Abstract
The molecular mechanisms of endothelial nitric oxide synthase (eNOS) regulation of microvascular permeability remain unresolved. Agonist-induced internalization may have a role in this process. We demonstrate here that internalization of eNOS is required to deliver NO to subcellular locations to increase endothelial monolayer permeability to macromolecules. Using dominant-negative mutants of dynamin-2 (dyn2K44A) and caveolin-1 (cav1Y14F), we show that anchoring eNOS-containing caveolae to plasma membrane inhibits hyperpermeability induced by platelet-activating factor (PAF), VEGF in ECV-CD8eNOSGFP (ECV-304 transfected cells) and postcapillary venular endothelial cells (CVEC). We also observed that anchoring caveolar eNOS to the plasma membrane uncouples eNOS phosphorylation at Ser-1177 from NO production. This dissociation occurred in a mutant- and cell-dependent way. PAF induced Ser-1177-eNOS phosphorylation in ECV-CD8eNOSGFP and CVEC transfected with dyn2K44A, but it dephosphorylated eNOS at Ser-1177 in CVEC transfected with cav1Y14F. Interestingly, dyn2K44A eliminated NO production, whereas cav1Y14F caused reduction in NO production in CVEC. NO production by cav1Y14F-transfected CVEC occurred in caveolae bound to the plasma membrane, and was ineffective in causing an increase in permeability. Our study demonstrates that eNOS internalization is required for agonist-induced hyperpermeability, and suggests that a mechanism by which eNOS is activated by phosphorylation at the plasma membrane and its endocytosis is required to deliver NO to subcellular targets to cause hyperpermeability.
Keywords: caveolae, endothelial nitric oxide synthase, endothelial permeability, inflammation, protein traffic
Nitric oxide (NO) is the signal for several outcomes in the control of circulation. Its main source in the cardiovascular system is endothelial (e)NOS. The enzyme is modified by N-myristoylation and palmitoylation, which targets it to the caveolae in the plasma membrane where it is kept in a basal state by binding to caveolin-1 through a consensus site (1, 2). Agonists activate eNOS through multiple mechanisms: phosphorylation/dephosphorylation of specific residues, interaction with different proteins, S-nitrosylation, and specific subcellular localization (1, 3–7). Agonists such as platelet-activating factor (PAF) and VEGF phosphorylate eNOS via Akt (8, 9). Despite advances in our understanding of the biochemistry of eNOS, the mechanisms by which these molecular modifications determine the functional outcome of eNOS activation remain unexplored.
NO derived from eNOS is a key mediator in the hyperpermeability response to PAF and VEGF (7, 10, 11). We previously showed that eNOS internalization (endocytosis) is a required step in the signaling cascade leading to PAF-induced hyperpermeability (7). In this study, we tested the hypothesis that caveolar internalization of eNOS is a requirement for localized NO production and NO delivery to a subcellular target to cause hyperpermeability. To address this hypothesis, we used ECV-304 cells (which lack endogenous eNOS) transfected with CD8-GFPeNOSmyr− (referred to hereafter as ECV-CD8eNOSGFPmyr−) (12). This eNOS is mutated in its myristoylation site and fused to the extracellular and transmembrane domain of the cell surface glycoprotein CD8. This mutated eNOS is unable to be myristoylated, but can be palmitoylated. However, because of the transmembrane domain of the CD8 protein, depalmitoylation does not cause internalization. We also anchored eNOS-containing caveolae to the plasma membrane with a caveolin-1 mutant that inhibits phosphorylation at Y14 (cav1Y14F-GFP) in ECV-CD8eNOSGFP and in bovine coronary postcapillary venular ECs. Transfection with cav1Y14F prevents scission of caveolae from the plasma membrane. Our results show a direct correlation between eNOS endocytosis by caveolae, NO production, and hyperpermeability, in support of our hypothesis. Surprisingly, experiments aimed at testing activation of eNOS in transfected cells revealed dissociation or uncoupling between eNOS phosphorylation and NO production. These unexpected results suggest a mechanism for endothelial hyperpermeability whereby eNOS is activated by phosphorylation at the plasma membrane, but production of efficacious NO occurs during or at the end of endocytosis.
