Abstract
It was previously reported that the ciliary epithelium (CE) of the mammalian eye contains a rare population of cells that could produce clonogenic self-renewing pigmented spheres in culture. Based on their ability to up-regulate genes found in retinal neurons, it was concluded that these sphere-forming cells were retinal stem cells. This conclusion raised the possibility that CE-derived retinal stem cells could help to restore vision in the millions of people worldwide who suffer from blindness associated with retinal degeneration. We report here that human and mouse CE-derived spheres are made up of proliferating pigmented ciliary epithelial cells rather than retinal stem cells. All of the cells in the CE-derived spheres, including the proliferating cells, had molecular, cellular, and morphological features of differentiated pigmented CE cells. These differentiated cells ectopically expressed nestin when exposed to growth factors and low levels of pan-neuronal markers such as beta-III-tubulin. Although the cells aberrantly expressed neuronal markers, they retained their pigmented CE cell morphology and failed to differentiate into retinal neurons in vitro or in vivo. Our results provide an example of a differentiated cell type that can form clonogenic spheres in culture, self-renew, express progenitor cell markers, and initiate neuronal differentiation that is not a stem or progenitor cell. More importantly, our findings highlight the importance of shifting the focus away from studies on CE-derived spheres for cell-based therapies to restore vision in the degenerating retina and improving techniques for using ES cells or retinal precursor cells.
Keywords: transdifferentiation, transplantation, neurospheres, differentiation
Over 40 million people worldwide are blind. Macular degeneration accounts for ≈8 million cases of blindness. Although the cause of macular degeneration is not known, 1 potential treatment is cell therapy. Stem cells hold great promise for regenerative medicine, and recently many efforts have been devoted to finding suitable candidates for retinal transplants. These candidates include photoreceptor progenitors (1) and embryonic stem cells (2, 3). Others have looked to the ciliary epithelium (CE) as a potential source of retinal stem cells (4, 5).
In 2000, Tropepe and colleagues revealed that the CE of the mouse eye can be dissociated, maintained in culture at clonal density and stimulated to form clonogenic spheres (5). The CE-derived spheres were pigmented, expressed nestin, and could be dissociated and replated to form spheres up to 8 times. When transferred to differentiation conditions in culture, cells from the CE-derived spheres up-regulated genes found in rod photoreceptors, bipolar neurons, and Müller glia (5). These findings were later extended to CE isolated from postmortem human eyes (4). Based on these and other data, it was suggested that the sphere-forming cells in the mammalian CE are retinal stem cells (RSCs) and, thus, hold promise for therapeutic replacement of retinal neurons lost to disease or degeneration.
Given the potential impact of cell replacement therapy to treat retinal degeneration, we sought to characterize the molecular and cellular mechanisms underlying the expansion and differentiation of human and murine RSCs. We present evidence that the clonogenic spheres derived from the mouse and human CE originate from differentiated pigmented CE cells rather than a rare stem cell population harbored in the ciliary epithelium. These cells proliferate in culture while retaining all of their features of differentiated pigmented CE cells. When transferred to culture conditions that promote differentiation, the pigmented CE cells ectopically up-regulate pan-neuronal markers. However, they do not form bona fide retinal neurons or glia, in vitro or in vivo. Therefore, the mouse and human CE does not contain retinal stem cells, but instead it contains a population of differentiated pigmented CE cells that can proliferate, clonally expand, and self-renew in stem cell medium and express some neuronal markers while retaining features of pigmented epithelial cells.
Results
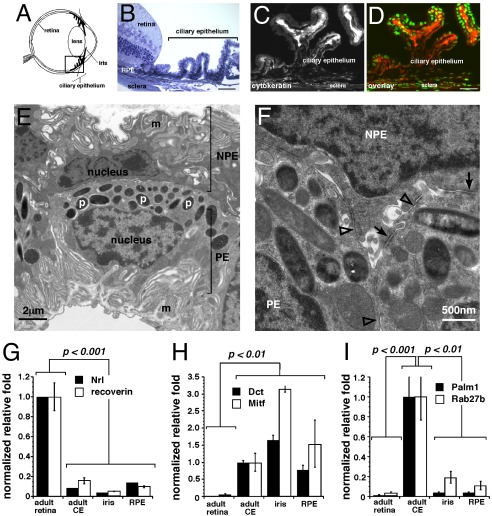
The CE of the mammalian eye (Fig. 1A) consists of a layer of pigmented epithelial cells adjacent to a layer of nonpigmented epithelial cells (Fig. 1B). Both types of CE cells express cytokeratin, which is not expressed in the retina (Fig. 1 C and D). Transmission electron microscopy (TEM) was used to visualize the melanosomes, basal and lateral membrane interdigitations, and epithelial cell–cell junctions in the CE (Fig. 1 E and F). Similar analyses of retinal progenitor cells and neural stem cells [supporting information (SI) Fig. S1] demonstrated that these properties are unique to pigmented CE cells. Real-time RT-PCR confirmed that CE cells have unique molecular profiles (Fig. 1 G–I).
Fig. 1.
