Abstract
Whether early hominins were adept tree climbers is unclear. Although some researchers have argued that bipedality maladapts the hominin skeleton for climbing, others have argued that early hominin fossils display an amalgamation of features consistent with both locomotor strategies. Although chimpanzees have featured prominently in these arguments, there are no published data on the kinematics of climbing in wild chimpanzees. Without these biomechanical data describing how chimpanzees actually climb trees, identifying correlates of climbing in modern ape skeletons is difficult, thereby limiting accurate interpretations of the hominin fossil record. Here, the first kinematic data on vertical climbing in wild chimpanzees are presented. These data are used to identify skeletal correlates of climbing in the ankle joint of the African apes to more accurately interpret hominin distal tibiae and tali. This study finds that chimpanzees engage in an extraordinary range of foot dorsiflexion and inversion during vertical climbing bouts. Two skeletal correlates of modern ape-like vertical climbing are identified in the ankle joint and related to positions of dorsiflexion and foot inversion. A study of the 14 distal tibiae and 15 tali identified and published as hominins from 4.12 to 1.53 million years ago finds that the ankles of early hominins were poorly adapted for modern ape-like vertical climbing bouts. This study concludes that if hominins included tree climbing as part of their locomotor repertoire, then they were performing this activity in a manner decidedly unlike modern chimpanzees.
Keywords: chimpanzee, tibia, talus
One of the defining attributes of the hominin* lineage is terrestrial bipedality. A fossil tibia from Kanapoi, Kenya, demonstrates that bipedality evolved as early as 4.12 million years ago (mya) in Australopithecus anamensis (1, 2). There is more tentative and fragmentary evidence that bipedalism may have evolved in the hominin genera Ardipithecus (3), Orrorin (4, 5), or Sahelanthropus (6) as early as 6 mya. Current molecular evidence suggests that the last common ancestor of humans and chimpanzees lived 4–8 mya (reviewed in ref. 7) and therefore terrestrial bipedalism may be a defining characteristic of the hominin lineage.
Although early hominins were apparently bipedal, whether they were obligate or facultative bipeds and whether early hominins also engaged in arboreal activities remain unclear. Some researchers have argued that the evolution of bipedality results in postcranial morphologies that preclude any significant amount of tree climbing (e.g., refs. 8–11). Others have regarded retained primitive morphologies of the early hominin postcranial skeleton as evidence for arboreality (e.g., refs. 12–15). Still others have argued that if early hominins were engaged in any significant amount of arboreality, then they were climbing in a manner kinematically distinct from any known anthropoid primate (16–19). In part, what has hindered a resolution to this debate is the complete absence of kinematic data on climbing in wild chimpanzees.
Although early hominins were not chimpanzees per se, some researchers have argued that the last common ancestor of humans and chimpanzees was probably quite chimpanzee-like (20), including in its locomotion (21, 22). Furthermore, when the prospects of climbing in hominins are discussed in the paleoanthropological literature, the model that is used is often a chimpanzee one (e.g., refs. 13–15). This tendency to regard early hominins as chimpanzee-like is becoming more and more prevalent (11), and I suggest here that climbing adaptations in early hominins have been promoted with this chimpanzee model in mind but without a rigorous test of the utility of this model.
This study presents the first kinematic data on climbing in wild chimpanzees, focusing specifically on the ankle joint. Climbing data were obtained on members of the Ngogo community of ≈150 wild chimpanzees, located in the Kibale National Park of western Uganda. The chimpanzees were filmed in lateral view during vertical climbing bouts, and the kinematics of climbing at the ankle joint was assessed (detailed in Materials and Methods). These data were used to identify skeletal correlates of vertical climbing in the distal tibia and talus of the African apes. The results of the skeletal analysis were applied to the fossil record (Table S1) to assess whether hominin distal tibiae or tali retain any adaptations for ape-like vertical climbing and to test the hypothesis that early hominins were capable tree climbers.
Results and Discussion
Biomechanics of Climbing in Wild Chimpanzees.
Wild chimpanzees dorsiflex at the ankle joint 45.5° ± 7.1° (n = 63) during vertical climbing bouts. Maximum dorsiflexion occurs during a “stance” phase of climbing in which the opposite foot is pushing off the substrate (Fig. 1). During this time, the ipsilateral hand is still in contact with the tree and the contralateral hand has released from the tree trunk to reach upwards. Although not directly measured, much of the body weight is likely supported on a single highly dorsiflexed ankle at this point during climbing. This dorsiflexion occurs solely at the ankle joint; dorsiflexion of the midfoot region, often referred to as the “midtarsal break,” occurs during the subsequent push-off phase of climbing. This high angle of dorsiflexion during vertical climbing occurs in captive populations of gorilla, orangutan, and gibbon as well (23, 24) and appears to be a strategy of climbing unique to the apes. Dorsiflexion is considerably less (≈15–25°) during climbing bouts in cercopithecoid monkeys (24, 25). Furthermore, maximum dorsiflexion during normal walking in modern humans is only 15–20° (26–28). Dorsiflexing the human ankle to 45° results in soft-tissue failure and severe injury (29, 30). Thus, the mean angle of dorsiflexion achieved by chimpanzees during vertical climbing bouts results in severe soft-tissue injury in the modern human ankle. Although difficult to accurately quantify, in all vertical climbing bouts (n = 166) chimpanzees could be qualitatively observed to engage in significant foot inversion while ascending a tree. Chimpanzees will grasp onto the vertical substrate with the abducted hallux and the lateral digits, while inverting the foot to increase the contact between the tree and the sole of the foot. Inversion of the foot was especially apparent when the chimpanzee was climbing smaller diameter trees and lianas.