Results
PAF Activates and Internalizes eNOS to Cause Hyperpermeability in ECV-CD8-GFPeNOSmyr−.
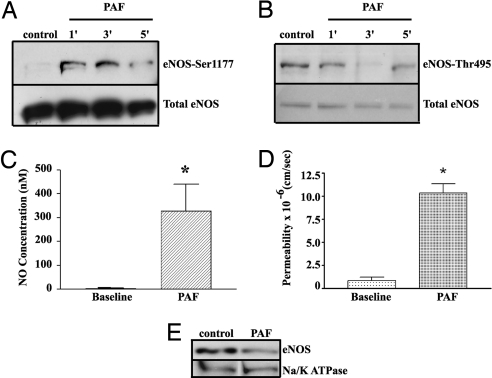
We verified by Western blotting that CD8-GFPeNOSmyr− was expressed after transfection in ECV-304 cells. Immunofluorescence microscopy demonstrated that it distributes preferentially in the plasma membrane and Golgi, in agreement with the eNOS distribution in ECs (6, 13, 14). To assess the functional state of the transfected protein, we investigated eNOS activation by measuring eNOS phosphorylation, NO production, and permeability of cell monolayers to macromolecules using PAF as an agonist. PAF significantly increased phosphorylation of eNOS at Ser-1177 as early as 1 min (Fig. 1A; p-eNOS/total eNOS ratio was control, 0.97 ± 0.07; 1 min, 1.32 ± 0.09; 3 min, 1.19 ± 0.16; and 5 min, 1.24 ± 0.32), and dephosphorylated eNOS at Thr-495 significantly at 1 min (Fig. 1B, p-eNOS/total eNOS ratio was control, 1.0 ± 0.01; 1 min, 0.74 ± 0.13; 3 min, 1.02 ± 0.21; 5 min, 0.93 ± 0.28). PAF increased pericellular NO concentration from 0 ± 5 to 320 ± 100 nM (n = 3, P < 0.05) (Fig. 1C). Last, PAF increased endothelial permeability to FITC-conjugated dextran (FITC-Dx-70) from 0.82 ± 0.3 × 10−6 to 10.0 ± 0.97 × 10−6 cm/s (Fig. 1D; P < 0.05). Because fusion to CD8 is expected to anchor eNOS to the plasma membrane in ECV-CD8-GFPeNOSmyr−, the PAF-induced hyperpermeability results were unexpected. To determine whether or not PAF induced traffic of CD8-GFPeNOSmyr− away from the cell surface, we used biotin labeling of cell surface proteins, followed by Neutravidin precipitation and Western blotting for eNOS. Fig. 1E shows that PAF induced internalization of CD8-GFPeNOSmyr−, as indicated by a reduction in eNOS expression in the plasma membrane by 31.6 ± 3.9%. Na/K ATPase, a marker for plasma membrane protein, was not affected by PAF, indicating that the effect was specific for eNOS.
Fig. 1.
Functional Characterization of ECV-CD8-GFPeNOSmyr−. (A) PAF significantly increases phosphorylation of eNOS at Ser-1177 as a function of time (n = 3). (B) PAF induces eNOS dephosphorylation at Thr-495 (n = 3). (C) PAF stimulates a robust increase in pericellular NO concentration (mean ± SEM; n = 6; *, P < 0.05). (D) 10−7 M PAF increases permeability to FITC-Dx-70 in CD8-GFPeNOSmyr− cells. Data are mean ± SEM. (*, P < 0.05, n = 4. (E) PAF induces eNOS translocation in CD8-GFPeNOSmyr− cells. The Western blotting shows that PAF induces the disappearance of eNOS from plasma membrane (n = 3).