Isolation and characterization of the mouse ciliary epithelium. (A) Diagram of the location of the ciliary epithelium (CE) in the mammalian eye. (B) Toluidine blue stained 1-μm thick plastic section of the adult C57BL/6 mouse CE. (C and D) Cytokeratin immunofluorescense in the adult mouse CE (red fluorescence) alone (C) and overlaid with nuclear Sytox green stain D. (E and F) Transmission electron microscopy of the mouse CE showing membrane interdigitations (m), pigment containing melanosomes (p), and epithelial junctions found in the CE including tight junctions (open arrowheads) and gap junctions (arrows). (G–I) Real time RT-PCR analysis of mouse CE, retina, retinal pigment epithelium, and iris using probes for retinal specific genes (Nrl, recoverin), pigmented cells (Dct, Mitf), and CE (Palmdelphin, Rab27b). Each bar represents the mean and standard deviation from duplicate experiments for at least 3 independent samples for each piece of tissue. Abbreviations: RPE, retinal pigment epithelium; CE, ciliary epithelium; m, membrane interdigitations; p, pigmented melanosomes; NPE, nonpigmented ciliary epithelium; PE, pigmented epithelium. (Scale bars in B–D, 10 μm.)
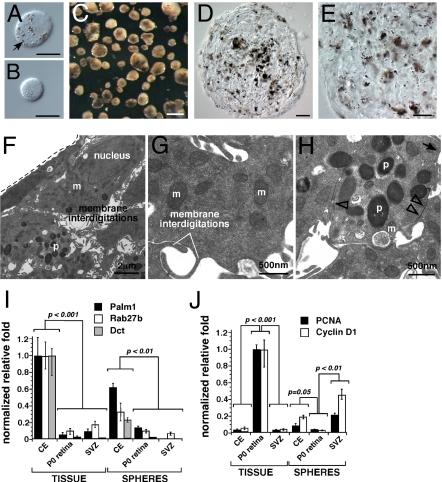
To determine whether murine CE-derived spheres contain RSCs and retinal progenitor cells as proposed (5), we performed molecular, cellular, and morphologic analyses of CE-derived spheres and compared those data to normal CE, retinal progenitor cells, retinal progenitor cell derived spheres, neural stem cells, and neural stem cell derived spheres. Dissociated CE cell preparations (Fig. 2 A and B) were maintained at clonal density (20 cells per μL) in stem cell medium for 7 days (4–6). Pigmented spheres (Fig. 2C) were identified at a frequency indistinguishable from previous results (5). TEM analysis on CE-derived spheres after 7 days in culture revealed that all of the cells in the CE-derived spheres appeared pigmented (Fig. 2 D and E) and had morphologic characteristics of normal pigmented CE cells (Fig. 2 F–H). Moreover, the cells did not have characteristics that were found in spheres derived from retinal progenitor cells or neural stem cells (Fig. S2, Fig. S3, and Table S1).
Fig. 2.
CE-derived spheres are pigmented. (A and B) Representative DIC images of pigmented and nonpigmented dissociated mouse CE cells. (C) Brightfield image of CE-derived spheres after 7 days in culture from the mouse. (D and E) Cryosections of the mouse CE-derived spheres imaged using DIC optics. (F–H) TEM analysis of mouse CE-derived spheres emphasizing membrane interdigitations (F and G) and epithelial junctions (H) found in the normal CE (tight junctions, open arrowheads; gap junctions, arrow). (I) Real time RT-PCR analysis of CE-derived spheres using probes for genes found in the normal CE (Palm1, Rab27b, Dct). The source of retinal progenitor cells was P0 mouse retina, and the source of neural stem cells was adult SVZ. (J) Genes normally expressed in proliferating cells (PCNA, Cyclin D1) were also analyzed in these samples using real time RT-PCR. Each bar represents the mean and standard deviation from duplicate experiments for at least 3 independent samples for each piece of tissue. Abbreviations: m, mitochondria; p, pigment; CE, ciliary epithelium; SVZ, subventricular zone. (Scale bars in A and B, 5 μm; C, 100 μm; D and E, 10 μm.)
To validate this finding, we analyzed gene expression in CE-derived spheres by real-time RT-PCR using TaqMan probes. Consistent with the TEM analysis, the CE-derived spheres expressed genes found in the primary CE (Fig. 2I) and those required for proliferation (Fig. 2J). They did not express genes found in retinal progenitor cells (Fig. S2) or neural stem cells (Fig. S3). Affymetrix gene expression microarray analyses of primary mouse CE, CE-derived spheres, and retinal samples confirmed the high level of similarity between the gene expression profiles of pigmented CE and the CE-derived spheres (Table S2).
We predicted that if pigmented CE cells are the cells of origin for CE-derived spheres, then tissue samples from mutant mice that aberrantly produce more pigmented CE cells will show increased sphere production. Previous studies have shown that in the Chx10orJ/orJ microphthalmic mouse strains, CE-sphere formation is increased (7). To determine whether this finding was the result of an increased number of pigmented CE cells, we analyzed the CE from Chx10orJ/orJ eyes (Fig. S4 A–D). Chx10orJ/orJ eyes gave rise to more spheres per eye (Fig. S4 F–H), and they had more pigmented CE cells per eye than did their wild-type littermates (Fig. S4 E–G). TEM analysis of serial sections of the spheres from the Chx10orJ/orJ eye revealed that each cell was a pigmented CE cell (Fig. S4I). These data suggest that the increase in CE cells in the Chx10orJ/orJ eye is sufficient to account for the increased efficiency of sphere production from these animals.
