Abstract
Drug addiction is mediated by complex neuronal processes that converge on the shell of the nucleus accumbens (NAcSh). The NAcSh receives inputs from the lateral hypothalamus (LH), where self-stimulation can be induced. Melanin-concentrating hormone (MCH) is produced mainly in the LH, and its receptor (MCH1R) is highly expressed in the NAcSh. We found that, in the NAcSh, MCH1R is coexpressed with dopamine receptors (D1R and D2R), and that MCH increases spike firing when both D1R and D2R are activated. Also, injecting MCH potentiates cocaine-induced hyperactivity in mice. Mice lacking MCH1R exhibit decreased cocaine-induced conditioned place preference, as well as cocaine sensitization. Using a specific MCH1R antagonist, we further show that acute blockade of the MCH system not only reduces cocaine self-administration, but also attenuates cue- and cocaine-induced reinstatement. Thus, the MCH system has an important modulatory role in cocaine reward and reinforcement by potentiating the dopaminergic system in the NAcSh, which may provide a new rationale for treating cocaine addiction.
Keywords: G protein-coupled receptor, drug abuse, neuropeptide, MCH1R knockout mice, MCH1R antagonist
The shell of the nucleus accumbens (NAcSh) is known to be an important center for reward and motivation. It is a major terminal area of the mesolimbic dopamingergic system, which projects from the ventral tegmental area (VTA) (1). The NAcSh neurons express both dopamine D1 and D2 receptors, which have opposite effects on cAMP-dependent signaling; however, motivated behaviors require concomitant D1 and D2 receptor signaling in the NAcSh (2). The NAcSh receives inputs from different brain areas, in particular, from the lateral hypothalamus (LH) (3). The importance of the LH in reward and motivation has been shown by Olds (4), who discovered that electrical activation of the LH induced an extraordinarily intense self-stimulation response in rats. However, the transmitter(s) responsible for this action have not been established definitively. We now know of 2 neuropeptides that are uniquely synthesized in the LH: the orexins/hypocretins (5), and melanin-concentrating hormone (MCH) (6–8). The orexin/hypocretin neurons do not project prominently to the NAcSh, whereas the MCH neurons do (9). This data suggested to us that the MCH system may regulate the activities of the NAcSh neurons to influence reward.
MCH is a cyclic, 19-amino acid peptide originally isolated from salmon pituitary as a melanophore-concentrating factor (10). It is also present in rats and humans (11, 12), where it is viewed as important in regulating nutritional homeostasis (8, 13). In mammals, MCH is expressed predominantly centrally, and occurs only in 2 nuclei: the perikarya of the LH area (LHA), and the zona incerta (ZI). However, MCH fibers project in many areas throughout the CNS (6, 9), indicating that the MCH system may have a broad range of physiological roles. In rodents, MCH is known to interact with one G protein-coupled receptor, MCH1R (14–18). MCH1R is expressed in many brain regions (9), and the NAcSh has one of the highest MCH1R expression levels. Indeed, the effects of the MCH system on feeding and mood behaviors already have been shown to depend on its activity in the NAcSh (19). Because motivated behaviors related to the NAcSh often require dopamine (2), the high MCH1R expression level in the NAcSh suggested to us that the MCH system might interact with the dopamine system in the NAcSh, and thereby regulate drug taking and/or seeking.
This report provides evidence for this hypothesis by showing that the dopamine and MCH systems can interact in the NAcSh at the level of their receptors, and that MCH administration can increase dopamine responses in vitro and in vivo. Also, chronic blockade of the MCH system attenuates motivation for cocaine, whereas acute blockade inhibits cocaine self-administration, as well as cue- and cocaine-induced reinstatement. Therefore, our results suggest that the MCH system is a powerful potentiator of the dopaminergic system in NAcSh neurons, exhibiting an important modulatory role in cocaine reward and reinforcement, which may provide a new rationale for treating cocaine addiction.
Results
Interactions Between the MCH and Dopamine Systems in the NAcSh.