PAF-Induced Hyperpermeability Requires eNOS Internalization in ECV-CD8-GFPeNOSmyr−.
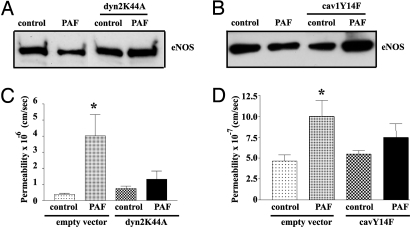
To further test the functional role of eNOS endocytosis in PAF-induced hyperpermeability, we inhibited eNOS internalization by 2 methods that block caveolar endocytosis: (i) transfection with dyn2K44A (15, 16); and (ii) transfection with cav1Y14F. Using the cell surface biotinylation technique, we confirmed that dyn2K44A blocks PAF-induced eNOS endocytosis, (Fig. 2A). Subsequently, we corroborated that transfection with cav1Y14F blocks PAF-induced eNOS endocytosis (Fig. 2B). Fig. 2C shows that dyn2K44A inhibited PAF-induced hyperpermeability. Transfection with cav1Y14F also significantly inhibited PAF-induced hyperpermeability (Fig. 2D). Cells transfected with empty vector served as controls in these experiments.
Fig. 2.
Inhibition of caveolar internalization decreases PAF-induced hyperpermeability in ECV-CD8-GFPeNOSmyr− cells. (A) Western blotting showing that cotransfection of dyn2K44A inhibits PAF-induced eNOS endocytosis. (B) Western blotting showing that cotransfection of cav1Y14F inhibits PAF-induced eNOS endocytosis. (C) Transfection of ECV-CD8-GFPeNOSmyr− cells with dyn2K44A inhibits PAF-induced hyperpermeability to FITC-DX-70 (*, P < 0.05 vs. control, n = 5). (D) Transfection of ECV-CD8-GFPeNOSmyr− cells with cav1Y14F inhibits PAF-induced hyperpermeability to FITC-DX-70. (*, P < 0.05 vs. control, n = 5).
Inhibition of eNOS Endocytosis Reduces Hyperpermeability Independent of Cell Type.
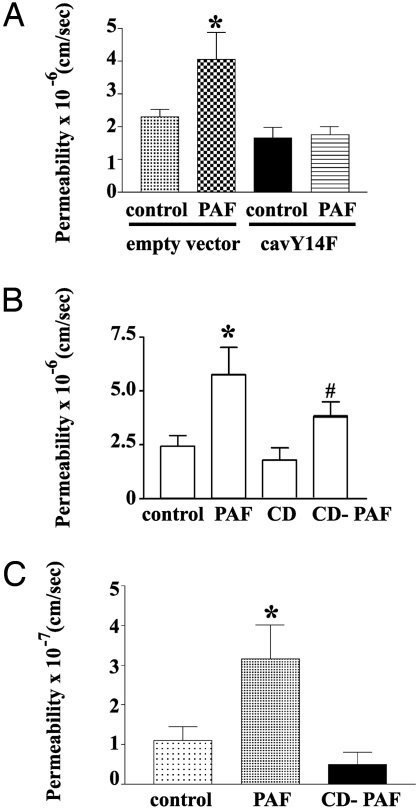
We used cav1Y14F to test our hypothesis in ECs derived from postcapillary venules (CVEC). This microvascular segment constitutes the main target for inflammatory agents, and, therefore, provides a clinically relevant correlation. Confirming our earlier observation that transfection of CVEC with dyn2K44A inhibits PAF-induced hyperpermeability (7), transfection of CVEC with cav1Y14F inhibited PAF-induced increase in permeability (Fig. 3A). We performed additional tests using cyclodextrin, an agent that removes cholesterol and causes disarray of caveolar structure (17). Topical administration of 5 mM cyclodextrin inhibited PAF-induced hyperpermeability in CVEC monolayers (Fig. 3B). Topical administration of 10 mM cyclodextrin inhibited PAF-induced hyperpermeability in ECV-eNOSGFP (ECV-304 transfected with wild-type eNOSGFP) (Fig. 3C). These results support our hypothesis regarding eNOS endocytosis, and indicate that this requirement is independent of cell type.
