Abstract
Oxidative stress plays an important role in the progression of vascular endothelial dysfunction. The two major systems generating vascular oxidative stress are the NADPH oxidase and the xanthine oxidase pathways. Allopurinol, a xanthine oxidase inhibitor, has been in clinical use for over 40 years in the treatment of chronic gout. Allopurinol has also been shown to improve endothelial dysfunction, reduce oxidative stress burden and improve myocardial efficiency by reducing oxygen consumption in smaller mechanistic studies involving various cohorts at risk of cardiovascular events. This article aims to explain the role of xanthine oxidase in vascular oxidative stress and to explore the mechanisms by which allopurinol is thought to improve vascular and myocardial indices.
Keywords: allopurinol, vascular oxidative stress, vascular endothelial dysfunction
Introduction
The role of oxidative stress in disease has always been a contentious issue because attempts to reduce oxidative stress using antioxidant vitamins such as in the Heart Outcomes Prevention Evaluation (HOPE) study have consistently failed to demonstrate a mortality benefit.1 There are many possible reasons for this, not least because of cohort selection and inappropriate dosing of antioxidants. Even if adequate doses were used, at many magnitudes higher than currently taken as part of multivitamin supplementation, there would be further issues with tolerability and safety. Therefore, recent research has concentrated on mechanisms to reduce the formation of reactive oxygen species (ROS) rather than a scavenging approach to already-formed ROS. The two major ROS generating systems are the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the xanthine oxidase (XO) systems.
Background
ROS consists of molecular oxygen and all of its aerobic cellular metabolites including superoxide (O2–) and hydroxyl radical (OH–). Other substances such as hydrogen peroxide (H2O2), peroxynitrite (ONOO–) and hypochlorous acid (HOCL) have oxidative properties although they are not free radicals.2 McCord and Fridovich were one of the first to describe the deleterious effects of ROS in inflammation in an article describing the enzyme superoxide dismutase.3 Since then, there has been an abundance of research demonstrating the role oxidative stress plays in the pathophysiology of abnormal vasorelaxation. There is also increasing evidence that oxidative stress plays a direct role in myocardial remodeling.4,5 above and beyond its effects on vasomotion.
Enhanced production of reactive oxygen species (ROS) is a major cause of endothelial dysfunction. This has been demonstrated in animal studies using p66SHC knockout mice which demonstrate reduced aortic endothelial cell superoxide production and a 30% prolonged lifespan.6 The precise role of p66SHC remains unclear but in studies looking at its effects on the p53 signaling pathway demonstrate that it is involved in stress-activated p53-induced elevation of intracellular oxidants, apoptosis and regulation of the intracellular redox state.7 This is consistent with the finding that ROS promotes apoptosis and microvascular rarefaction in spontaneously hypertensive rats.8 Furthermore, in chronic heart failure (CHF) patients with the Glu298Asp variant (Glu to Asp amino acid substitution for codon 298 of endothelial nitric oxide synthase (eNOS)) have a significantly shorter eNOS half-life. This translates to a significantly lower event-free survival.9 There is also evidence that the same polymorphism results in blunting of the endothelial-dependent vasodilation in healthy individuals.10
NADPH oxidase catalyzes the reduction of oxygen through electron donation from either NADH (predominantly) or NADPH to generate superoxide (O2–). This system is thought to be the predominant driver of O2– formation. The most potent inducer of the NADPH oxidase system is angiotensin II,11,12 and there are angiotensin II antagonists in the form of ACE inhibitors, angiotensin II receptor antagonists and direct renin inhibitors (DRA) in clinical use. The other enzyme system, XO, has been largely ignored with regards to managing oxidative stress despite the availability of XO inhibitors allopurinol, oxypurinol and febuxostat. This is primarily because most of the data for the beneficial effect of these agents have come from smaller mechanistic studies and the lack of large clinical trial evidence to support widespread use. The two larger studies using the XO inhibitor oxypurinol either showed neutral.13 (in LA-PLATA) or negative results (OPT-CHF)14 which may have been due to an insufficient dose-effect or patient selection. A subsequent follow-up study14 found a significant benefit in patients with high baseline urate, which could imply either high XO activity or that urate itself is detrimental. A recent study in patients with type II diabetes suggests that urate lowering per se does not improve endothelial function.15
Xanthine oxidase (XO)
Xanthine oxidoreductase (XOR) is part of a group of enzymes known as the molybdenum iron-sulfur flavin hydroxylases. It was first discovered in milk by Schardinger in 190216 and is thought to be involved in reactions that produce ROS such as nitrite which enable newborn infants to overcome gut-associated bacterial gastroenteritis.17,18 XOR is widely distributed throughout various organs including the liver, gut, lung, kidney, heart, brain and plasma19 with the highest levels being found in the gut and the liver.20 In the myocardium, it is localized to the capillary endothelial cells.21 The gene encoding for XOR is located at the short arm of chromosome 2.22 It exists in two inter-convertible forms known as XO (EC 1.1.3.22) and xanthine dehydrogenase (XDH) (EC 1.17.1.4).23 Both enzymes consist of two identical subunits of 145 kDa.