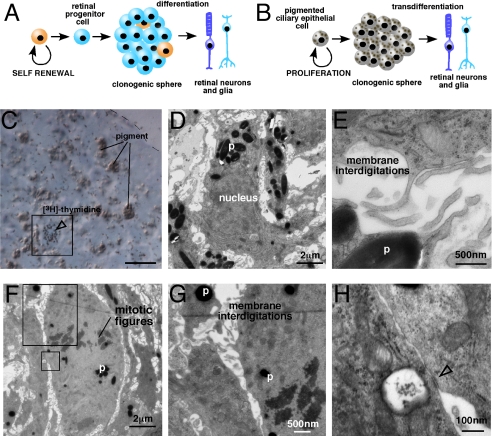
On the basis of the data presented above, we propose that the murine CE–derived spheres are made up of proliferating pigmented CE cells rather than RSCs or progenitors (Fig. 3 A and B). To test this hypothesis, we identified and characterized the proliferating cells in the CE-derived spheres. We labeled S-phase cells by incubating the spheres with [3H]-thymidine for 1 h on Day 3. The spheres were then immediately harvested, and a 1-μm section was collected, stained with toluidine blue, and overlaid with autoradiographic emulsion to detect the [3H]-thymidine+ cells (Fig. 3C). Adjacent to the 1-μm section, ten 50-nm serial sections were collected and processed for TEM imaging. By aligning the 1-μm and 50-nm sections, we were able to characterize the morphologic features of the S-phase cells (Fig. 3 D and E). Every cell analyzed had all of the morphologic features of pigmented CE cells including pigment, membrane interdigitations, and epithelial junctions (Table S3 and Fig. 3E). TEM analysis of M-phase cells revealed that they also had the same features as pigmented CE cells (Fig. 3 F–H).
Fig. 3.
Pigmented CE cells are proliferating in CE-derived spheres. (A and B) Comparision of the retinal stem cell model and the transdifferentiation model. (C) S-phase cells were labeled by incubating Day 3 spheres with [3H]-tymidine for 1 h. Immediately after labeling, the spheres were fixed and a 1-μm thick section was collected from the center of the sphere. This section was stained with toluidine blue and overlaid with autoradiographic emulsion to detect the cells in S-phase (box, open arrowhead). (D and E) Immediately after the 1-μm section, 10 serial 50-nm sections were collected for transmission electron microscopy. These sections were imaged and aligned to the [3H]-tymidine–labeled section to characterize the morphology of the cells in S-phase. The cell shown in (C) is also shown in (D and E) by aligning the sections. (F–H) Cells in M-phase were identified by the presence of condensed chromosomes (mitotic figures). These cells also contained pigment, membrane interdigitations, and epithelial junctions (open arrowhead). Abbreviations: p, pigment. (Scale bar in C, 10 μm.)
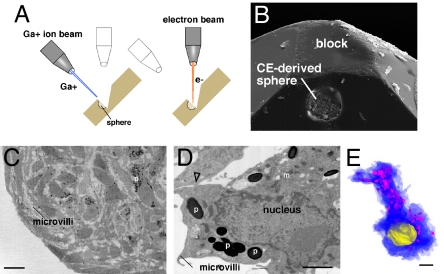
Although these data indicated that the proliferating cells in CE-derived spheres are pigmented CE cells, they did not exclude the possibility of a rare stem cell population. To analyze every cell in the CE-derived spheres, we performed serial electron microscopic analysis of entire spheres using dual-beam focused ion beam technology (Fig. 4 A and B, and Movies S1–S4). This imaging technique allowed us to serially query the entire sphere in 70- to 150-nm slices with resolution similar to that of standard TEM (Fig. 4 C–E).
Fig. 4.
Dual beam focused ion beam electron microscopic analysis of every cell in a CE-derived sphere. (A) Diagram of dual beam focused ion beam (FIB) technology. This procedure is based on alternating ion (Ga+) and electron beams. The ion beam mills the sample at 70 nm per round and the electron beam provides TEM quality images. (B) A scanning electron microscopic image of a sphere in a block halfway through the analysis. (C and D) Low power and high power images of representative sections in the sphere demonstrating the resolution of the analysis and pigment (p), membrane interdigitations (m), and epithelial junctions (open arrowhead). (E) Example of a 3D reconstruction of a single cell from the dual-beam FIB dataset showing the cytoplasm in blue, the pigment in pink, and the nucleus in yellow. Abbreviations: p, pigment; m, membrane interdigitations. (Scale bars in C, 10 μm; D and E, 1 μm.)