Fig. 3.
Inhibition of eNOS endocytosis blocks hyperpermeability independent of cell type. Different treatments that prevent caveolar endocytosis of eNOS in different cells block PAF-induced hyperpermeability. (A) Transfection of CVEC with cav1Y14F. (B) Treatment of CVEC with 5 mM cyclodextrin (CD). (C) Treatment of ECVeNOSGFP cells with 10 mM cyclodextrin.
Inhibition of eNOS Endocytosis and Activation of eNOS.
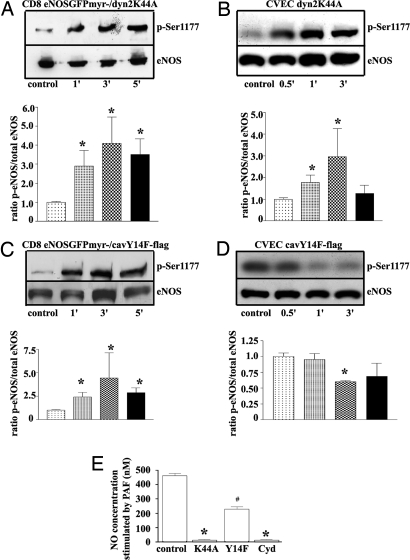
Because inhibition of caveolar endocytosis may impair the ability of eNOS to produce NO, we tested eNOS activation by measuring eNOS phosphorylation at Ser-1177 and NO production in the transfected cells. PAF caused phosphorylation of eNOS at Ser-1177 in ECV-CD8-GFPeNOSmyr− cotransfected with dyn2K44A (Fig. 4A) and in CVEC transfected with dyn2K44A (Fig. 4B), as well as in ECV-CD8-GFPeNOSmyr− cotransfected with cav1Y14F (Fig. 4C). In contrast, PAF caused dephosphorylation of eNOS at Ser-1177 in CVEC transfected with cav1Y14F (Fig. 4D). Fig. 4E shows PAF-induced NO production in CVEC transfected with dyn2K44A, transfected with cav1Y14F-GFP (GFP served to identify the transfected cells) or treated with 5 mM cyclodextrin. We observed a strong inhibition in PAF-induced NO production in the presence of dyn2K44A and with cyclodextrin treatment, whereas only partial inhibition was observed in the presence of the caveolin mutant. Because cav1Y14F reduces NO production, whereas dyn2K44A and cyclodextrin abolish NO production, these results demonstrate a previously undescribed uncoupling or dissociation between eNOS phosphorylation at Ser-1177 and NO production.
Fig. 4.
Effect of inhibition of PAF-induced eNOS endocytosis in ECV-CD8-GFPeNOSmyr− cells and CVEC. Except for control, all measurements were performed after stimulation with 10−7 M PAF. (A–D) The times on the bar graphs are in the same order as in the Western blotting panels. (A) Phosphorylation of CD8-GFPeNOSmyr− in the presence of the dynamin mutant. (B) Phosphorylation of eNOS in CVEC in the presence of the dynamin mutant. (C) Phosphorylation of CD8-GFPeNOSmyr− in the presence of the caveolin mutant. (D) Phosphorylation of eNOS in CVEC in the presence of the caveolin mutant. (E) NO production in CVEC transfected with dyn2K44A, cav1Y14F, or treated with 5 mM cyclodextrin (Cyd).
Inhibition of eNOS Endocytosis Inhibits VEGF-Induced Hyperpermeability.
