetes Obes Metab. 2006;8:197–205. doi: 10.1111/j.1463-1326.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 69.Einhorn D, Rendell M, Rosenzweig J, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther. 2000;22:1395–1409. doi: 10.1016/s0149-2918(00)83039-8. [DOI] [PubMed] [Google Scholar]
- 70.Rosenblatt S, Miskin B, Glazer NB, Prince MJ, Robertson KE. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001;12:413–423. doi: 10.1097/00019501-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Mattoo V, Eckland D, Widel M, et al. Metabolic effects of pioglitazone in combination with insulin in patients with type 2 diabetes mellitus whose disease is not adequately controlled with insulin therapy: results of a six-month, randomized, double-blind, prospective, multicenter, parallel-group study. Clin Ther. 2005;27:554–567. doi: 10.1016/j.clinthera.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Berhanu P, Kipnes MS, Khan MA, et al. Effects of pioglitazone on lipid and lipoprotein profiles in patients with type 2 diabetes and dyslipidaemia after treatment conversion from rosiglitazone while continuing stable statin therapy. Diab Vasc Dis Res. 2006;3:39–44. doi: 10.3132/dvdr.2006.005. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 74.Derosa G, D’Angelo A, Ragonesi PD, et al. Metformin–pioglitazone and metformin–rosiglitazone effects on non-conventional cardiovascular risk factors plasma level in type 2 diabetic patients with metabolic syndrome. J Clin Pharm Ther. 2006;31:375–383. doi: 10.1111/j.1365-2710.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 75.Deeg MA, Buse JB, Goldberg RB, et al. for the GLAI Study Investigators Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30:2458–2464. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- 76.Lawrence JM, Reid J, Taylor GJ, Stirling C, Reckless JP. Favorable effects of pioglitazone and metformin compared with gliclazide on lipoprotein subfractions in overweight patients with early type 2 diabetes. Diabetes Care. 2004;27:41–46. doi: 10.2337/diacare.27.1.41. [DOI] [PubMed] [Google Scholar]
- 77.Perez A, Khan M, Johnson T, Karunaratne M. Pioglitazone plus a sulfonylurea or metformin is associated with increased lipoprotein particle size in patients with type 2 diabetes. Diabetes Vasc Dis Res. 2004;1:44–50. doi: 10.3132/dvdr.2004.006. [DOI] [PubMed] [Google Scholar]
- 78.Derosa G, D’Angelo A, Ragnonesi PD, et al. Effects of rosiglitazone and pioglitazone combined with metformin on the prothrombotic state of patients with type 2 diabetes mellitus and metabolic syndrome. J Int Med Res. 2006;34:545–555. doi: 10.1177/147323000603400513. [DOI] [PubMed] [Google Scholar]
- 79.Goldstein BJ, Weissman PN, Wooddell MJ, Waterhouse BR, Cobitz AR. Reductions in biomarkers of cardiovascular risk in type 2 diabetes with rosiglitazone added to metformin compared with dose escalation of metformin: an EMPIRE trial sub-study. Curr Med Res Opin. 2006;22:1715–1723. doi: 10.1185/030079906X115720. [DOI] [PubMed] [Google Scholar]
- 80.Pfutzner A, Marx N, Lubben G, et al. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the PIONEER study. J Am Coll Cardiol. 2005;45:1925–1931. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 81.Yang WS, Jeng CY, Wu TY, et al. Synthetic peroxisome proliferator-activated receptor-γ agonist, rosiglitazone, increases plasma levels of adiponectin type 2 diabetic patients. Diabetes Care. 2002;25:376–380. doi: 10.2337/diacare.25.2.376. [DOI] [PubMed] [Google Scholar]
- 82.Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296:2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 83.Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 84.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 85.Wilcox R, Bousser M-G, Betteridge DJ, et al. for the PROactive Investigators Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38:865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- 86.Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM, for the PROactive Investigators The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction. Results from the PROactive (PROactive 05) study. J Am Coll Cardiol. 2007;49:1772–1780. doi: 10.1016/j.jacc.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 87.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 88.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 89.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duckworth WC.American Diabetes Association 68th Scientific Sessions: VA Diabetes Trial SymposiumPresented June82008 [Google Scholar]
- 91.Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J, for the Rosiglitazone Clinical Trials Study Group A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226–1232. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 92.Basu A, Jensen MD, McCann F, Mukhopadhyay D, Joyner MJ, Rizza RA. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care. 2006;9:510–514. doi: 10.2337/diacare.29.03.06.dc05-2004. [DOI] [PubMed] [Google Scholar]
- 93.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 94.Ryan, et al. Macular Edema associated with glitazone. Retina. 2006;26:562–570. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Liazos, et al. Spontaneous resolution of diabetic macular edema after discontinuation of thizolidinediones. Diabet Med. 2008;25:860–862. doi: 10.1111/j.1464-5491.2008.02491.x. [DOI] [PubMed] [Google Scholar]
- 96.Karalliedde J, Buckingham R, Starkie M, Lorand D, Stewart M, Viberti G, for the Rosiglitazone Fluid Retention Study Group Effect of various diuretic treatments on rosiglitazone-induced fluid retention. J Am Soc Nephrol. 2006;17:3482–3490. doi: 10.1681/ASN.2006060606. [DOI] [PubMed] [Google Scholar]
- 97.Grey A, Bolland M, Gamble G, et al. The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 98.Berberoglu Z, Gursoy A, Bayraktar N, Yazici AC, Bascil Tutuncu N, Guvener Demirag N. Rosiglitazone decreases serum bone-specific alkaline phosphatase activity in postmenopausal diabetic women. J Clin Endocrinol Metab. 2007;92:3523–3530. doi: 10.1210/jc.2007-0431. [DOI] [PubMed] [Google Scholar]
- 99.Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann AP. Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2008;93:1696–1701. doi: 10.1210/jc.2007-2249. [DOI] [PubMed] [Google Scholar]
- 100.Meymeh RH, Wooltorton E. Diabetes drug pioglitazone (Actos): risk of fracture. CMAJ. 2007;177:723–724. doi: 10.1503/cmaj.071177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yaturu S, Bryant B, Jain SK. Thiazolidinediones treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care. 2007;30:1574–1576. doi: 10.2337/dc06-2606. [DOI] [PubMed] [Google Scholar]
- 102.Lau A, Harper W. Thiazolidinediones and their effect on bone metabolism: a review. Can J Diabetes. 2007;31:378–383. [Google Scholar]
- 103.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:1–11. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]