After all images were collected, we performed morphometric analysis on 5 datasets per sphere from 3 independent spheres to provide a random sampling of cells at all eccentricities of the sphere. Each dataset consisted of 21 sequential sections selected randomly and scored by 2 independent investigators. Every cell in each section was scored. Of the total 383 cells analyzed, all contained pigment and had features of differentiated CE cells (Fig. 4 D and E, 383/383 pigment +, 383/383 interdigitations+).
Scoring every cell in all of the spheres was impossible because of the labor-intensive nature of this approach; therefore, we wrote a computer program (SI Materials and Methods) to detect nonpigmented cells that resembled RSCs or progenitors in the digital 3D dataset (Movie S2). Our analysis eliminated the possibility that a nonpigmented RSC or neural progenitor was present in CE-derived spheres.
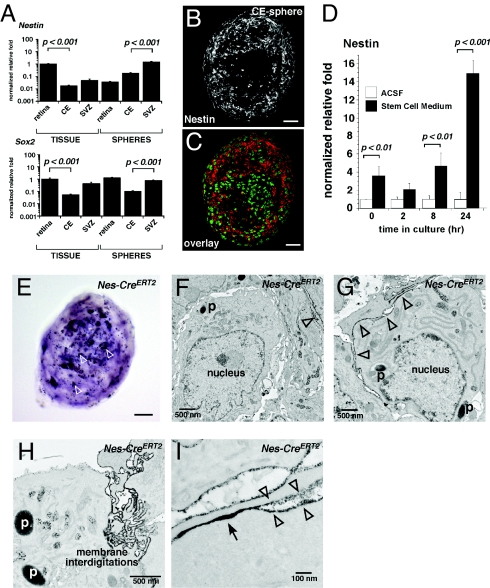
It was previously reported that CE-derived spheres express Nestin, a gene found in neural progenitor and stem cells (12). Our real-time RT-PCR analysis confirmed these previous findings (Fig. 5A). Immunofluorescent analysis of CE-derived spheres confirmed Nestin protein expression in a subset of cells in CE-derived spheres (Fig. 5B, Table S4). These data raised the possibility that when dissociated and maintained in culture in stem cell medium, a subset of CE cells expressing nestin can clonally expand to form spheres of pigmented CE cells. This observation would be consistent with the identification of a pigmented epithelial stem cell in the CE. Alternatively, pigmented CE cells may ectopically up-regulate stem cell markers upon dissociation and/or exposure to stem cell medium, despite their differentiated state. To distinguish between these 2 possibilities, we analyzed changes in Nestin expression in dissociated CE cells at several time points during the first 24 h in stem cell medium and compared that to cultures in the absence of growth factors (Fig. 5D and Table S5). Exposure to stem cell medium led to the rapid up-regulation of Nestin expression before the cells had time to clonally proliferate. Thus, dissociation and exposure to the stem cell medium was sufficient to cause these changes in gene expression rather than selective growth of an epithelial or retinal stem cell population.
Fig. 5.
Expression of stem/progenitor cell markers in CE-derived spheres. (A) Real time RT-PCR for Nestin and Sox2 in CE-derived spheres and neural stem cells and retinal progenitor cells. (B and C) Immunofluorescence confirmed the expression of nestin (red fluorescence) in CE-derived spheres with green fluorescence overlay. (D) Timecourse of nestin induction in dissociated CE cells cultured in stem cell medium or artificial cerebrospinal fluid (ACSF). (E) Spheres derived from the Nes-CreERT2−IRES−hPLAP strain expressed (AP) as detected with the NBT/BCIP substrate. (F–I) TEM analysis using lead citrate staining to detect the AP expressing (nestin+) cells. Using this method, lead citrate staining of AP expressing cells appears as a dark electron dense precipitate in TEM images along the membrane (open arrowheads). (H and I) These nestin-expressing cells in CE-derived spheres contained pigment, membrane interdigitations, and epithelial junctions (arrow). Abbreviations: SVZ, subventricular zone; ACSF, artificial cerebrospinal fluid; p, pigment. (Scale bars in B, C, and E, 25 μm.)
To further investigate the morphologic features of the Nestin-expressing cells in CE-derived spheres and confirm these findings, we examined a transgenic mouse strain that expresses the human placental alkaline phosphatase gene (AP) under the control of the nestin promoter and enhancer in the second intron, which regulates nestin expression in neural stem/progenitor cells (8) (Nes-CreERT2−IRES−hPLAP). We grew spheres from dissociated CE of these mice and analyzed them with AP TEM by using lead citrate staining as described in ref. 9. We found that the Nestin-expressing (AP+) cells contained pigment, membrane interdigitations, and epithelial junctions similar to differentiated pigmented CE cells (Fig. 5 E–I). Therefore, pigmented CE cells ectopically induced the expression of the stem/progenitor cell gene Nestin, but this profile did not mark a stem cell population in the CE or in the CE-derived spheres based on morphological or molecular criteria.