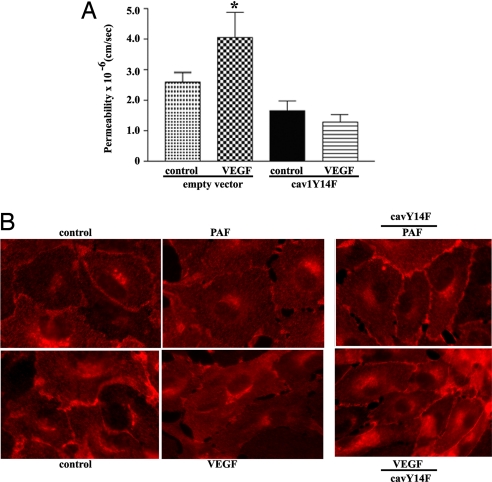
To test whether or not the association between eNOS endocytosis and hyperpermeability is a universal functional requirement, we measured the impact of transfecting CVEC with cav1Y14F on VEGF-induced hyperpermeability. In Fig. 5A, we demonstrate that inhibition of caveolar endocytosis also effectively abolishes VEGF-induced hyperpermeability. Fig. 5B shows a series of images demonstrating that: (i) PAF and VEGF induce translocation of eNOS from the plasma membrane (caveolae) to the cytosol in postcapillary ECs; and (ii) transfection of ECs with cav1Y14F inhibits the translocation of eNOS. Translocation of eNOS is indicated mainly by the disappearance of eNOS from the cell plasma membrane and the enhanced brightness in the cytosol (6, 7).
Fig. 5.
Inhibition of permeability and eNOS translocation by cav1Y14F. (A) Inhibition of eNOS endocytosis abolishes VEGF-induced hyperpermeability. Transfection of CVEC with cav1Y14F significantly inhibited VEGF-induced hyperpermeability to FITC-DX-70 (*, P < 0.05 vs. control, n = 5). (B) Transfection of CVEC with cav1Y14F blocks eNOS translocation to cytosol. PAF and VEGF decrease the brightness of eNOS (red fluorescence) from the plasma membrane relative to control. Transfection with cav1Y14F inhibits the PAF- and VEGF-induced movement of eNOS away from the plasma membrane.
Discussion
Our results demonstrate that eNOS location and internalization are important factors for determining endothelial function. We tested our hypothesis in the functional context of endothelial monolayer permeability to macromolecules. We demonstrate that eNOS endocytosis via caveolae to an as yet undetermined subcellular target is a necessary signaling step for NO production and NO delivery in the development of hyperpermeability in response to at least 2 proinflammatory agonists, PAF and VEGF. We also report a previously undescribed experimental dissociation between eNOS phosphorylation at Ser-1177 and NO production, which occurs when eNOS is activated under conditions that anchor caveolae to the plasma membrane.
Endothelial NOS Endocytosis and Permeability.
ECV-CD8-GFPeNOSmyr− transfected cells were used as an initial cell model to assess the significance of anchoring eNOS to the plasma membrane in the regulation of endothelial monolayer permeability. These cells show (i) eNOS distribution similar to endogenous eNOS in ECs; (ii) eNOS phosphorylation at Ser-1177, and dephosphorylation at Thr 495; (iii) NO production in response to PAF; and (iv) increase in permeability after PAF stimulation. Also, PAF causes internalization of eNOS to the cytosol as it does in ECVeNOS-GFP cells and CVEC (6, 7). Anchoring eNOS to the caveolae via dyn2K44A and cav1Y14F transfection or by causing caveolar disarray with cyclodextrin inhibited PAF-induced hyperpermeability. The fact that ECV-CD8-GFPeNOSmyr− is attached to the membrane by the fused CD8 (12) indicates that eNOS is not internalized by a depalmitoylation mechanism in these cells, and supports the role of caveolar endocytosis of eNOS in the control of hyperpermeability responses.
Our results complement current knowledge about regulation of eNOS, and advance the field by proposing a step that determines a defined functional outcome. Currently, several agonists are known to stimulate the same pathways in EC and lead to changes in phosphorylation of key consensus sites (18) and initiation of NO production. However, these biochemical studies have not addressed the regulation of eNOS in terms of microvascular function. We provide several lines of evidence in support of eNOS internalization via caveolae as an important determinant in regulating endothelial monolayer permeability.