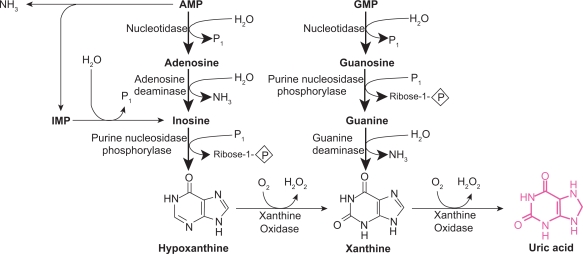
Mammalian XOR is present in vivo as the dehydrogenase form but is easily converted to XO by oxidation of the sulfhydryl residues or by proteolysis.19 Although XDH has a much greater affinity for NAD+ compared to oxygen (and therefore is practically incapable of directly producing ROS), both XO and XDH can oxidize NADH which results in ROS formation.24 Physiologically, XOR is involved in the hydroxylation of purines, pterins, and aldehydes but its primary role is as the rate-limiting enzyme in the conversion of hypoxanthine to xanthine and xanthine to urate (Figure 1). XOR is the only enzyme capable of catalyzing the formation of urate in man.25 In lower mammals, an enzyme, urate oxidase further metabolizes uric acid to allantoin but this enzyme is inactivated in primates.26 There is also a suggestion from teleological studies that urate may have even evolved as a compensatory mechanism in higher primates that have lost the capacity to generate other antioxidants like ascorbate in vivo.27
Figure 1.
The purine degradation pathway. Reproduced with permission from Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004; 555(Pt 3):589–606.16 Copyright © Blackwell Publishing.
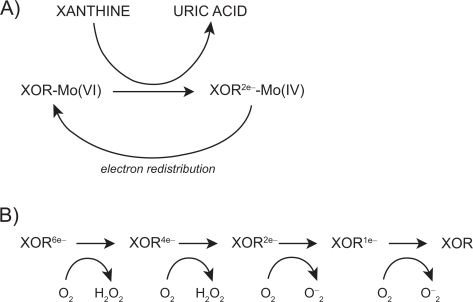
The mechanism by which XOR catalyzes hypoxanthine and xanthine conversion is extremely complex and has been previously described in detail.16,28 A fully reduced XO contains six electrons and its re-oxidation involves electron transfer to oxygen molecules which generates two H2O2 and two O2– species28 for every fully reduced XO molecule (Figure 1).
There is a large variability in human XOR expression which can be up to three-fold and on average 20% higher in men than in women.29 Although basal expression of XOR is low in humans, hypoxia, IL-1, IL-6, TNF-α, lipo-polysaccharides as well as steroid treatment have been shown to upregulate transcription.16 XO is significantly elevated in a variety of conditions including limb ischemia,30 major surgery31 coronary artery disease (CAD)32 and heart failure.33 Circulating XO binds to glycosaminoglycans on the surface of endothelial cells where it is thought it acquires modified kinetics (higher Km and Ki, oxidant producing capacity, and increased stability).34 There are suggestions that this form of induced, circulating and depositing XO appears to be more important in the pathogenesis of endothelial injury compared with XO constitutively produced from endothelial cells.35
Despite this, the activity of endothelial bound XO is increased by more than 200% in patients with CHF.36 Furthermore, studies using electron spin resonance have demonstrated that endothelial oxygen tension is thought to regulate XO activity at a post-translational level as demonstrated by a doubling in XOR activity post exposure to hypoxia without any increase in mRNA expression for 24 hours in bovine aortic endothelial cells.37 Cells produce a marked elevation in XO levels when exposed to ischemia38 and XDH conversion to XO is also accelerated in hypoxia.39 When infused acutely, XO produces a marked decrease in cardiac contractility, cardiac index and left ventricular systolic pressure.40 In atherosclerotic plaques, urate levels are found to be elevated six-fold, reflecting accelerated purine oxidation within these plaques. Therefore XO production may not necessarily be reflected by systemic levels of XO metabolites.41
The XO inhibitor allopurinol
Recent evidence indicates that allopurinol improves endothelial dysfunction in high risk primary prevention patients such as those with metabolic syndrome.42
Allopurinol has also been shown to normalize endothelial dysfunction in type 2 diabetics with mild hypertension and reduced plasma malondialdehyde (MDA) levels.43 MDA results from acid hydrolysis of lipid peroxides which are formed by free radical attack on plasma lipoproteins. It is therefore used as an indirect measure of oxidized low-density lipoprotein (LDL).