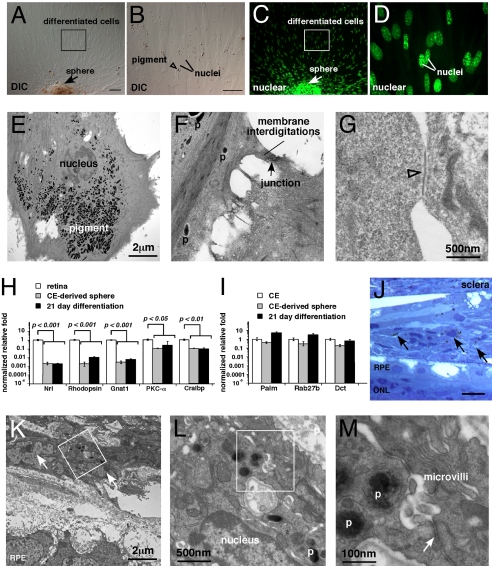
When CE-derived spheres are transferred to medium containing serum and plated on laminin, they were reported to produce cells expressing genes found in rods, bipolar cells, and Müller glia (5). Thus, we propose that these cells can initiate transdifferentiation or reprogramming from pigmented CE cells rather than differentiate from RSCs or progenitors. This model is consistent with previous studies showing robust transdifferentiation between CE, retina, and retinal pigment epithelium in various species (10–14). To test this hypothesis, we maintained spheres on laminin–coated coverslips for 21 days, as described in refs. 4 and 5. The spheres adhered to the coverslip, and pigmented cells migrated from them (Fig. 6 A–D). Even under differentiation conditions, every cell retained pigment (Fig. 6 A–D). TEM analysis revealed that after 21 days, the cells retained all morphologic features of CE cells (Fig. 6 E–G) and none of retinal neurons (e.g., processes, synaptic densities, synaptic vesicles, or synaptic ribbons).
Fig. 6.
Differentiated mouse CE-derived spheres resemble pigmented CE cells. (A–D) CE-derived spheres were plated on laminin coated coverglass and grown in differentiation medium for 21 days. The spheres adhered to the coverglass and spread out from there. All of the cells appeared to be pigmented in DIC images (A and B) when compared with the pattern of nuclear staining (C and D). (E–G) TEM analysis of CE-derived sphere differentiation cultures. (H and I) Real time RT-PCR analysis of differentiated CE cells for genes found in retinal neurons or glia (H) and pigmented CE cells (I). (J–M) CE-derived spheres were injected into the subretinal space of newborn rats to study their transdifferentiation into retinal neurons and glia. Tissue was analyzed 21 days after injection. (J) In and around the site of injection, pigmented cells were readily detected in histological sections (arrows). (K–M) TEM analysis of these sections revealed that the cells remained differentiated as pigmented CE cells. The injected cells retained pigment, membrane interdigitations (microvilli), and epithelial junctions (arrow in M). Abbreviations: DIC, differential interference contrast microscopy; p, pigment; RPE, retinal pigment epithelium; ONL, outer nuclear layer. (Scale bars in A, B, and J, 10 μm.)
To characterize the changes in gene expression over the course of the differentiation culture, we performed real-time RT-PCR on samples harvested every 2 days for 21 days. Genes that are normally expressed in rods (Nrl, Rhodopsin, Gnat1), bipolar cells (PKCα) and Müller glia (Cralbp) were not induced compared with levels seen in the normal retina (Fig. 6H). However, genes expressed in the differentiated CE (Palmdelphin, Rab27b) increased in these cultures (Fig. 6I).
If retinal differentiation is a very rare event in these cultures, it may be difficult to detect using real time RT-PCR. Therefore, we tested this possibility by immunostaining the cells with antibodies to markers of various retinal cells. The vast majority of cells were cytokeratin+ (97/100, 93/100; 95 ± 2.8%) (Fig. S5 A and B) consistent with their differentiation as pigmented CE cells. As mentioned above, a subset of the cells were nestin+ (15/100, 19/100; 17 ± 2.8%) (Fig. S5 C and D, compared with SVZ controls in Fig. S5 E and F), but were pigmented and had the features of differentiated pigmented CE cells. The pan-neuronal marker β-III tubulin (Tuj1) labeled ≈20% of the cells (22/100, 19/100; 20 ± 2.1%) in the CE-sphere differentiation cultures; those cells showed morphologic features of pigmented adherent epithelial cells. Occasionally (1–2 cells per well), an epithelial cell elongated and resembled a neuron (Fig. S9G); however, those cells also contained pigment (Fig. S5H). Dissociated cortex was used as a control for the Tuj1 immunostaining (Fig. S9J) (16/100, 11/100; 13 ± 3.5%). No cells in the CE differentiation cultures expressed rhodopsin (Fig. S5 K–M), but occasionally, nonspecific immunofluorescence was observed along the distorted edge of the pigmented epithelial cells that resembled a rod photoreceptor (Fig. S5 M and N). These “false rods” did not contain nuclei and appeared to be the curled-up membrane of pigmented epithelial cells that had trapped the primary antibody. Rhodopsin+ cells from adult retinae controls always contained a nucleus (Fig. S5 O and P). PKCα+ cells (bipolar cells) were readily detected in dissociated adult retinae but were absent from CE-derived sphere differentiation cultures. Together, these data suggest that at least 17% of cells become nestin positive, but fail to maintain a program to become retinal neurons. There is an ectopic up-regulation of immature neuron markers in the pigmented cells, such as Tuj1; however, very few cells express markers of bipolar neurons or photoreceptors. Without exception, all labeled cells retained the pigment and morphology of CE cells.