The target molecule or acceptor of eNOS-derived NO in the subcellular compartment remains to be identified. Soluble guanylyl cyclase is a candidate, because it is the main receptor for NO, and participates in the signaling cascade that leads to hyperpermeability (19). The mechanisms by which eNOS internalization and NO production, possibly via soluble guanylyl cyclase, modify junctional proteins to induce hyperpermeability to proteins remain unresolved. It is plausible that NO production in the cytosol may influence cytoskeletal proteins; thus, promoting EC contraction and hyperpermeability (20–22). One may alternatively speculate that NO-driven S-nitrosylation of proteins may induce changes in trafficking and permeability. Increased S-nitrosylation of proteins has been reported in increased permeability (23). NO could modify endothelial junctional proteins and increase permeability either promoting their internalization or inhibiting their arrival. S-nitrosylation modifies proteins either by enhancing their phosphorylation (23) or inhibiting phosphorylation (24). Because some junctional proteins are phosphorylated when hyperpermeability is activated, it is plausible that S-nitrosylation may regulate the phosphorylation of junctional proteins.
Activation of eNOS and NO Production.
PAF activates eNOS and causes phosphorylation of the enzyme in vivo and in vitro (6, 25). A key phosphorylation site is Ser-1177 (3). We determined that anchoring eNOS to the plasma membrane caveolae with dyn2K44A, or destroying the caveolar structure with cyclodextrin, still allowed PAF-induced phosphorylation of eNOS at Ser-1177, but blocked PAF-induced NO production. The reasons for these results are unknown. The mutation at K44 is located far away from the amino acids in dynamin-2 that interact directly with eNOS (26); thus, direct interference is unlikely. We cannot a priori disregard (i) the possibility that, due to the mutation, structural changes in the dynamin2 can occur and affect the interaction between dynamin and eNOS leading to impaired NO production, or (ii) the speculation that caveolar scission and endocytosis are necessary for PAF-induced NO production. Nonetheless, these experiments do reveal a novel uncoupling between eNOS phosphorylation and NO production.
To clarify the above issue, we used cav1Y14F to anchor caveolae to the plasma membrane. This construct works by blocking phosphorylation of caveolin-1 at Y14 (27), a site far away from the caveolin scaffolding that inhibits eNOS function (2). Interestingly, we observed cell line-dependent differences with this construct. PAF caused phosphorylation of eNOS at Ser-1177 in ECV-CD8-eNOSGFPmyr−, whereas it caused dephosphorylation of eNOS at Ser-1177 in CVEC (Fig. 4C). Also, PAF caused a reduced NO production in CVEC transfected with cav1Y14F relative to control CVEC. As with dyn2K44A, the mechanisms of these observations remain unknown, and the same speculations apply. However, together, the data unquestionably show an experimental dichotomy between eNOS phosphorylation and NO production.
Importantly, the fact that PAF induced reduced NO production and no increase in permeability to macromolecules in monolayers of CVEC transfected with cav1Y14F supports our argument that location of NO delivery is fundamental for microvascular function (6). Presumably, because cav1Y14F anchors caveolae to the plasma membrane the eNOS-derived NO in these cells is of “plasma membrane” origin and is not delivered at the appropriate concentration to the subcellular target that signals for permeability outcomes.
In summary, we report (i) data in support of the hypothesis that eNOS location and internalization are key signals in the regulation of permeability, and (ii) experimental uncoupling between eNOS phosphorylation and NO production. Phosphorylation of eNOS has been classically associated with production of NO (1, 3). We demonstrate that eNOS phosphorylation at Ser-1177 and NO production can be separated experimentally by anchoring caveolar eNOS to the plasma membrane. We suggest that phosphorylation alone does not constitute an absolute index of eNOS activation, and that traffic of eNOS and NO production should be determined for assessment of eNOS activity. Because the eNOS signaling pathways of PAF and VEGF are shared with bradykinin and histamine (9, 19, 28), our proposed mechanisms are likely to be a universal characteristic of ECs. In a broader context, our data contribute to the advancement of knowledge of the regulation of microvascular permeability in response to proinflammatory agents, and may impact the fields of cardiovascular medicine and surgery.