In the experimental murine myocardial infarction model, allopurinol significantly attenuated LV dilatation, hypertrophy, fibrosis and dysfunction. Once again, XO expression (as determined by electron spin resonance spectroscopy) and myocardial ROS generation were markedly increased in the post-mycardial infarction ischemic model.44 This suggests a role for allopurinol in LV remodeling, a possibility that we are investigating at present in our unit. Allopurinol has also been shown to be beneficial in conditions such as post coronary artery bypass surgery where it reduced ischemic events and produced less ST segment depression45 as well as in hypercholesterolemic patients.46 There are mixed data from ischemic-reperfusion studies. Pacher et al,19 in an excellent in-depth review on this topic, have summarized the data in the Table 1.
Table 1.
Effects of xanthine oxidase (XO) inhibitors in myocardial ischemia-reperfusion injury
| Model | Disease or trigger | Mode of XO inhibition | Effects of XO inhibition | Reference |
|---|---|---|---|---|
| Human (169 patients) | Coronary bypass surgery | Allopurinol | Decreased hospital mortality rate, increased cardiac index | Johnson et al52 |
| Human (90 patients) | Coronary bypass surgery | Allopurinol | Reduced arrhythmias, need for inotropes and perioperative myocardial infarction in patients | Rashid and William-Olsson53 |
| Human (140 patients) | Myocardial I | Allopurinol | Increased incidence of infarct extensions in the treatment group | Parmley et ala54 |
| Human (80 patients) | Ischemic heart disease | Allopurinol + erinit | Decreases in serum and daily urinary levels of uric acid and lipid peroxidation antioxidative system and an improvement of central hemodynamics | Kaliakin and Mit'kin55 |
| Human (50 patients) | Coronary bypass surgery | Allopurinol | Improves postoperative recovery and reduces lipidperoxidation in patients undergoing coronary artery bypass grafting | Coghlan et al56 |
| Human (20 patients) | Coronary bypass surgery | Allopurinol | Failed to demonstrate a cardioprotective effect of allopurinol in patients with good left ventricular function undergoing elective coronary artery surgery | Taggart et ala57 |
| Human (20 patients) | Coronary bypass surgery | Allopurinol | Allopurinol suppressed the reaction rate of XO; therefore, the levels of intermediates, hypoxanthine and xanthine, were high, and the level of the final product, uric acid, was low – however, allopurinol had no efficacy for the level of lactate, pyruvate, CK, and CK-MB | Yamazaki et ala58 |
| Human (33 patients) | Coronary bypass surgery | Allopurinol | Better recovery of cardiac output and left ventricular stroke work after bypass surgery and reduction of plasma XO activity and concentrations of uric acid | Castelli et al59 |
| Human (52 patients) | Coronary bypass surgery | Allopurinol | Allopurinol failed to improve left ventricular stroke work after cardiopulmonary bypass surgery | Coetzee et ala60 |
| Human (38 patients) | Percutaneous transluminal coronary angioplasty in patients with acute myocardial infarction | Allopurinol | Allopurinol pretreatment was effective in inhibiting generation of oxygen-derived radicals during reperfusion and in the recovery of left ventricular function | Guan et al61 |
Adapted with permission from Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006; 58(1):87–114.19 Copyright © 2006 American Society for Pharmacology and Experimental Therapeutics.
Studies concluded with negative results.
Abbreviations: I/R, ischemia-reperfusion; I, ischemia; CK, creatine kinase.