Fig. 1.
Lateral view of vertical climbing in an adult male chimpanzee. Notice the extreme dorsiflexion of the right ankle. The left leg has already pushed off the tree, and the left arm is reaching upward, meaning that much of the body weight is being supported on the highly flexed right ankle.
Hanna et al. (31) have found that climbing efficiency is invariant across a wide range of body sizes and morphologies in primates, and therefore these joint movements of extreme dorsiflexion and inversion are likely critical adaptations for safe climbing rather than for energetically efficient climbing. Pontzer and Wrangham (32) have also suggested that climbing adaptations in chimpanzees are related to safety rather than energetic costs. When any animal is vertically climbing, a torque, or moment, is produced and is the product of the mass of the animal, the downward acceleration due to gravity, and the distance that the animal is from the tree. This moment must be countered by the grasping forces of the hand and foot of the climbing animal. These muscular forces acting against gravity can be minimized by reducing one of the variables increasing the torque. Because the force of gravity and the mass of the animal cannot be altered during climbing, reducing the horizontal distance that the animal is from the tree will reduce the torque and thus reduce the muscular forces counteracting gravity, thereby ensuring a safer vertical ascent. This strategy of being close to the vertical substrate during climbing has been theoretically discussed (33–36) and has been actually observed in animals ranging from geckos (37) to the great apes (38, 39). Flexion at the hip and knee has been discussed as a means to keep climbing apes close to the substrate (38, 39), although these are the first data demonstrating a similar strategy for the ankle.
Ankle Dorsiflexion.
Stress on a joint is a complex function of the magnitude of the force crossing the joint, its surface area, and its range of motion (40). Stress can be reduced by an increased joint surface area. Because climbing apes load their ankles in extreme dorsiflexion and engage in frequent vertical climbing activities (39, 41), the ape ankle joint should be adapted for positions of extreme dorsiflexion. Work on human cadavers has demonstrated that the contact area between the tibia and the talus shifts anteriorly during dorsiflexion (42, 43), and thus suspecting that the same would be true for the African apes would be reasonable. Therefore, the anterior aspect of the distal tibia in climbing hominoids is predicted to be mediolaterally expanded.
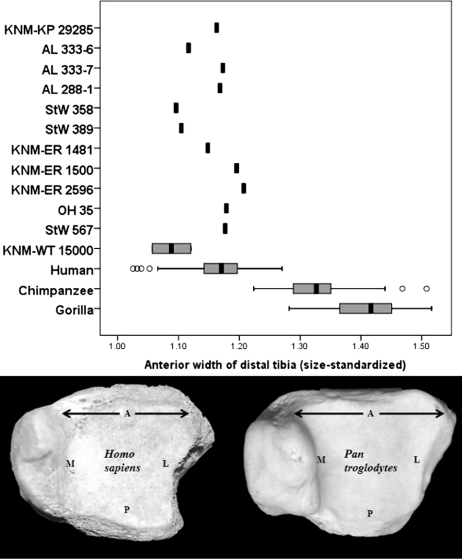
A size-standardized approach was used to assess the distribution of bone on the articular surface of the distal tibia (see Materials and Methods for details). Compared with the African apes, humans have a statistically equivalent anteroposterior length at the midpoint of the distal tibia and a statistically identical mediolateral width at the midpoint of the tibial articular facet (Table S2). These results imply that anteroposterior and mediolateral measurements taken at the midpoint of the tibial articular surface do not distinguish African apes and humans and differences instead exist at the edges of the ankle joint. As hypothesized, the mediolateral width of the anterior aspect of the distal tibia is dramatically longer in African apes than that in humans (two-tailed, t = 26.8, P < 0.0001). Additionally, the anteroposterior length on the medial side is significantly longer in African apes than that in humans (two-tailed, t = 7.97, P < 0.0001). In contrast, humans have an anteroposteriorly longer lateral aspect of the distal tibia than African apes (two-tailed, t = 16.97, P < 0.0001) and have a broader mediolateral width of the posterior aspect of the bone as well (two-tailed, t = 15.03, P < 0.0001).
The distribution of bone on the talar articular surface on 12 relatively complete hominin fossils from 4.12 mya (Australopithecus anamensis) to 1.53 mya (Homo erectus) was assessed using the size-standardized approach (see Materials and Methods for details). All 12 of the fossil hominin tibiae have articular surface geometries like those of modern humans and lack the mediolaterally broad anterior surface typical of modern African ape tibiae (Table S2). Interestingly, the fossils do not fall between the modern human and modern African ape distributions and therefore are unambiguous in terms of how the joint was being loaded in these extinct hominins (Fig. 2). These data suggest that the range of motion and the loading of the ankle in all known hominins for which distal tibiae have been recovered are like those found in modern humans and not African apes.
Fig. 2.
Width of the anterior aspect of the distal tibia in humans, African apes, and extinct hominins. The distal tibiae of chimpanzees and gorillas are mediolaterally wide along the anterior aspect and as a result have a trapezoid-like appearance in inferior view (see chimpanzee on bottom right). Humans, in contrast, have a more square-shaped articular surface to the distal tibia in inferior view (bottom left). All of the hominin tibiae studied (n = 12) are human-like and lack the wide anterior rim found in the distal tibiae of climbing apes. The box plots show the median (black bar), interquartile range (gray box), and overall ranges (whiskers). Outliers (circles) are defined as >1.5 times the interquartile range. Letters on the distal tibiae indicate anterior (A), posterior (P), medial (M), and lateral (L) aspects of the bone.