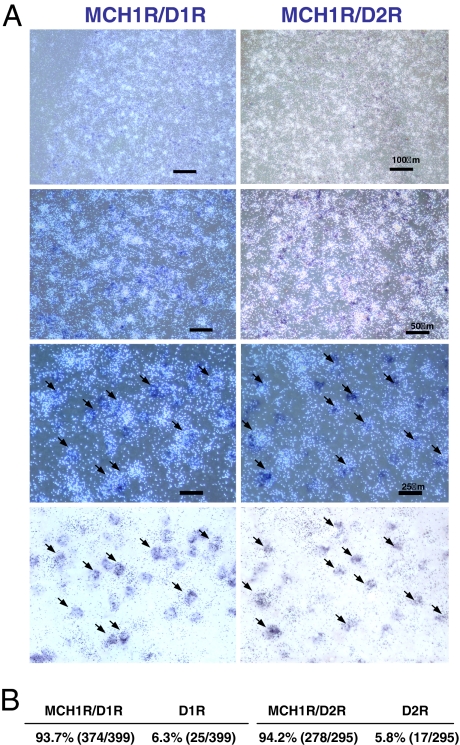
Previously, we and others showed that MCH1R is expressed in many areas of the brain (9, 16). In particular, MCH1R is highly expressed in the NAcSh, an important region in regulating reward behavior due to dopamine transmission. We then determined whether the MCH1R is coexpressed with dopamine receptors in the NAcSh using double in situ hybridization. Two separate experiments were performed to analyze D1R/MCH1R and D2R/MCH1R expression (Fig. 1A). We found that the vast majority of the cells positive for D1R- or D2R- coexpress MCH1R (Fig. 1 A and B), in agreement with the data of Georgescu et al. (19), which showed that MCH1R is colocalized with both dynorphin- and enkephalin-containing cells in the NAcSh. Our localization data suggest that the MCH system in the NAcSh may modulate dopamine-related responses and dopamine-mediated addictive behaviors.
Fig. 1.
Colocalization of MCH1R with D1R and D2R in the NAcSh. (A) Dark field images of MCH1R mRNA (white) with D1R mRNA or D2R mRNA (dark blue) in NAcSh. The panels at the bottom are bright field images of MCH1R (black grains) and D1R/D2R (blue). Arrows indicate examples of MCH1R-expressing cells that are colocalized either with D1R or D2R. (B) Percentages of cells expressing either MCH1R/D1R or D1R and MCH1R/D2R or D2R. D1R and D2R cells were counted first, and then matched for MCH1R expression (no. of colocalized cells/no. of D1R or D2R expressing cells counted).
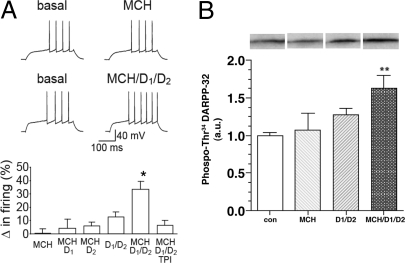
To understand whether the MCH system interacts functionally with the dopamine system in the NAcSh, we used whole-cell patch-clamp electrophysiology to monitor spike firing in NAcSh neurons. Application of MCH (2.5 μM) increased spike firing in the presence of threshold concentrations of a combination of D1 and D2 agonists (3 μM of SKF 81297 and quinpirole, respectively) (20), whereas MCH alone or MCH in combination with only the D1 or the D2 agonist had no effect (D1 or D2 agonist alone previously have been shown to have no effect) (20). Also, the MCH/D1/D2-induced increase in firing was reduced by the MCH1R antagonist, TPI 1361-17 (2 μM) (Fig. 2A). These results indicate that the MCH and dopamine systems can interact to elevate spike firing in the NAcSh, and that blockade of the MCH system would inhibit that increase in spike firing. This hypothesis was confirmed by using a biochemical approach. Because dopamine receptor activation is known to increase DARPP-32 phosphorylation at Thr-34 in the dorsal striatum (21), we monitored DARPP-32 phosphorylation at Thr-34 by MCH alone (2.5 μM) or after coincubation of MCH with D1 and D2 agonists (3 μM of SKF 81297 and quinpirole, respectively) in NAcSh slices. Although MCH or D1 plus D2 agonists had no significant or few effects, MCH increased DARPP-32 phosphorylation when combined with both D1 and D2 agonists (**, P < 0.01 vs. control; see Fig. 2B). Together, these experiments led us to conclude that MCH and dopamine receptors can act in concert to enhance the activity of NAcSh neurons.