Allopurinol in CHF was assessed by Doehner et al47 and by Farquharson et al.48 Doehner et al showed that the degree of improvement in forearm blood flow correlated with the degree of urate lowering. Interestingly, they also measured allantoin, a marker of oxygen free radical generation, which was reduced by 20% following 300 mg/day allopurinol. Farquharson et al48 from our unit, found a 181% change in forearm blood flow with 300 mg allopurinol. They also found a 33% reduction in plasma MDA levels in patients treated with 300 mg allopurinol suggesting that the improvement in endothelial function and NO bioavailability seen was due (at least in part) to a reduction of ROS generation or oxidative stress burden. Allopurinol also reduced B-type natriuretic peptide (BNP) in stable CHF patients, although the reduction did not correlate with the fall in urate.49
We have shown previously that high dose allopurinol is a very effective antioxidant in the vasculature because it abolishes the vitamin C-sensitive component of oxidative stress on vascular endothelial function, ie, in patients on high dose allopurinol, there is insufficient vascular oxidative stress being formed for vitamin C to neutralize the oxidative stress and further improve endothelial function.50 This is further strengthened by evidence that the beneficial effect of vitamin C co-infusion in patients with CHF was greatest in patients with the highest levels of oxidative stress as measured by extracellular superoxide dismutase (ecSOD)36 and XO activity.
Interestingly, it is the ability of allopurinol to inhibit XO, and therefore ROS, that results in its inclusion as a constituent in the University of Wisconsin solution for organ transport prior to transplantation.51
The biomarker of lipid peroxidation, F2-isoprostane, is an indirect but validated marker of oxidative stress. Data from our group (unpublished) also demonstrate the ability of allopurinol to significantly reduce F2-isoprostane levels in patients with high baseline oxidative stress, further confirming the potent antioxidant effect of allopurinol. This also reflects XO activity within the vascular milieu in these patients because for the same degree of urate lowering as those with low baseline oxidative stress, only patients with high pre-existing oxidative stress were found to have a significant reduction in F2-isoprostanes. This is consistent with the known cascade effect of multiple superoxide and hydrogen peroxide generation for every urate molecule formation that is catalyzed by XO.16,62 In fact, NO and O2– react at a three-fold greater rate than the rate at which antioxidant defense mechanisms such as SOD can eliminate O2–.63
In chronic diseases such as CHF, sustained high levels of ROS may exceed the capacity of cellular enzymatic and non-enzymatic antioxidants64 to counter its effects. Using electron spin resonance, Spiekermann et al demonstrated that both NADPH oxidase and xanthine oxidase are up-regulated in patients with coronary artery disease.32 Others have demonstrated increased levels in CHF.36,65
Direct antioxidant action of allopurinol
Allopurinol directly scavenges free radicals as demonstrated by Das et al and others66–68 in in vitro hearts where evidence of free radical scavenging occurred in the absence of XO activity. Animal studies in experimentally induced uveitis show that at very high doses (up to 50 mg/kg), allopurinol behaves as a free radical scavenger with intrinsic antioxidant properties. Crucially, this was only achieved far beyond the XO inhibition dose of 10 mg/kg and not at that dose itself. Further evidence for a possible direct antioxidant effect of allopurinol comes from models of experimental colitis where tungsten (a potent XO inhibitor) failed to improve symptoms whereas allopurinol did.69 Augustin et al suggested that this direct effect was only seen at higher doses.70 This was also seen in mice paracetamol toxicity models where lower doses (sufficient to block XO activity) of allopurinol failed to show antioxidant protection but higher doses did.71 There have been other non-XO effects of allopurinol suggested such as copper chelation, preventing LDL oxidation as described above,72 inhibition of heat shock protein (hsp) expression73 and calcium sensitization (below). Allopurinol treatment reduces early changes in inflammation such as leukocyte activation by reducing adherence, rolling and extravasation.74
Mechanoenergetic uncoupling