Whereas African apes are adapted for loading the ankle in dorsiflexion and inversion, the square-shaped human tibiae may be adapted for a more uniform distribution of forces across the joint surface and to maintain joint congruence throughout the range of motion. Data from Winter (44) indicate that during normal walking the foot is in slight plantarflexion at heel strike and the first 20% of stance phase. The foot begins dorsiflexing at this time and typically reaches an angle of dorsiflexion of 7° (44) to 14° (45) by 70% of stance phase. During the final 30% of stance phase, the foot plantarflexes again to prepare for the push-off phase of walking and achieves a maximum position of plantarflexion of between 14° (44) and 20° (45) at push-off. Vertical ground reaction forces at the ankle are the highest at 20% of stance phase, when the foot is in slight (≈5°) plantarflexion but beginning to dorsiflex, and 80%, when the foot is in slight (≈5°) dorsiflexion but beginning to plantarflex. Although not nearly as broad as that found in African apes, humans still possess a slightly wider anterior than posterior aspect of the tibial articular surface. Internal studies of trabecular bone have found that the posterior portion of the distal tibia is both stronger (46) and has an increased bone volume density, trabecular number, thickness, and orientation relative to the anterior portion of the distal tibia (47), perhaps an internal bony adaptation to the reduction in contact area, and thus increase in stress, between the tibia and the talus during plantarflexion (48). This combination of external and internal bony architecture may reflect adaptations to the high forces incurred throughout the ankle during the stance phase of walking. The reasons for the broader lateral aspect of the human distal tibial joint are not as clear, although the rapid pronation of the foot after heel contact (49), which shifts the contact area between the talus and the tibia to a lateral position (48), may be a factor. Nevertheless, by having more bone along the lateral and posterior portions of the distal tibia than African apes do, humans necessarily reduce the relative amount of bone that is along the anterior and medial portion of the joint surface. There may be a trade-off in the distal tibia in which adaptations for efficient force distribution through the talocrural joint surface during bipedalism renders the bone maladapted for joint movements and force distribution incurred during bouts of modern ape-like vertical climbing.
Ankle Inversion.
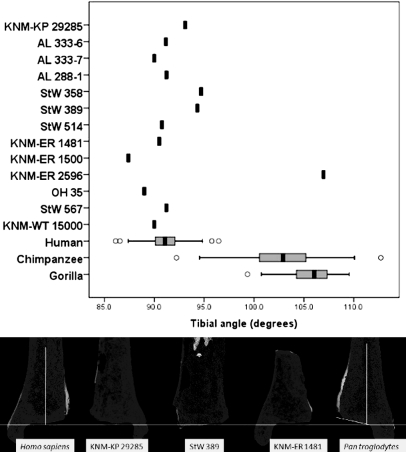
Inversion is an important joint motion for positioning the sole of the foot against the vertical substrate during climbing bouts in wild chimpanzees. Much of this motion occurs at the subtalar and transverse tarsal joints (50); however, the geometry of the ankle joint is important in positioning the foot in inversion as well (8). Nonhuman primates have a straight or a bowed femur and an obliquely oriented tibia relative to the horizontal plane of the ankle joint. This anatomy positions the knees lateral to the center of gravity and places the free foot in an inverted set. In contrast, humans possess an obliquely oriented femur with a bicondylar angle and a tibia in which the long axis is perpendicularly oriented relative to the plane of the ankle joint. This morphology positions both the knees and the ankles directly under the center of gravity, an important adaptation for terrestrial bipedalism (8, 51). The sole of the human foot is directed plantarly rather than medially when in a neutral position. There are thus relationships among the geometries of the femur, tibia, and foot in primates.
A skeletal correlate of foot inversion is the angle between the long axis of the tibia and the plane of the ankle joint, which has been found to differentiate human and African ape distal tibia (8). This angle has been used in functional interpretations of three Australopithecus afarensis tibiae from Hadar (8) and the KNM-ER 1500 and KNM-KP 29285 hominin tibiae as well (52). This present study expands this approach and examines all of the published hominin distal tibiae. The fossil tibiae were digitally cross-sectioned using three-dimensional (3D) scans acquired with a portable desktop laser scanner. The angle formed between the long axis of the tibia and the articular surface at the distal end of the bone is 91.1° ± 2.4° in humans (n = 28), 102.6° ± 4.4° in chimpanzees (n = 31), and 105.7° ± 2.5° in gorillas (n = 29). The difference between the angle in humans and the African apes is statistically significant (two-tailed, t = 16.35, P < 0.001). All of the fossil hominin tibiae, except one, KNM-ER 2596, are decidedly human-like in possessing a perpendicularly oriented ankle joint relative to the long axis of the tibia (Fig. 3). The A. anamensis tibia has a slight tilt to its articular surface of 93.1°, and two A. africanus tibiae also have slight tilts of 94.7° and 94.3° for StW 358 and StW 389, respectively, although these values are well within the modern range of variation. All of the other hominin tibiae are within one standard deviation of the modern human mean, except for KNM-ER 1500, which has a lower angle of 87.4°, although this too is within the modern human range (Fig. 3). These data suggest that hominins did not have an inverted “set” to the ankle joint and would have had a compromised ability to invert their foot against an arboreal substrate. Without this ability to invert the foot against a tree, early hominins would have needed an even greater capacity for extreme dorsiflexion to keep themselves close to the vertical substrate. Given the data presented here and ligamentous data presented elsewhere (24), extreme dorsiflexion in the early hominin ankle is not likely. The one tibia with an inverted “set,” KNM-ER 2596, has a human-like expanded metaphysis and is therefore a hominin and not a hominoid or cercopithecoid. However, the tibia is most likely from a pathological individual that suffered an unhealed tibial or fibular fracture as a juvenile, which can result in an adult valgus ankle in modern humans (53, 54).