To determine whether transplantation of CE-derived spheres into the developing retina promotes their transdifferentiation, we injected pigmented CE-derived spheres (10 spheres/1 μL vehicle) into the subretinal space of 7 newborn albino rat pups. Twenty-one days later, we identified the pigmented cells in and around the injection site. There was no evidence of their integration into the developing retina (Fig. 6J). TEM analysis of the injected eyes confirmed these results (Fig. 6 K–M). Our analysis shows that the injected pigmented cells grouped together and formed a basal lamina, thus isolating the transplanted cells from the surrounding tissue. In all cases, the injected cells failed to integrate within the retina, however, some cells did migrate from the subretinal space to the outer nuclear layer (ONL). Detailed analyses of these transplantations are shown in supplemental data (Fig. S9).
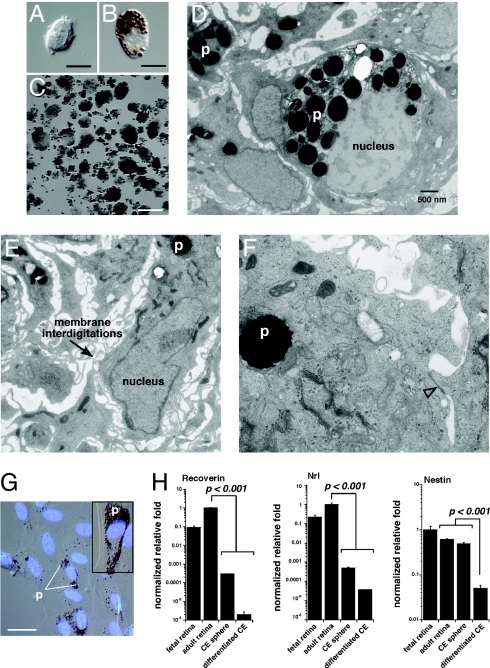
To determine whether human CE-derived spheres are also made up of proliferating pigmented CE cells, we performed a similar series of experiments to those described for the murine CE-derived spheres. TEM analysis revealed that the cells in the human CE-derived spheres had interdigitating processes, pigment, and epithelial junctions (Fig. 7 D–F). Furthermore, differentiation cultures of human spheres were also made up of pigmented cells that expressed genes found in normal pigmented CE cells (Fig. 7 G and H).
Fig. 7.
Human CE-derived spheres are made up of pigmented CE cells. (A and B) Postmortum human eyes were used as a source of CE cells for culture experiments. Dissociated human CE cell preparations were made up of pigmented and nonpigmented cells as for the mouse CE. (C) Clonogenic spheres from postmortum human CE samples. (D–F) TEM analysis of human CE-derived spheres showing pigment (p), membrane interdigitations, and epithelial junctions (open arrowhead). (G) Human CE-derived spheres were differentiated for 21 days coverslides as described for mouse CE-derived spheres. The cells spread out along the coverglass and took on the morphology of pigmented epithelial cells as for the mouse samples. (H) Real time RT-PCR analysis of human CE-differentiation cultures revealed that these cells did not express photoreceptor genes (recoverin, Nrl) or neural progenitor cell genes (nestin). Each bar represents the mean and standard deviation from duplicate experiments for at least 3 independent samples for each piece of tissue. Abbreviations: p, pigment. (Scale bars in A and B, 5 μm; C, 1 mm; G, 10 μm.)
Discussion
The CE of the mammalian eye was previously reported to contain retinal stem cells. Here, we reinterpret these previous findings and extend those studies to show that the CE-derived spheres do not originate from stem cells. Instead, the pigmented CE cells have the unexpected ability to form clonogenic spheres made up of proliferating differentiated pigmented CE cells. Importantly, to form spheres the pigmented CE cells do not harbor a retinal stem cell, nor do they become a retinal stem cell; instead the differentiated pigmented CE cells proliferate to form clonogenic spheres in culture. The pigmented CE cells in the spheres express neural progenitor/stem cell markers, such as nestin, and can be dispersed and replated to form new spheres with the same properties of differentiated pigmented CE cells. When transferred to differentiation conditions, the CE-derived spheres begin to up-regulate markers of neurons; however, they retain most, if not all, of the molecular, cellular, and morphological features of pigmented CE cells. These data are consistent with another study showing that CE cells can express neural markers but not retinal markers (15).
To rule out the possibility of a rare stem cell in these CE-derived spheres, we performed serial TEM through entire spheres by using focused ion beam electron microscopy. We also showed that the CE-derived spheres expressed genes (real time RT-PCR and Affymetrix gene expression arrays) found in differentiated pigmented CE cells rather than genes normally expressed in retinal progenitor cells or neural stem cells. There were a few exceptions to this trend. In addition, we directly demonstrated that the proliferating cells in M-phase or S-phase of the cell cycle were differentiated pigmented CE cells. Taken together, these data suggest that the pigmented spheres derived from the mouse and human CE were actually the outgrowth of differentiated pigmented CE cells that are stimulated to reenter the cell cycle when dissociated and plated in stem cell medium.