Fig. 2.
Interaction of the MCH1R with D1R and D2R in the NAcSh. (A) Changes in NAcSh spike firing induced by MCH (2.5 μM) alone, D1 agonist (SKF 81297 3 μM) plus MCH, D2 agonist (Quinpirole 3 μM) plus MCH, D1+D2 agonists, D1+D2 agonists plus MCH, and D1+D2 agonists plus MCH and TPI 1361-17 (2 μM) (*, P < 0.05 for MCH/D1/D2 vs. each other condition, F (5, 25) = 6.66; *, P < 0.001, for 1-way ANOVA, followed by Bonferroni multiple comparison test; n = 4–6). Resting potential for MCH example was −82.4 and −83.2 mV before and after MCH, and resting potential for MCH/D1/D2 example was −80.2 and −79.6 mV before and after agonists. (B) Phosphorylation of DARPP-32 (Thr 34) in NAcSh slices after MCH (2.5 μM) alone, D1+D2 agonists (SKF 81297 3 μM and Quinpirole 3 μM), and D1+D2 agonists plus MCH (**, P < 0.01 for MCH/D1/D2 vs. control, ANOVA followed by Bonferroni multiple comparison test; n = 6–10). Immunoblots for detection of phospho-Thr-34 DARPP-32 are shown at the top. The levels of phospho-Thr-34 DARPP-32 were normalized to values obtained from control slices (a.u., arbitraty unit).
Effect of MCH Administration on Cocaine-Induced Locomotor Activity.
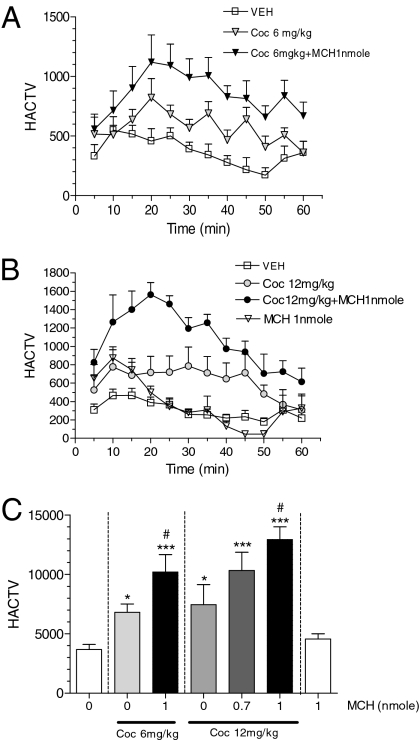
To analyze whether MCH system activation can potentiate the dopamine system in vivo, we investigated whether cocaine-induced hyperactivity is enhanced by exogenous MCH administration. Mice were injected either with vehicle or MCH (0.7 or 1 nmol i.c.v.) 5 min before cocaine injection (6 or 12 mg/kg i.p.), and their locomotor activity was measured for 60 min. Time course of horizontal activity after vehicle, MCH (1 nmol i.c.v.), cocaine (6 or 12 mg/kg i.p.), or MCH/cocaine (1 nmol i.c.v., and 6 or 12 mg/kg i.p., respectively) are shown in Fig. 3 A and B. As expected, 6 and 12 mg/kg of cocaine increased locomotor activity. However, i.c.v. injections of MCH potentiated the hyperactivity induced by cocaine injection in a dose-related manner (*, P < 0.05; ***, P < 0.001 vs. vehicle group; and #, P < 0.05 vs. cocaine alone group; see Fig. 3C), showing that central injection of MCH can potentiate cocaine effects in vivo.
Fig. 3.