Fig. 3.
Angle between the plane of the ankle joint and the long axis of the tibia in humans, African apes, and fossil hominins. Chimpanzees and gorillas have an obliquely oriented tibia relative to the plane of the ankle joint, positioning the foot in inversion. Humans have a perpendicularly oriented tibia relative to the horizontal plane of the ankle joint. All fossil hominins, except for the pathological KNM-ER 2596, are decidedly human-like for this feature. The box plots show the median (black bar), interquartile range (gray box), and overall ranges (whiskers). Outliers (circles) are defined as >1.5 times the interquartile range. Under the graph, digital cross-sections of (left to right) human, KNM-KP 29285 (A. anamensis), StW 389 (A. africanus), KNM-ER 1481 (Homo sp.), and chimpanzee are shown. A line has been drawn perpendicular to the long axis of these tibiae to show the oblique tilt of the chimpanzee articular surface and the approximately perpendicular orientation of the articular surface in hominin and modern human tibiae.
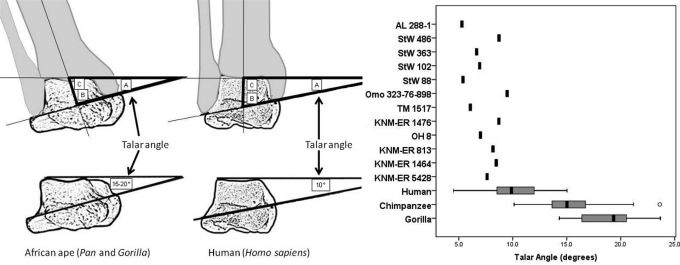
With the geometric relationships calculated by Latimer et al. (8), the ankle joint of African apes and humans can also be distinguished by the angle formed between the horizontal plane of the ankle joint and the axis of rotation of the ankle joint. Because the axis of rotation runs through the most inferior aspects of the malleolar facets on a talus (55) and the horizontal plane of the ankle joint runs mediolaterally through the most superior aspect of the talus, the general geometry of the ankle can be estimated using isolated tali (Fig. 4). This angle is 15.5° ± 2.9° in chimpanzees and 18.8° ± 2.5° in lowland gorillas. In contrast, this angle in the human talus is 10.2° ± 2.3°, significantly distinct from the average value measured in the talus of the African apes (two-tailed, t = 12.1, P < 0.001). All 12 hominin fossils measured are within the range of variation found in modern humans, although interestingly all 12 have values below the human mean and are thus quite distinct from the African ape condition (Fig. 4). When these values are converted to a measure of the angle that the long axis of the tibia forms with its articular surface, the 12 hominin tali give a range of 90.3–94.5°, well within the range of the modern human ankle. This suggests that isolated tali can be used to reliably reconstruct the orientation of the long axis of the tibia over the foot and indirectly determine whether the knee was in a varus or valgus position. These data imply that an isolated talus may be used to reconstruct the general geometry of the entire lower limb, from the orientation of the tibia over the foot to the position of the knee. Specimens for which a distal femur is also present (KNM-ER 1481, KNM-ER 1500, and KNM-WT 15000) corroborate this hypothesis.
Fig. 4.
Lines drawn through the long axis of the tibia, the axis of rotation of the ankle, and the superior plane of the ankle form a triangle. The angle formed between the long axis of the tibia and the axis of rotation of the ankle (B) is conserved between humans and the African apes (8). Thus, the angle formed between the axis of rotation of the ankle and the plane of the ankle (A), even if taken on an isolated talus, can be used to calculate the angle that the long axis of the tibia formed with the articular surface of the tibia (C). All of the hominin tali (n = 12) are human-like in possessing a low angle between the axis of rotation and the horizontal plane of the ankle, implying that these individuals would have also possessed a human-like perpendicularly oriented tibia. African apes, in contrast, have a larger talar angle, and thus an obliquely oriented tibia. The box plots show the median (black bar), interquartile range (gray box), and overall ranges (whiskers). Outliers (circles) are defined as >1.5 times the interquartile range. [Redrawn from Latimer et al. (8).]
These results collectively suggest that early hominins did not have an inverted “set” to the ankle joint, nor did they load their ankles in dorsiflexion. Together with the absence of a grasping hallux (56–58) (but see ref. 59), this ankle morphology in early hominins would have compromised their ability to position their foot against an arboreal substrate and precludes safe climbing in an ape-like manner. However, observing that body mass may affect the kinematic strategy of a climbing primate is important. As noted previously, cercopithecoid monkeys do not climb with a dorsiflexed ankle and instead pull themselves close to the vertical substrate via dorsiflexion at the midfoot (24). Although early hominins were quite small, with females only ≈30 kg (60), they possessed a stiff, nongrasping midfoot, a morphology inconsistent with a monkey-like style of climbing (24). Given, therefore, how maladapted the ankle and foot were for climbing, for these early hominins to vertically climb and engage in arboreal activities with any frequency would have been unsafe. Early hominins may have climbed trees like modern humans can and occasionally do today; however, this study suggests that vertical climbing and arboreality were not significant parts of their locomotor repertoire.
Conclusion
Modern chimpanzees safely and effectively climb trees in part because they are capable of extreme dorsiflexion and inversion at the ankle joint. Skeletal adaptations correlated with loading in these joint positions include a mediolaterally expanded anterior aspect of the distal tibia and an inverted set to the ankle joint. Although early hominins have been hypothesized to be adept tree climbers, none of the 29 known fossil tibiae or tali from 4.12 to 1.53 mya possesses the combination of features functionally correlated with vertical climbing in modern chimpanzees. On the basis of these data, if early hominins were engaging in any substantial amount of arboreal climbing, then they were doing it in a manner kinematically distinct from modern chimpanzees. Caution should thus be used when employing the chimpanzee as a model for understanding locomotion in the early hominins.