This phenomenon refers to an imbalance between left ventricular performance and myocardial energy consumption.75 The role of XO inhibition may either be to maintain cardiac output while reducing myocardial oxygen consumption or even increase cardiac output without increasing myocardial oxygen consumption. In dogs with pacing-induced heart failure, allopurinol improved myocardial contractility and eff iciency in oxygen utilization, prevented increases in systemic vasoconstriction and ameliorated reductions in myocardial contractility.65,76,77 In murine post-ischemic cardiomyopathy models, allopurinol attenuated the increase in end-systolic and end-diastolic volumes,78 increased survival, augmented ventricular function as well as reduced products of lipid peroxidation.79 Khan et al found a direct protein-protein interaction between XO and neuronal NOS in the sarcoplasmic reticulum of cardiac myocytes.80 Allopurinol improved myofilament calcium sensitivity as contraction force increased without a concomitant rise in systolic Ca2+ influx. The effects were not seen in endothelial NOS deficient mice, suggesting a role for neuronal NOS preventing XO inhibition of cardiac excitation-contraction coupling.80 The finding that allopurinol is a potent myofilament Ca2+ sensitizer, particularly in the setting of ischemia, is thought to be due to the inhibition of basal XO production. As with the previous study by Khan et al, Perez et al found an almost exclusive increase in force generation without a lowering of inward transient Ca2+.81
Despite the small sample size (n = 9), Cappola et al showed using cardiac catheterization that direct intracoronary infusions of allopurinol in these patients resulted in a marked decrease in myocardial oxygen consumption (MVO2) with no decrease in the rate of left ventricular pressure rise (dP/dT), stroke work or ventricular load.82 Patients post coronary artery bypass grafting given allopurinol have also been shown to require less inotropic support.45
Conclusion
The evidence for the role of oxidative stress in disease cannot be disputed. However, there are many questions that remain such as (1) exactly what is the contribution of oxidative stress to overall endothelial dysfunction; (2) at what stage should intervention take place; (3) what agents can we employ to effectively deal with this ubiquitous problem, which will be both safe and tolerable to our patients? The emerging evidence from therapies such as allopurinol are encouraging and should be put to the test in larger randomized studies to determine if the interesting data garnered from smaller mechanistic studies actually translate to a survival advantage with these agents.
Figure 2.
Mechanism of xanthine oxidoreductase XOR reaction with xanthine; A) reductive half reaction; B) oxidative half reaction. Reproduced with permission from Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004; 555(Pt 3):589–606.16 Copyright © Blackwell Publishing.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 2.Fenster BE, Tsao PS, Rockson SG. Endothelial dysfunction: clinical strategies for treating oxidant stress. Am Heart J. 2003;146(2):218–226. doi: 10.1016/S0002-8703(02)94796-4. [DOI] [PubMed] [Google Scholar]
- 3.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 4.Zhang M, Shah AM. Role of reactive oxygen species in myocardial remodeling. Curr Heart Fail Rep. 2007;4(1):26–30. doi: 10.1007/s11897-007-0022-5. [DOI] [PubMed] [Google Scholar]
- 5.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49(2):241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 6.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110(18):2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 7.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, et al. A p53–p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21(24):3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N, DeLano FA, Schmid-Schonbein GW. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2005;25(10):2114–2121. doi: 10.1161/01.ATV.0000178993.13222.f2. [DOI] [PubMed] [Google Scholar]
- 9.McNamara DM, Holubkov R, Postava L, Ramani R, Janosko K, Mathier M, et al. Effect of the Asp298 variant of endothelial nitric oxide synthase on survival for patients with congestive heart failure. Circulation. 2003;107(12):1598–1602. doi: 10.1161/01.CIR.0000060540.93836.AA. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey V, Chan SL, Cassidy A, Butler R, Choy A, Fardon T, et al. The functional consequence of the Glu298Asp polymorphism of the endothelial nitric oxide synthase gene in young healthy volunteers. Cardiovasc Drug Rev. 2007;25(3):280–288. doi: 10.1111/j.1527-3466.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- 11.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74(6):1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 12.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91(3A):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 13.Cingolani HE, Plastino JA, Escudero EM, Mangal B, Brown J, Perez NG. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J Card Fail. 2006;12(7):491–498. doi: 10.1016/j.cardfail.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Cleland JGF, Coletta AP, Clark AL. Clinical trials update from the Heart Failure Society of America meeting: FIX-CHF-4, selective cardiac myosin activator and OPT-CHF. Eur J Heart Fail. 2006;8(7):764–766. doi: 10.1016/j.ejheart.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Lowering serum urate does not improve endothelial function in patients with type 2 diabetes. Diabetologia. 2007;50(12):2572–2579. doi: 10.1007/s00125-007-0817-7. [DOI] [PubMed] [Google Scholar]