Effect of MCH on cocaine induced locomotor activity. (A) Time course of horizontal activity after vehicle, cocaine (6 mg/kg i.p.) or MCH/cocaine (1 nmol i.c.v. and 6 mg/kg i.p., respectively). (B) Time course of horizontal activity after vehicle, MCH (1 nmol i.c.v.), cocaine (12 mg/kg i.p.), or MCH/cocaine (1 nmol and 12 mg/kg, respectively). (C) Cumulative horizontal activity. Effect of MCH on cocaine induced hyperactivity for 60 min (*, P < 0.05; ***, P < 0.001 vs. vehicle group; #, P < 0.05 vs. cocaine alone group; n = 6–17 per dose; ANOVA followed by Bonferroni multiple comparison test).
Decreased Sensitivity to the Rewarding Properties of Cocaine in MCH1R KO Mice.
To test whether the endogenous MCH system contributes to dopamine-mediated reward in vivo, MCH1R KO mice were tested for their cocaine-seeking behavior in the conditioned place preference (CPP) paradigm and for any changes in their locomotor activity during cocaine sensitization. Cocaine-induced CPP paradigm measures the ability of cocaine to produce a positive association with environmental cues, a response that is characteristic of rewarding/reinforcing stimuli, such as those created by drugs of abuse. We used a 2-chamber, nonbiased, CPP model. Mice were injected either with vehicle or cocaine on alternate days, and confined in one of the chambers during conditioning sessions. During the postconditioning session, increased time spent in the cocaine-paired chamber was taken to indicate increased cocaine-seeking behavior. We found that cocaine induced CPP in WT mice, as shown by the increase in the time spent in the cocaine-associated compartment during the postconditioning session (Fig. 4A,#, P < 0.05 vs. saline). However, cocaine did not significantly induce CPP in MCH1R KO mice. Also, WT mice displayed a significant preference for the cocaine-paired compartment when compared with MCH1R KO mice (**, P < 0.01, at 12 mg/kg vs. MCH1R KO mice).
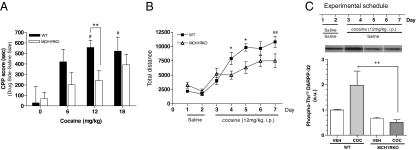
Fig. 4.
Behavioral responses to cocaine in MCH1R KO mice. (A) Cocaine-induced place preference in WT and MCH1R KO mice. CPP score: Time spent on the drug side minus time spent on the saline side during post conditioning (#, P < 0.05 vs. saline, ANOVA followed by Dunnett's test for multiple comparison; **, P < 0.01 vs. MCH1R KO mice, unpaired t test; n = 5–9). (B) Cocaine sensitization in WT and MCH1R KO mice (##, P = 0.0022 vs. day 3, paired t test; *, P < 0.05 vs. MCH1R KO mice, unpaired t test; n = 5–6). (C) Changes of phosphorylation of DARPP-32 (Thr 75) in WT and MCH1R KO mice after 5 days of saline or cocaine treatment. The data were normalized to saline treated WT group (a.u., arbitraty unit) (**, P < 0.01 vs. WT/coc mice, ANOVA followed by Bonferroni multiple comparison test; n = 4–6).
MCH1R KO mice also were tested for sensitization of locomotor activity in response to repeated cocaine administration. WT mice showed significantly increased locomotor activity after repeated daily cocaine injections (Fig. 4B, ##, P = 0.0022 vs. day 3), whereas MCH1R KO mice did not exhibit any significant changes in activity, in agreement with the previous report (22). Together, these results indicate that the lack of MCH1R results in a decreased sensitivity to the rewarding properties of cocaine in vivo. Therefore, MCH system activation is involved in the rewarding effect of cocaine.
Chronic exposure to cocaine has been shown to increase DARPP-32 phosphorylation at Thr 75 in the caudate putamen and nucleus accumbens (23). Here, we repeated this experiment, and show that WT mice exhibit an increased DARPP-32 phosphorylation level at Thr 75 in NAcSh with repeated cocaine administration (Fig. 4C). In contrast, we found that MCH1R KO mice do not exhibit a similar change in phosphorylation level after chronic cocaine treatment. Indeed, the phosphorylation level of DARPP-32 at Thr 75 in NAcSh was significantly reduced in MCH1R KO mice when compared with the level in WT mice (**, P < 0.01 vs. WT/coc group), consistent with the decreased cocaine sensitivity of MCH1R KO mice found in the behavioral assays (Fig. 4B). Therefore, MCH system activation impacts the DARPP-32 pathway in response to chronic cocaine treatments.