Materials and Methods
Chimpanzee Kinematics.
Observations of wild chimpanzees of the Ngogo community in the Kibale National Park, Uganda, were made during 3 weeks in June 2006 and July–August 2007. The Ngogo community of common chimpanzees (Pan troglodytes schweinfurthii) is exceptionally large, with ≈150 individuals. The unusual size of the community facilitates finding and following chimpanzees daily.
Chimpanzees were filmed opportunistically with a Canon GL2 hand-held digital video recorder. Observations were made primarily on adult males, although some juveniles and females were studied as well. Chimpanzee vertical climbing was filmed as the animal made its ascent from the forest floor to the highest height achieved in the forest canopy. The distance between the video camera and climbing chimpanzee varied because climbing episodes were filmed in real time as they occurred. The distance between the observer and climbing chimpanzee was typically between 5 and 10 m. In total, 166 separate climbing bouts were filmed. The usability of the video was assessed using the program Windows Movie Maker. Frames of vertical climbing were viewed individually with a temporal resolution of 70 ms. Several criteria were applied to identify video that could be used to assess dorsiflexion at the talocrural joint within a reasonably accurate range. First, the animal had to be in lateral view to estimate dorsiflexion at the talocrural joint. Dorsiflexion at the talocrural joint was always assessed as the maximum angle achieved by the ankle nearest to the observer. Videos in which the opposite hip or shoulder of the chimpanzee could be easily seen or was completely obscured by the tree during climbing were eliminated. Only video in which the far hip and shoulder were obscured by the near hip and shoulder were considered lateral. Second, videos of lateral views had to be captured within 2–5 m from the ground to minimize the angular errors that can be introduced by height. Of the 166 videos obtained from vertically climbing chimpanzees, 63 met the above criteria and were measured.
Video stills were imported into the program ImageJ. A knee to heel line was drawn as an approximate bisection of the tibia, whereas the long axis of the foot followed the skin/hair line that runs along the lateral side of the foot. The angle tool was used to measure the angle of dorsiflexion at the talocrural joint by drawing a straight line from the knee to the heel and another straight line from the heel through the metatarsophalangeal joint of the fifth metatarsal. The angle was then subtracted from 90° to make it comparable to results from the literature. Dorsiflexion angles of the same video stills measured a month apart suggest that maximum measurement error is ±5°. Foot inversion associated with vertical climbing was assessed qualitatively from both lateral and posterior views of climbing from all 166 vertical climbing bouts captured on film.
Skeletal Comparisons.
The right distal tibiae (n = 97) and tali (n = 95) of adult wild-shot African apes (Pan troglodytes, Pan paniscus, and Gorilla gorilla gorilla) were studied at the Cleveland Museum of Natural History (CMNH), Harvard Museum of Comparative Zoology, American Museum of Natural History (New York), National Museum of Natural History (Washington, DC), Peabody Museum (Yale University), and Field Museum (Chicago). The human tibiae (n = 136) and tali (n = 45) were from the 9th–12th century Paleoindian Libben collection housed at Kent State University (61), the Hamann–Todd collection at the CMNH, and an unprovenienced sample of human tibia from the Department of Anthropology, University of Michigan. For all measures, the three populations were first treated as separate groups, and only when they did not statistically differ for any measure were the results combined. Fossil hominin tibia and tali were studied at the Transvaal Museum in Pretoria, South Africa, the Department of Anatomy at the University of Witwatersrand in Johannesburg, South Africa, the Kenya National Museum in Nairobi, and the Tanzania National Museum and House of Culture in Dar es Salaam. High-quality research casts of the Hadar A. afarensis tibiae and tali and the Omo tali were measured at the CMNH, the Harvard Peabody Museum, and the Department of Anthropology, University of Michigan. All linear measurements on fossil and extant tibiae and tali were taken with digital calipers. Significance was assessed for all measures in this study using a t test.
Six measures were taken on the articular surface of the distal tibia: the maximum mediolateral length of the anterior, posterior, and midpoint dimensions of the articular surface and the maximum anteroposterior width of the most medial, lateral, and midpoint aspects of the articular surface. Measurement error, assessed by repeating these measures on 40 specimens a month after the original measurements were taken, was within 5%. Each raw measure was then normalized by the geometric mean, following a size adjustment protocol (62). The anterolateral corner of A.L. 333–7 was damaged, and thus the mediolateral length of the anterior surface and the anteroposterior width of the lateral aspect of the bone were estimated. The anterior aspect of StW 181 and the posterior region of StW 514 were both sheared away, and thus the dimensions of these tibiae could not be assessed with any accuracy.
An inverted set to the talocrural joint was assessed in two different ways. The angle that the long axis of the tibia forms with the distal articular surface of the tibia was measured using a carpenter's contour guide on chimpanzee (n = 31), lowland gorilla (n = 29), and modern human tibiae (n = 28) from the Hamann–Todd Collection at the CMNH. The tibiae were pressed into the carpenter's contour guide with care taken to be sure that the contour pins were parallel to the long axis of the tibial shaft. The impression of the articular surface made on the contour guide was then laid flat and photographed with a Nikon D100 digital camera. The images were imported into the program ImageJ, and the angle formed between the plane formed by the contour pins and the long axis of the tibia as inferred by the unmoved straight contour pins was measured.