- 16.Berry CE, Hare JM.Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications J Physiol 2004. 16;555(Pt 3): 589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock JT, Salisbury V, Ovejero-Boglione MC, Cherry R, Hoare C, Eisenthal R, et al. Antimicrobial properties of milk: dependence on presence of xanthine oxidase and nitrite. Antimicrob Agents Chemother. 2002;46(10):3308–3310. doi: 10.1128/AAC.46.10.3308-3310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens CR, Millar TM, Clinch JG, Kanczler JM, Bodamyali T, Blake DR. Antibacterial properties of xanthine oxidase in human milk. Lancet. 2000;356(9232):829–830. doi: 10.1016/s0140-6736(00)02660-x. [DOI] [PubMed] [Google Scholar]
- 19.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58(1):87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parks DA, Granger DN. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- 21.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, et al. Elevated serum uric acid levels are associated with diastolic dysfunction in patients with dilated cardiomyopathy. Am Heart J. 2002;143(6):1107–1111. doi: 10.1067/mhj.2002.122122. [DOI] [PubMed] [Google Scholar]
- 22.Ichida K, Amaya Y, Noda K, Minoshima S, Hosoya T, Sakai O, et al. Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the gene. Gene. 1993;133(2):279–284. doi: 10.1016/0378-1119(93)90652-j. [DOI] [PubMed] [Google Scholar]
- 23.Della Corte E, Gozzetti G, Novello F, Stirpe F. Properties of the xanthine oxidase from human liver. Biochim Biophys Acta. 1969;191(1):164–166. doi: 10.1016/0005-2744(69)90327-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Blake DR, Stevens CR, Kanczler JM, Winyard PG, Symons MC, et al. A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donor. Free Radic Res. 1998;28(2):151–164. doi: 10.3109/10715769809065801. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer KD, Huecksteadt TP, Hoidal JR. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J Immunol. 1994;153(4):1789–1797. [PubMed] [Google Scholar]
- 26.Usuda N, Reddy MK, Hashimoto T, Rao MS, Reddy JK. Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Lab Invest. 1988;58(1):100–111. [PubMed] [Google Scholar]
- 27.Wilkinson SR, Prathalingam SR, Taylor MC, Horn D, Kelly JM. Vitamin C biosynthesis in trypanosomes: A role for the glycosome. Proc Natl Acad Sci U S A. 2005;102(33):11645–11650. doi: 10.1073/pnas.0504251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hille R, Massey V. Studies on the oxidative half-reaction of xanthine oxidase. J Biol Chem. 1981;256(17):9090–9095. [PubMed] [Google Scholar]
- 29.Guerciolini R, Szumlanski C, Weinshilboum RM. Human liver xanthine oxidase: nature and extent of individual variation. Clin Pharmacol Ther. 1991 Dec;50(6):663–672. doi: 10.1038/clpt.1991.205. [DOI] [PubMed] [Google Scholar]
- 30.Tan S, Gelman S, Wheat JK, Parks DA. Circulating xanthine oxidase in human ischemia reperfusion. South Med J. 1995;88(4):479–482. doi: 10.1097/00007611-199504000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Pesonen EJ, Linder N, Raivio KO, Sarnesto A, Lapatto R, Hockerstedt K, et al. Circulating xanthine oxidase and neutrophil activation during human liver transplantation. Gastroenterology. 1998;114(5):1009–1015. doi: 10.1016/s0016-5085(98)70321-x. [DOI] [PubMed] [Google Scholar]
- 32.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, et al. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107(10):1383–1389. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 33.de Jong JW, Schoemaker RG, de Jonge R, Bernocchi P, Keijzer E, Harrison R, et al. Enhanced expression and activity of xanthine oxidoreductase in the failing heart. J Mol Cell Cardiol. 2000;32(11):2083–2089. doi: 10.1006/jmcc.2000.1240. [DOI] [PubMed] [Google Scholar]
- 34.Radi R, Rubbo H, Bush K, Freeman BA. Xanthine Oxidase Binding to Glycosaminoglycans: Kinetics and Superoxide Dismutase Interactions of Immobilized Xanthine Oxidase-Heparin Complexes. Arch Biochem Biophys. 1997;339(1):125–135. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 35.Panus PC, Wright SA, Chumley PH, Radi R, Freeman BA. The contribution of vascular endothelial xanthine dehydrogenase/oxidase to oxygen-mediated cell injury. Arch Biochem Biophys. 1992;294(2):695–702. doi: 10.1016/0003-9861(92)90743-g. [DOI] [PubMed] [Google Scholar]