We confirmed earlier findings that CE-derived spheres express nestin (5), but these cells were pigmented CE cells rather than RSCs or progenitors. Our data suggest that the growth factors in the stem cell medium induce nestin expression in differentiated pigmented CE cells rather than the selection and clonal expansion of a retinal stem cell. These data are consistent with accumulating evidence that markers previously thought to be stem cell markers are not enriched exclusively in stem cell populations in every tissue (16). Overall, the expression of a small number of stem/progenitor cell markers in our CE-derived spheres does not reflect the presence of a stem or progenitor cell but the ectopic induction of these genes in differentiated cells by the growth factors in the medium.
Our studies indicate that a subset of cells could up-regulate pan-neuronal markers, such as β-III tubulin after 21 days in differentiation cultures, but these cells remained pigmented and did not become retinal neurons. It is possible that with further refinement, researchers may be able to induce pigmented CE cells to form retinal neurons. This would not be surprising considering that the neural retina, retinal pigment epithelium, and the CE are derived from the same embryonic tissue during eye morphogenesis. Indeed, key examples of transdifferentiation have been reported for retinal pigmented epithelium, neural retina, and ocular CE (10–14).
However, even if future studies show that it is possible to induce pigmented CE transdifferentiation, it may be more effective to use ES cells, retinal progenitor cells, or photoreceptor precursor cells for cell-based therapies to restore vision in patients with retinal degeneration. Importantly, the differentiated cells expressing pan-neuronal markers retained pigment in our experiments in vitro and in vivo. Unlike most other regions of the CNS, there are no pigmented neurons in the mammalian retina. Indeed, the presence of pigment in the retina could limit efficient detection of light in the outer segments of retinal photoreceptors to defeat the purpose of cell-based therapy to restore vision.
The initial report of a retinal stem cell population in the mouse and human eyes was met with enthusiasm because of its potential for cell-based therapies to treat millions of people with retinal degeneration worldwide. The data presented here do not eliminate the possibility of using CE-derived clonogenic spheres from postmortem eyes for cell-based therapies to treat retinal degeneration; however, they suggest that efforts should be directed toward the production of replacement neurons through reprogramming or transdifferentiation of pigmented CE, because neither mouse nor human CE contains true stem cells. Recent work from the Svendsen lab has indicated that factors secreted by the RPE may promote the expression of retinal progenitor cell genes in human retinal neurospheres, ultimately leading to the expression of genes found in retinal neurons (17). Transdifferentiation of glia and retinal pigment epithelium has been described and perhaps represents a more attractive candidate than the CE for the production of functional retinal neurons. Other promising alternatives include the use of photoreceptor precursor cells from fetal retinae (18–20) or the use of ES cells (21, 22). Of particular interest is the recent report that retinal neurons can be induced to reenter the cell cycle and make more retinal neurons without the need for stem cells or progenitor cells (23). If these studies can be extended to photoreceptors, then we might be able to generate new neurons in situ in a degenerating retina (23).
Materials and Methods
Animals.
Timed, pregnant Sprague–Dawley rats were obtained from Charles River Laboratories. All retinal samples and CE cultures were from C57BL/6 male mice 6–10 weeks of age. Chx10orJ and Mitfmi mice were obtained from Jackson Labs. The Nes-CreERT2−IRES−hPLAP mice were obtained from Dr. S. Baker (St. Jude Children's Research Hospital). The St. Jude Children's Research Hospital Institutional Animal Care and Use Committee approved all of the animal experiments.
Human Tissue.
Adult postmortem eyes were obtained from the Mid-South Eyebank, and fetal eyes were obtained from ABR, Inc. All experiments involving human tissue were approved by the St. Jude Children's Research Hospital IRB.
Histology and Immunostaining.
We immunolabeled retinal sections with attached CE and SVZ sections cut on a vibratome and dissociated retinae and SVZ as described in refs. 24 and 25). The list of antibodies used and their dilutions is provided (SI Materials and Methods).
Real Time RT-PCR.
Real time RT-PCR experiments were performed as described in ref. 23. Individual probe and primer sequences can be found in the SI Materials and Methods, Table S5.
[3H]-thymidine Labeling and Detection.
To label S-phase retinal progenitor cells, we incubated freshly dissected retinae in 1 mL explant culture medium containing [3H]thy (5 μCi/mL; 89 μCi/mmol) for 1 h at 37°C. Autoradiography was carried out as described in refs. 24 and 25.
Microscopy.
Bright-field and single-cell fluorescent images were obtained using a Zeiss Axioplan-2 fluorescent microscope with the Zeiss AxioCam digital camera. Fluorescent images of sections were obtained by using a Leica TCSNT confocal microscope. Electron microscopy was carried out as described in refs. 9 and 26. Detailed protocols and procedures are presented in SI Text.
CE, SVZ, and Retinal Dissociation and Culture.
Cultures of CE, SVZ, and Retinae to generate clonogenic spheres was carried out as described in refs. 4, 5, 27–30. Detailed protocols are available in SI Materials and Methods.