Two other methods were used to measure this angle on the tibia as well. Research casts of hominin tibiae were molded using a high-quality room temperature vulcanizing silicone rubber (GI-1100; Silicones, Inc.), deaerated before pouring each side of the multipiece molds. Plaster casts of the tibiae were produced from these molds and sectioned in the coronal plane with a handsaw. The angle formed between the tibial axis and the articular surface was then measured directly with a protractor. This approach allowed the results of this study to be compared directly to the results of Latimer et al. (8), who used a similar cast sectioning method to measure the angle that the long axis of the tibia forms with the ankle. The results from the carpenter's contour guide method were within 1° of the angles measured on sectioned casts. In addition, original fossil tibiae were scanned with a NextEngine portable 3D desktop laser scanner at the maximum resolution possible of 0.1 mm. The 3D models were imported into the program ScanStudio, and with the crop tool, the bones were digitally sectioned in the coronal plane. Images of the digitally sectioned fossils were imported into ImageJ, where the angle formed between the long axis of the tibia and the articular joint surface was measured with the angle tool as described above. Measured angles were within 1° of one another for specimens in which all three methods were used (KNM-KP 29285, KNM-ER 1481, KNM-ER 1500, and KNM-ER 2596), allowing results from the three methods to be used interchangeably.
The set of the tibia on the talus was estimated using isolated tali as well from Homo sapiens (n = 45), Pan troglodytes (n = 51), and Gorilla gorilla gorilla (n = 45). The tali were positioned using sculpting clay such that the plantar edges of the calcaneal facets and the inferior aspect of the talar head were in the same plane. In this orientation, the malleolar facets are in the same coronal plane as the superior aspect of the trochlear talus, and both can be seen in a distal view. The tali were photographed with a Nikon D100 digital camera. The images were imported into the program ImageJ, and the angle between the most inferior extent of the facets for the medial and lateral malleoli and the superior surface of the talus was measured with the angle tool. Twenty randomly selected specimens were measured a second time a month after the original measurement to assess repeatability. The average difference between the two measures was 1° ± 0.5°, with a maximum difference between two measures of 1.9°. The original fossil hominin tali StW 347, SKX 42695, and KNM-ER 803 were too badly damaged along the medial or lateral aspect of the talar body to accurately take this angle.
Supplementary Material
Acknowledgments.
I am grateful to J. Mitani, J. Lwanga, and D. Watts for guidance while at Ngogo. D. Guillot provided assistance with film analysis. I am grateful to those who allowed me to study skeletal specimens in their care: E. Westwig (American Museum of Natural History), L. Gordan (National Museum of Natural History), K. Zyskowski (Yale Peabody), and W. Stanley and M. Schulenberg (Field Museum). I especially thank J. Chupasko (Harvard Museum of Comparative Zoology), L. Jellema (CMNH), and O. Lovejoy (Libben Collection at Kent State). I am grateful to Y. Haile-Selassie, D. Pilbeam, and M. Morgan for access to hominin casts. Fossils were studied in Tanzania thanks to the Tanzanian Commission for Science and Technology, A. Kwekason, and Dr. P. Msemwa; in Kenya thanks to the Kenyan Ministry of Education, Science, and Technology and Drs. I. Omar Farah and E. Mbua; in Johannesburg, South Africa, thanks to B. Zipfel, and in Pretoria, South Africa, thanks to F. Thackeray and S. Potze. I am extremely grateful to L. MacLatchy and to the rest of my thesis committee: J. Mitani, M. Wolpoff, D. Fisher, and W. Sanders. Field work in Uganda was made possible by the Uganda Wildlife Authority and Uganda National Council for Science and Technology. Funding for this work was provided by the Leakey Foundation.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
Hominin is defined as those species more closely related to modern humans than to the African apes.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900270106/DCSupplemental.
References
- 1.Leakey MG, Feibel CS, McDougall I, Walker A. New four-million-year-old hominid species from Kanapoi and Allia Bay, Kenya. Nature. 1995;376:565–571. doi: 10.1038/376565a0. [DOI] [PubMed] [Google Scholar]
- 2.Ward CV, Leakey MG, Walker A. Morphology of Australopithecus anamensis from Kanapoi and Allia Bay, Kenya. J Hum Evol. 2001;41:255–368. doi: 10.1006/jhev.2001.0507. [DOI] [PubMed] [Google Scholar]
- 3.Haile-Selassie Y. Late Miocene hominids from the Middle Awash, Ethiopia. Nature. 2001;412:178–181. doi: 10.1038/35084063. [DOI] [PubMed] [Google Scholar]
- 4.Pickford M, Senut B, Gommery D, Treil J. Bipedalism in Orrorin tugenensis revealed by its femora. CR Palevol. 2002;1:191–203. [Google Scholar]