Effect of Acute MCH Blockade on Cocaine Self-Administration.
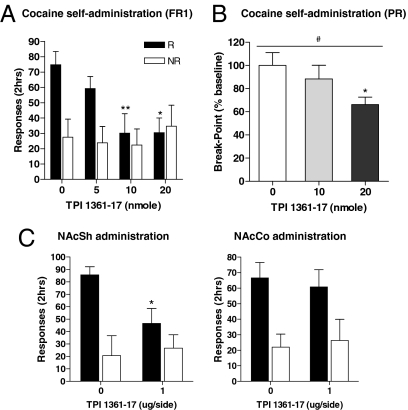
Cocaine self-administration is the most common paradigm for studying drugs of abuse. We studied how pharmacological blockade of the MCH system affects cocaine self-administration. These experiments used a high-affinity MCH1R antagonist, TPI 1361-17. This antagonist inhibits MCH1R with a nanomolar affinity, it is highly specific, showing no affinity for a panel of receptors and channels, is active in vivo, and does not exert unwanted side effects (24). The cocaine self-administration paradigm measures the primary reinforcing effect of cocaine. Animals were trained to self-administer cocaine (0.5 mg/kg per injection) on a fixed ratio 1 (FR1) schedule of reinforcement for 2 h/d for 4 days. Vehicle or TPI 1361-17 (5, 10, or 20 nmol, i.c.v.) was injected 5 min before the session, and the nose-poke responses were measured during the session. TPI 1361-17 inhibited cocaine self-administration dose-dependently (*, P < 0.05; **, P < 0.01 vs. control; see Fig. 5A). TPI 1361-17 also was tested in rats working on a progressive ratio (PR) schedule. Under this schedule, rats are required to increase the number of nose-pokes required for each successive cocaine injection. The breakpoint was defined as the number of completed nose pokes before a 30-min period when no infusions were obtained by the rat. An increase in the breakpoint after TPI 1361-17 injection indicates that blockade of the MCH system increases the reinforcing properties of cocaine, whereas a decrease indicates that MCH blockade reduces the motivation for cocaine. The steps of the exponential progression were the same as those used previously (25). As shown in Fig. 5B, pharmacological blockade of the MCH system decreased breakpoint (percentage baseline) dose-dependently (#, P < 0.05, repeated measures 1-way ANOVA; *, P < 0.05 vs. control, repeated measures 1-way ANOVA followed by Dunnett's post hoc comparison; see Fig. 5B). The decrease in PR breakpoint suggests that blockade of the MCH system leads to a reduction in the rewarding efficacy of cocaine and in motivational properties for cocaine-taking behavior.
Fig. 5.
Effect of MCH system blockade on cocaine self-administration. (A) TPI 1361-17 (5–20 nmol i.c.v.) inhibition of cocaine self-administration under a FR1 measured by the number of cocaine infusions obtained (■) and the number of inactive responses (□) (*, P < 0.05; **, P < 0.01 vs. control, ANOVA followed by Dunnett's test for multiple comparison; n = 6–11). (B) Effect of TPI 1361-17 on cocaine self-administration under a PR schedule in rats (n = 6). Data are presented as the breakpoint (percentage baseline) (mean ± SEM). There was a significant TPI 1361-17 effect on breakpoint (#, P < 0.05, repeated measures ANOVA). Significant differences were determined by repeated measures 1-way ANOVA with Dunnette's post hoc comparison (*, P < 0.05 vs. control). (C) Cocaine self-administration on TPI 1361-17 administration into the NAcSh (1 μg/side) or into NAcCo (*, P < 0.05, unpaired t test; n = 6–7). R and NR indicate reinforced (■) and nonreinforced responses (□), respectively. Injection placements for NAcSh (○) and NAcCo (●) shown in Fig. S1.