Materials and Methods
Drugs and Antibodies.
PAF and methyl-beta-cyclodextrin were from Sigma-Aldrich. VEGF was from R & D Systems. Antibodies recognizing TGN-46 was from Serotec; flag was from Sigma-Aldrich; eNOS, eNOS-Ser-1177, eNOS-T495, and GFP were from BD Biosciences.
Cell Culture and Transfection.
ECV-304, ECV-eNOSGFP (provided by William C. Sessa, Yale University, New Haven, CT) and CVEC cultures were grown according to published protocols (6, 7). ECV-304 were transfected with a CD8-GFPeNOSmyr− expression plasmid (provided by T. Michel, Harvard Medical School, Boston) by using Lipofectamine and following the instructions of the manufacturer (Invitrogen). ECV-CD8-GFPeNOSmyr− and CVEC were transfected with dyn2K44A-GFP, cav1Y14F-GFP (both provided by M. McNiven, Mayo Clinic College of Medicine, Rochester, MN) or cav1Y14F-flag (provided by M. del Pozo, National Center of Cardiovascular Research, Madrid) expression plasmids by electroporation by using the manufacturer's instructions (Amaxa Biosystems). All of the experiments were run 72 h after transfection.
Monolayer Permeability Experiments.
We determined control and PAF- or VEGF-stimulated permeability across cellular monolayers using an established method (6, 29).
Immunoblot Analysis.
We extracted protein from cells grown to confluence in 100-mm plates and detected the proteins of interest, as previously described (6, 7). We used the National Institutes of Health Image J Program to quantify Western blottings.
Cell Surface Biotinylation.
Confluent cells growing in a 60-mm plate were serum starved overnight. Cells were left untreated or 10−7M PAF was applied to the cells for 5 min. They were washed 2 times with PBS and incubated with 1 mL of EZ-Link Sulfo-NHS-Biotin (Pierce) for 30min at 4 °C. The monolayers were washed 3 times with Tris buffered saline, scraped in 500 μL lysis buffer (50 mM Tris/150 mM NaCl/0.1 mM EDTA/0.1 mM EGTA/1% Triton X-100/protease inhibitor mixture), and incubated on ice with shaking for 30 min. Precleared lysates were obtained by centrifugation at 10,000 × g for 5 min at 4 °C, and incubated afterward for 2 h at 4 °C with 50 μL NeutrAvidin-coated agarose beads (Pierce). Beads were collected by centrifugation at 14,000 × g and washed 6 times with lysis buffer. The beads with biotinylated proteins were resuspended in loading buffer and run in PAGE-SDS gels. Separated proteins were blotted to nitrocellulose and detected with antibodies specific for eNOS.
NO Measurements.
We measured NO production using NO-sensitive recessed-tip microelectrodes and published protocols (30, 31). Coverslips containing confluent cells were placed in a perfusion chamber. The cells were superfused at a rate of 1 mL/min (shear stress, 1.0 × 10−4 dyne/cm2). PAF was added through a side-port in the perfusion line to achieve a concentration of 10−7 M in the chamber.
Statistical Analysis.
Data are presented as mean ± SD or SEM. Groups were analyzed for differences by 1-way ANOVA followed by Tukey-Kramer's test. Paired t test was applied when appropriate. Significance was accepted at P < 0.05.
Acknowledgments.