- 36.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106(24):3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 37.Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR.Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia Am J Physiol. 1996270(6 Pt 1):L941–L946. [DOI] [PubMed] [Google Scholar]
- 38.Zweier JL, Kuppusamy P, Lutty GA. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doehner W, Anker SD. Uric acid in chronic heart failure. Semin Nephrol. 2005;25(1):61–66. doi: 10.1016/j.semnephrol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Prasad K, Kalra J, Bharadwaj L. Cardiac depressant effects of oxygen free radicals. Angiology. 1993;44(4):257–270. doi: 10.1177/000331979304400401. [DOI] [PubMed] [Google Scholar]
- 41.Baldus S, Koster R, Chumley P, Heitzer T, Rudolph V, Ostad MA, et al. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39(9):1184–1190. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yiginer O, Ozcelik F, Inanc T, Aparci M, Ozmen N, Cingozbay BY, et al. Allopurinol improves endothelial function and reduces oxidant-inflammatory enzyme of myeloperoxidase in metabolic syndrome. Clin Res Cardiol. 2008;97(5):334–340. doi: 10.1007/s00392-007-0636-3. [DOI] [PubMed] [Google Scholar]
- 43.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35(3):746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 44.Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M, et al. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;110(15):2175–2179. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- 45.Sisto T, Paajanen H, Metsa-Ketela T, Harmoinen A, Nordback I, Tarkka M. Pretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass grafting. Ann Thorac Surg. 1995;59(6):1519–1523. doi: 10.1016/0003-4975(95)00197-s. [DOI] [PubMed] [Google Scholar]
- 46.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Quyyumi AA, Panza JA.Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients Hypertension 199730(1 Pt 1):57–63. [DOI] [PubMed] [Google Scholar]
- 47.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 48.Farquharson CAJ, Butler R, Hill A, Belch JJF, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106(2):221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 49.Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91(6):749–753. doi: 10.1136/hrt.2004.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George J, Carr E, Davies J, Belch JJF, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 51.Jamieson NV, Lindell S, Sundberg R, Southard JH, Belzer FO. An analysis of the components in UW solution using the isolated perfused rabbit liver. Transplantation. 1988;46(4):512–516. doi: 10.1097/00007890-198810000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Johnson WD, Kayser KL, Brenowitz JB, Saedi SF.A randomized controlled trial of allopurinol in coronary bypass surgery Am Heart J 1991121(1 Pt 1):20–24. [DOI] [PubMed] [Google Scholar]
- 53.Rashid MA, William-Olsson G. Influence of allopurinol on cardiac complications in open heart operations. Ann Thorac Surg. 1991;52(1):127–130. doi: 10.1016/0003-4975(91)91433-v. [DOI] [PubMed] [Google Scholar]
- 54.Parmley LF, Mufti AG, Downey JM. Allopurinol therapy of ischemic heart disease with infarct extension. Can J Cardiol. 1992;8(3):280–286. [PubMed] [Google Scholar]
- 55.Kaliakin IE, Mit’kin AF. [Effects of allopurinol on uric acid metabolism and lipid peroxidation in ischemic heart disease patients with stable angina] Kardiologiia. 1993;33(2):15–17. [PubMed] [Google Scholar]
- 56.Coghlan JG, Flitter WD, Clutton SM, Panda R, Daly R, Wright G, et al. Allopurinol pretreatment improves postoperative recovery and reduces lipid peroxidation in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1994;107(1):248–256. [PubMed] [Google Scholar]
- 57.Taggart DP, Young V, Hooper J, Kemp M, Walesby R, Magee P, et al. Lack of cardioprotective efficacy of allopurinol in coronary artery surgery. Br Heart J. 1994;71(2):177–181. doi: 10.1136/hrt.71.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamazaki I, Soma T, Ichikawa Y, Iwai Y, Kondo J, Matsumoto A. [Usefulness of allopurinol for prevention of myocardial reperfusion injury in open heart surgery] Nippon Kyobu Geka Gakkai Zasshi. 1995;43(1):26–31. [PubMed] [Google Scholar]
- 59.Castelli P, Condemi AM, Brambillasca C, Fundaro P, Botta M, Lemma M, et al. Improvement of cardiac function by allopurinol in patients undergoing cardiac surgery. J Cardiovasc Pharmacol. 1995;25(1):119–125. doi: 10.1097/00005344-199501000-00019. [DOI] [PubMed] [Google Scholar]