In Vivo Sphere Injections.
Spheres were harvested by gravity and resuspended at a density of 10 spheres/μL. Injections of 1 μL of spheres was performed as described in ref. 31.
Supplementary Material
Acknowledgments.
We thank Dr. Jiakun Zhang for technical assistance with the research; Angie McArthur for editing the manuscript; Fara Sudlow and Jackie Craft for expertise with TEM; Dr. Marina Kedrov for assistance with TEM image analysis and 3D reconstruction of serial TEM images; and David Finkelstein for providing assistance with gene expression array analysis. This work was supported by the National Institutes of Health Grant (to M.A.D.); Cancer Center Support from the National Cancer Institute; American Cancer Society; Research to Prevent Blindness; Pearle Vision Foundation; International Retinal Research Foundation; and American Lebanese Syrian Associated Charities (ALSAC). M.A.D. is a Pew Scholar. S.A.C. is a Hartwell Foundation Fellow.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901596106/DCSupplemental.
References
- 1.MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 2.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coles BL, et al. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci USA. 2004;101:15772–15777. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tropepe V, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 6.Wachs FP, et al. High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest. 2003;83:949–962. doi: 10.1097/01.lab.0000075556.74231.a5. [DOI] [PubMed] [Google Scholar]
- 7.Coles BL, Horsford DJ, McInnes RR, van der Kooy D. Loss of retinal progenitor cells leads to an increase in the retinal stem cell population in vivo. Eur J Neurosci. 2006;23:75–82. doi: 10.1111/j.1460-9568.2005.04537.x. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DA, Donovan SL, Dyer MA. Mosaic deletion of Rb arrests rod differentiation and stimulates ectopic synaptogenesis in the mouse retina. J Comp Neurol. 2006;498:112–128. doi: 10.1002/cne.21059. [DOI] [PubMed] [Google Scholar]
- 10.Azuma N, et al. Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Hum Mol Genet. 2005;14:1059–1068. doi: 10.1093/hmg/ddi098. [DOI] [PubMed] [Google Scholar]
- 11.Del Rio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Dev Dyn. 2003;226:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- 12.Fischer AJ, Reh TA. Transdifferentiation of pigmented epithelial cells: A source of retinal stem cells? Dev Neurosci. 2001;23:268–276. doi: 10.1159/000048710. [DOI] [PubMed] [Google Scholar]
- 13.Lee CS, May NR, Fan CM. Transdifferentiation of the ventral retinal pigmented epithelium to neural retina in the growth arrest specific gene 1 mutant. Dev Biol. 2001;236:17–29. doi: 10.1006/dbio.2001.0280. [DOI] [PubMed] [Google Scholar]
- 14.Rowan S, Chen CM, Young TL, Fisher DE, Cepko CL. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development. 2004;131:5139–5152. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- 15.Engelhardt M, Bogdahn U, Aigner L. Adult retinal pigment epithelium cells express neural progenitor properties and the neuronal precursor protein doublecortin. Brain Res. 2005;1040:98–111. doi: 10.1016/j.brainres.2005.01.075. [DOI] [PubMed] [Google Scholar]
- 16.Fargeas CA, Florek M, Huttner WB, Corbeil D. Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem. 2003;278:8586–8596. doi: 10.1074/jbc.M210640200. [DOI] [PubMed] [Google Scholar]
- 17.Gamm DM, et al. Regulation of prenatal human retinal neurosphere growth and cell fate potential by retinal pigment epithelium and Mash1. Stem Cells. 2008;26:3182–3193. doi: 10.1634/stemcells.2008-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humayun MS, et al. Human neural retinal transplantation. Invest Ophthalmol Vis Sci. 2000;41:3100–3106. [PubMed] [Google Scholar]
- 19.Ehinger B, et al. Ultrastructure of human retinal cell transplants with long survival times in rats. Exp Eye Res. 1991;53:447–460. doi: 10.1016/0014-4835(91)90162-8. [DOI] [PubMed] [Google Scholar]
- 20.Aramant RB, Seiler MJ. Human embryonic retinal cell transplants in athymic immunodeficient rat hosts. Cell Transplant. 1994;3:461–474. doi: 10.1177/096368979400300603. [DOI] [PubMed] [Google Scholar]
- 21.Osakada F, et al. Corrigendum: Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:352. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 22.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24:274–283. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajioka I, et al. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyer MA, Cepko CL. p57(Kip2) regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development. 2000;127:3593–3605. doi: 10.1242/dev.127.16.3593. [DOI] [PubMed] [Google Scholar]
- 25.Dyer MA, Cepko CL. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J Neurosci. 2001;21:4259–4271. doi: 10.1523/JNEUROSCI.21-12-04259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson D, et al. Neuronal differentiation and synaptogenesis in retinoblastoma. Cancer Res. 2007;67:2701–2711. doi: 10.1158/0008-5472.CAN-06-3754. [DOI] [PubMed] [Google Scholar]
- 27.Morshead CM, et al. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 28.Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251:367–379. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- 29.Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 30.Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259:225–240. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 31.Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.