- 5.Richmond BG, Jungers WL. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science. 2008;319:162–1665. doi: 10.1126/science.1154197. [DOI] [PubMed] [Google Scholar]
- 6.Brunet M, et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature. 2002;418:145–151. doi: 10.1038/nature00879. [DOI] [PubMed] [Google Scholar]
- 7.Bradley BJ. Reconstructing phylogenies and phenotypes: A molecular view of human evolution. J Anat. 2008;212:337–353. doi: 10.1111/j.1469-7580.2007.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latimer B, Ohman JC, Lovejoy CO. Talocrural joint in African hominoids: Implications for Australopithecus afarensis. Am J Phys Anthropol. 1987;74:155–175. doi: 10.1002/ajpa.1330740204. [DOI] [PubMed] [Google Scholar]
- 9.Latimer B. In: Origin(s) of Bipedalism in Hominids. Coppens Y, Senut B, editors. Paris: Centre National Center for Scientific Research; 1991. pp. 169–176. [Google Scholar]
- 10.Lovejoy CO. The natural history of human gait and posture. Part 2. Hip and thigh. Gait Posture. 2005;21:113–124. doi: 10.1016/j.gaitpost.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Sayers K, Lovejoy CO. The chimpanzee has no clothes. A critical examination of Pan troglodytes in models of human evolution. Curr Anthropol. 2008;49:87–114. [Google Scholar]
- 12.Stern JT, Susman RL. The locomotor anatomy of Australopithecus afarensis. Am J Phys Anthropol. 1983;60:279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- 13.Susman RL, Stern JT, Jungers WL. Arboreality and bipedality in the Hadar hominins. Folia Primatol. 1984;43:113–156. doi: 10.1159/000156176. [DOI] [PubMed] [Google Scholar]
- 14.Senut B. Climbing as a crucial preadaptation for human bipedalism. Int J Skeletal Res. 1988;14:35–44. [Google Scholar]
- 15.Preuschoft H, Witte H. In: Origin(s) of Bipedalism in Hominids. Coppens Y, Senut B, editors. Paris: National Center for Scientific Research; 1991. pp. 59–77. [Google Scholar]
- 16.Jungers WL. Lucy's limbs: Skeletal allometry and locomotion in Australopithecus afarensis. Nature. 1982;297:676–678. [Google Scholar]
- 17.McHenry HM. In: Origin(s) of Bipedalism in Hominids. Coppens Y, Senut B, editors. Paris: National Center for Scientific Research; 1991. pp. 133–141. [Google Scholar]
- 18.MacLatchy LM. Another look at the australopithecine hip. J Hum Evol. 1996;31:455–476. [Google Scholar]
- 19.Sanders WJ. Comparative morphometric study of the australopithecine vertebral series Stw-H8/H41. J Hum Evol. 1998;34:249–302. doi: 10.1006/jhev.1997.0193. [DOI] [PubMed] [Google Scholar]
- 20.Wrangham RW, Pilbeam D. In: All Apes Great and Small: African Apes. Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J, editors. Vol 1. New York: Kluwer; 2001. pp. 5–17. [Google Scholar]
- 21.Gebo DL. Climbing, brachiation, and terrestrial quadrupedalism: Historical precursors of hominin bipedalism. Am J Phys Anthropol. 1996;101:55–92. doi: 10.1002/(SICI)1096-8644(199609)101:1<55::AID-AJPA5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Richmond BG, Begun DR, Strait DS. Origin of human bipedalism: The knuckle-walking hypothesis revisited. Yearb Phys Anthropol. 2001;44:70–105. doi: 10.1002/ajpa.10019.abs. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki N, Ishida H. A biomechanical study of vertical climbing and bipedal walking in gibbons. J Hum Evol. 1984;13:563–571. [Google Scholar]
- 24.DeSilva JM. Ann Arbor, MI: Univ of Michigan; 2008. Vertical climbing adaptations in the ape ankle and midfoot. Implications for locomotion in Miocene catarrhines and Plio-Pleistocene hominins. PhD dissertation. [Google Scholar]
- 25.Hirasaki E, Kumakura H, Matano S. Kinesiological characteristics of vertical climbing in Ateles geoffroyi and Macaca fuscata. Folia Primatol. 1993;61:148–156. doi: 10.1159/000156742. [DOI] [PubMed] [Google Scholar]
- 26.Siegler S, Chen J, Schneck CD. The three-dimensional kinematics and flexibility characteristics of the human ankle and subtalar joints—Part I: Kinematics. J Biomech Eng. 1988;110:364–373. doi: 10.1115/1.3108455. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg A, Goldie I, Kalin B, Selvik G. Kinematics of the ankle/foot complex: Plantarflexion and dorsiflexion. Foot Ankle. 1989;9:194–200. doi: 10.1177/107110078900900409. [DOI] [PubMed] [Google Scholar]
- 28.Rome K. Ankle joint dorsiflexion measurement studies. A review of the literature. J Am Podiatr Med Assoc. 1996;86:205–211. doi: 10.7547/87507315-86-5-205. [DOI] [PubMed] [Google Scholar]
- 29.Begeman PC, Prasad P. Human ankle impact response in dorsiflexion. Proceedings of the 34th Stapp Car Crash Conference; Warrendale, PA: Society of Automotive Engineers Inc; 1990. pp. 39–54. No. 902308. [Google Scholar]
- 30.Parenteau CS, Viano DC, Petit PY. Biomechanical properties of human cadaveric ankle-subtalar joints in quasi-static loading. J Biomech Eng. 1998;120:105–111. doi: 10.1115/1.2834289. [DOI] [PubMed] [Google Scholar]
- 31.Hanna JB, Schmitt D, Griffin TM. The energetic cost of climbing in primates. Science. 2008;320:898. doi: 10.1126/science.1155504. [DOI] [PubMed] [Google Scholar]