- 60.Coetzee A, Roussouw G, Macgregor L. Failure of allopurinol to improve left ventricular stroke work after cardiopulmonary bypass surgery. J Cardiothorac Vasc Anesth. 1996;10(5):627–633. doi: 10.1016/s1053-0770(96)80141-8. [DOI] [PubMed] [Google Scholar]
- 61.Guan W, Osanai T, Kamada T, Hanada H, Ishizaka H, Onodera H, et al. Effect of allopurinol pretreatment on free radical generation after primary coronary angioplasty for acute myocardial infarction. J Cardiovasc Pharmacol. 2003;41(5):699–705. doi: 10.1097/00005344-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Munkres KD. Ageing of Neurospora crassa IV. Induction of senescence in wild type by dietary amino acid analogs and reversal by antioxidants and membrane stabilizers. Mech Ageing Dev. 1976;5(3):171–191. doi: 10.1016/0047-6374(76)90017-8. [DOI] [PubMed] [Google Scholar]
- 63.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 64.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 65.Amado LC, Saliaris AP, Raju SV, Lehrke S, St John M, Xie J, et al. Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failure. J Mol Cell Cardiol. 2005;39(3):531–536. doi: 10.1016/j.yjmcc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 66.Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS. Role of xanthine oxidase inhibitor as free radical scavenger: a novel mechanism of action of allopurinol and oxypurinol in myocardial salvage. Biochem Biophys Res Commun. 1987;148(1):314–319. doi: 10.1016/0006-291x(87)91112-0. [DOI] [PubMed] [Google Scholar]
- 67.Hoey BM, Butler J, Halliwell B. On the specificity of allopurinol and oxypurinol as inhibitors of xanthine oxidase. A pulse radiolysis determination of rate constants for reaction of allopurinol and oxypurinol with hydroxyl radicals. Free Radic Res Commun. 1988;4(4):259–263. doi: 10.3109/10715768809055151. [DOI] [PubMed] [Google Scholar]
- 68.Ricardo SD, Bertram JF, Ryan GB. Podocyte architecture in puromycin aminonucleoside-treated rats administered tungsten or allopurinol. Exp Nephrol. 1995;3(5):270–279. [PubMed] [Google Scholar]
- 69.Keshavarzian A, Morgan G, Sedghi S, Gordon JH, Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut. 1990;31(7):786–790. doi: 10.1136/gut.31.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Augustin AJ, Boker T, Blumenroder SH, Lutz J, Spitznas M. Free radical scavenging and antioxidant activity of allopurinol and oxypurinol in experimental lens-induced uveitis. Invest Ophthalmol Vis Sci. 1994;35(11):3897–3904. [PubMed] [Google Scholar]
- 71.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62(2):212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 72.Malkiel S, Har-el R, Schwalb H, Uretzky G, Borman JB, Chevion M. Interaction between allopurinol and copper: possible role in myocardial protection. Free Radic Res Commun. 1993;18(1):7–15. doi: 10.3109/10715769309149909. [DOI] [PubMed] [Google Scholar]
- 73.Nishizawa J, Nakai A, Matsuda K, Komeda M, Ban T, Nagata K. Reactive oxygen species play an important role in the activation of heat shock factor 1 in ischemic-reperfused heart. Circulation. 1999;99(7):934–941. doi: 10.1161/01.cir.99.7.934. [DOI] [PubMed] [Google Scholar]
- 74.Granger DN, Benoit JN, Suzuki M, Grisham MB.Leukocyte adherence to venular endothelium during ischemia-reperfusion Am J Physiol 1989257(5 Pt 1):G683–G688. [DOI] [PubMed] [Google Scholar]
- 75.Kittleson MM, Hare JM. Xanthine oxidase inhibitors: an emerging class of drugs for heart failure. Eur Heart J. 2005;26(15):1458–1460. doi: 10.1093/eurheartj/ehi321. [DOI] [PubMed] [Google Scholar]
- 76.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, et al. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85(5):437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 77.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart Circ Res 2002. 22;903297–304. [DOI] [PubMed] [Google Scholar]
- 78.Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marban E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am J Physiol Heart Circ Physiol. 2006;290(2):H837–H843. doi: 10.1152/ajpheart.00831.2005. [DOI] [PubMed] [Google Scholar]
- 79.Stull LB, Leppo MK, Szweda L, Gao WD, Marban E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ Res. 2004;95(10):1005–1011. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 80.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101(45):15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perez NG, Gao WD, Marban E. Novel myofilament Ca2+-sensitizing property of xanthine oxidase inhibitors. Circ Res. 1998;83(4):423–430. doi: 10.1161/01.res.83.4.423. [DOI] [PubMed] [Google Scholar]
- 82.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, et al. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104(20):2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]