This work was supported by National Institutes of Health Grants 5R01 HL070634 and 1R01 HL088479, and by institutional grants from the Department of Pharmacology and Physiology, the New Jersey Medical School Dean's Biomedical Research Support, and the Foundation of the University of Medicine and Dentistry of New Jersey.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Fulton D, et al. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in golgi and plasma membrane defines the existence of two pools of active enzyme. J Biol Chem. 2002;277:4277–4284. doi: 10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Cardena G, et al. Dissecting the interaction between nitric oxide synthase (nos) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 3.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scotland RS, et al. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res. 2002;90:904–910. doi: 10.1161/01.res.0000016506.04193.96. [DOI] [PubMed] [Google Scholar]
- 5.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic s-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez FA, et al. Functional significance of differential eNOS translocation. Am J Physiol Heart Circ Physiol. 2006;291:H1058–H1064. doi: 10.1152/ajpheart.00370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez FA, Kim DD, Durán RG, Meininger CJ, Durán WN. Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules. Am J Physiol Heart Circ Physiol. 2008;295:H1642–H1648. doi: 10.1152/ajpheart.00629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauwels A, Janssen B, Buys E, Sips P, Brouckaert P. Anaphylactic shock depends on Pi3K and eNOS-derived NO. J Clin Invest. 2006;116:2244–2251. doi: 10.1172/JCI25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lal BK, Varma S, Pappas PJ, Hobson RW, II, Durán WN. VEGF increases permeability of the endothelial cell monolayer by activation of pkb/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res. 2001;62:252–262. doi: 10.1006/mvre.2001.2338. [DOI] [PubMed] [Google Scholar]
- 10.Hatakeyama T, et al. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol. 2006;574:275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukumura D, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakar P, Cheng V, Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J Biol Chem. 2000;275:19416–19421. doi: 10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goetz RM, et al. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1999;96:2788–2793. doi: 10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledesma MD, Simons K, Dotti CG. Neuronal polarity: Essential role of protein-lipid complexes in axonal sorting. Proc Natl Acad Sci USA. 1998;95:3966–3971. doi: 10.1073/pnas.95.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 19.Varma S, et al. P42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res. 2002;63:172–178. doi: 10.1006/mvre.2001.2381. [DOI] [PubMed] [Google Scholar]
- 20.Reynoso R, et al. A role for long chain myosin light chain kinase (mlck-210) in microvascular hyperpermeability during severe burns. Shock. 2007;28:589–595. doi: 10.1097/SHK.0b013e31804d415f. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and rock signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13:237–247. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 22.Guo M, Breslin JW, Wu MH, Gottardi CJ, Yuan SY. Ve-cadherin and beta-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am J Physiol Cell Physiol. 2008;294:C977–C984. doi: 10.1152/ajpcell.90607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pei DS, et al. Exogenous nitric oxide negatively regulates c-jun n-terminal kinase activation via inhibiting endogenous no-induced s-nitrosylation during cerebral ischemia and reperfusion in rat hippocampus. J Neurochem. 2008;106:1952–1963. doi: 10.1111/j.1471-4159.2008.05531.x. [DOI] [PubMed] [Google Scholar]
- 24.Whalen EJ, et al. Regulation of beta-adrenergic receptor signaling by s-nitrosylation of g-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Durán WN, et al. Stimulation of NO production and of eNOS phosphorylation in the microcirculation in vivo. Microvasc Res. 2000;60:104–111. doi: 10.1006/mvre.2000.2250. [DOI] [PubMed] [Google Scholar]
- 26.Cao S, et al. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem. 2001;276:14249–14256. doi: 10.1074/jbc.M006258200. [DOI] [PubMed] [Google Scholar]
- 27.del Pozo MA, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. [PubMed] [Google Scholar]
- 29.Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW, II, Durán WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284:H92–H100. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- 30.Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol. 1998;275:H542–H550. doi: 10.1152/ajpheart.1998.275.2.H542. [DOI] [PubMed] [Google Scholar]
- 31.Kim DD, Sánchez FA, Durán RG, Kanetaka T, Durán WN. Endothelial nitric oxide synthase is a molecular vascular target for the chinese herb danshen in hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H2131–H2137. doi: 10.1152/ajpheart.01027.2006. [DOI] [PubMed] [Google Scholar]