- 32.Pontzer H, Wrangham RW. Climbing and the daily energetic cost of locomotion in wild chimpanzees: Implications for hominoid locomotor evolution. J Hum Evol. 2004;46:317–335. doi: 10.1016/j.jhevol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Preuschoft H. In: The Chimpanzee. Bourne GH, editor. Vol 3. Basel: Karger; 1970. pp. 221–294. [Google Scholar]
- 34.Cartmill M. In: Primate Locomotion. Jenkins FA, editor. New York: Academic; 1972. pp. 45–83. [Google Scholar]
- 35.Cartmill M. In: Functional Vertebrate Morphology. Hildebrand M, Bramble DM, Liem KF, Wake BD, editors. Cambridge, MA: Harvard Univ Press; 1985. pp. 73–88. [Google Scholar]
- 36.Preuschoft H, Witte H, Demes B. In: Topics in Primatology, Evolutionary Biology, Reproductive Endocrinology and Virology. Matano S, Tuttle RH, Ishida H, Goodman M, editors. Vol 3. Tokyo: Univ of Tokyo Press; 1992. pp. 259–289. [Google Scholar]
- 37.Autumn K, et al. Dynamics of geckos running vertically. J Exp Biol. 2006;209:260–272. doi: 10.1242/jeb.01980. [DOI] [PubMed] [Google Scholar]
- 38.Isler K. Zürich, Switzerland: Univ of Zürich; 2003. 3D-kinematics of vertical climbing in hominoids. PhD thesis. [DOI] [PubMed] [Google Scholar]
- 39.Isler K. 3D-kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126:66–82. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- 40.Currey JD. Bones: Structure and Mechanics. Princeton: Princeton Univ Press; 2002. [Google Scholar]
- 41.Hunt KD. In: The Evolution of Thought. Russon AE, Begun DR, editors. Cambridge, UK: Cambridge Univ Press; 2004. pp. 172–189. [Google Scholar]
- 42.Driscoll HL, Christensen JC, Tencer AF. Contact characteristics of the ankle joint. Part 1. The normal joint. J Am Podiatr Med Assoc. 1994;84:491–498. doi: 10.7547/87507315-84-10-491. [DOI] [PubMed] [Google Scholar]
- 43.Corazza F, Stagni R, Castelli VP, Leardini A. Articular contact at the tibiotalar joint in passive flexion. J Biomech. 2005;38:1205–1212. doi: 10.1016/j.jbiomech.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 44.Winter DA. Biomechanics and Motor Control of Human Movement. New York: Wiley; 2005. [Google Scholar]
- 45.Czerniecki JM. Foot and ankle biomechanics in walking and running. A review. Am J Phys Med Rehabil. 1988;67:246–252. [PubMed] [Google Scholar]
- 46.Hvid I, Rasmussen O, Jensen NC, Nielsen S. Trabecular bone strength profiles at the ankle joint. Clin Orthop Relat Res. 1985;199:306–312. [PubMed] [Google Scholar]
- 47.Lai YM, Qin L, Yeung HY, Lee KKH, Chan KM. Regional differences in trabecular BMD and micro-architecture of weight-bearing bone under habitual gait loading- a pQCT and microCT study in human cadavers. Bone. 2005;37:274–282. doi: 10.1016/j.bone.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 48.Kura H, Kitaoka HB, Luo Z-P, An K-N. Measurement of surface contact area of the ankle joint. Clin Biomech. 1998;13:365–370. doi: 10.1016/s0268-0033(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 49.Matsusaka N. Control of the medial-lateral balance in walking. Acta Orthop Scand. 1986;57:555–559. doi: 10.3109/17453678609014793. [DOI] [PubMed] [Google Scholar]
- 50.Lewis OJ. The joints of the evolving foot. Part II. The intrinsic joints. J Anat. 1980;130:833–857. [PMC free article] [PubMed] [Google Scholar]
- 51.Heiple KG, Lovejoy CO. The distal femoral anatomy of Australopithecus. Am J Phys Anthropol. 1971;35:75–84. doi: 10.1002/ajpa.1330350109. [DOI] [PubMed] [Google Scholar]
- 52.Ward CV, Leakey MG, Walker AC. The new hominid species Australopithecus anamensis. Evol Anthropol. 1999;7:197–205. [Google Scholar]
- 53.Wiltse LL. Valgus deformity of the ankle: A sequel to acquired or congenital abnormalities of the fibula. J Bone Joint Surg Am. 1972;54:595–606. [PubMed] [Google Scholar]
- 54.Gibson V, Prieskorn D. The valgus ankle. Foot Ankle Clin. 2007;12:15–27. doi: 10.1016/j.fcl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Inman VT. The Joints of the Ankle. Baltimore: Williams & Wilkins; 1976. [Google Scholar]
- 56.White TD, Suwa G. Hominid footprints at Laetoli: Facts and interpretations. Am J Phys Anthropol. 1987;72:485–514. doi: 10.1002/ajpa.1330720409. [DOI] [PubMed] [Google Scholar]
- 57.Latimer BM, Lovejoy CO. Hallucal tarsometatarsal joint in Australopithecus afarensis. Am J Phys Anthropol. 1990;82:125–134. doi: 10.1002/ajpa.1330820202. [DOI] [PubMed] [Google Scholar]
- 58.McHenry HM, Jones AL. Hallucial convergence in early hominids. J Hum Evol. 2006;50:534–539. doi: 10.1016/j.jhevol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Harcourt-Smith WEH, Aiello LC. Fossils, feet and the evolution of human bipedal locomotion. J Anat. 2004;204:403–416. doi: 10.1111/j.0021-8782.2004.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McHenry HM. Body size and proportions in early hominids. Am J Phys Anthropol. 1992;87:407–431. doi: 10.1002/ajpa.1330870404. [DOI] [PubMed] [Google Scholar]
- 61.Lovejoy CO, et al. Paleodemography of the Libben Site, Ottawa County, Ohio. Science. 1977;198:291–293. doi: 10.1126/science.198.4314.291. [DOI] [PubMed] [Google Scholar]
- 62.Darroch JN, Mosimann JE. Canonical and principal components of shape. Biometrika. 1985;72:241–252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.