The strong inhibitory effect of TPI 1361-17 on important aspects of cocaine addiction, as well as high levels of MCH1Rs in the NAcSh suggest that the MCH system may modulate dopamine-related responses in the NAcSh. To analyze this possibility, we first tested whether TPI 1361-17 elicits its inhibitory action by acting in the NAcSh. TPI 1361-17 was injected into the NAcSh or into the closely located nucleus accumbens core (NAcCo). We found that TPI 1361-17 reduced cocaine self-administration only when injected into the NAcSh (*, P < 0.05; see Fig. 5C), showing that the NAcSh was a primary target of the modulatory role of the MCH system.
Effects of MCH Blockade on Cue-, Cocaine-, and Stress-Induced Reinstatement.
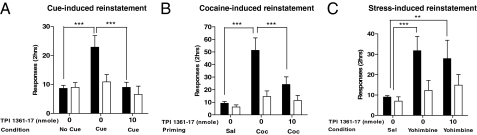
Relapse is an important aspect of drug addiction (26). It is generally studied after cocaine self-administration and then extinction of response to cocaine; after this time, cocaine response can be reinstated by exposing the animals to cocaine-associated cues, priming injection of cocaine or stress. In our experiments, the cue was a house light that predicted drug availability and a 5.6-s light that was associated with cocaine infusion; 10 to 12 days of cocaine self-administration under these conditions were followed by an extinction phase, in which nose poking had no consequences and the house light was turned off. Rats were allowed to reach an extinction baseline for 3 consecutive days (25% of their responses during cocaine self-administration) before being tested on cue-induced reinstatement. In these tests, the house light was turned on at the start of the session, and every nose poke resulted in activation of the discrete cue light, but not in cocaine delivery. We found that exposure to the cocaine-associated cues reinstated extinguished response on the previously cocaine-paired hole (***, P < 0.001), and that TPI 1361-17 was able to significantly reduce this response (***, P < 0.001; see Fig. 6A).
Fig. 6.
Effect of MCH system blockade on cue-, cocaine-, and stress-induced reinstatements. (A) Cue-induced reinstatement: TPI 1361-17 inhibition of reinstatement induced by cocaine-associated cues, measured by the number of responses in the previously cocaine-paired hole (■) and in the inactive hole (□) (***, P < 0.001; n = 7–10). (B) Cocaine-induced reinstatement: TPI 1361-17 inhibition of reinstatement induced by priming injection of cocaine (10 mg/kg i.p.) (***, P < 0.001; n = 8). (C) Stress-induced reinstatement: TPI 1361-17 inhibition of reinstatement induced by priming injection of yohimbine (2.5 mg/kg i.p.) (**, P < 0.01; ***, P < 0.001; n = 7–8). Data expressed as mean ± SEM.
Next, we determined the effect of TPI 1361-17 on reinstatement of cocaine-seeking primed by injections of cocaine. Cocaine self-administration training and extinction were carried out as described above. However, on the test days, rats were primed by an injection of cocaine (10 mg/kg i.p.) before being put in the test chamber. The cocaine injection reinstated response on the previously cocaine-paired hole (***, P < 0.001), an effect that was inhibited by TPI 1361-17 (***, P < 0.001; see Fig. 6B). These 2 experiments indicate that the endogenous MCH system is critical for relapse induced by cocaine-associated cues, as well as a priming injection of cocaine.
Another stimulus that triggers relapse-like behavior in rats is brief exposure to stress. Therefore, we tested the effect of TPI 1361-17 on stress-induced reinstatement of cocaine seeking. To trigger stress, we used yohimbine, an α2 adrenoreceptor antagonist, which induces stress-like symptoms in humans and laboratory animals (27). Priming injections of yohimbine (2.5 mg/kg i.p.) reinstated responses to the previously cocaine-paired hole (***, P < 0.001). However, in contrast to the data above, this effect was not affected by the injections of TPI 1361-17 (P > 0.05; see Fig. 6C). Thus, MCH1R activation does not appear to contribute to relapse induced by a stressor.
Together, our results strongly suggest that endogenous MCH has a critical role during reinstatement of cocaine-seeking by a cocaine-associated cue or by a cocaine priming injection, but not by stress.
Discussion
The MCH system has been implicated in modulating several behavioral responses (13); most notably, MCH has been shown to induce increases in food intake when administered centrally (8). This effect has been linked to a high MCH1R expression level in the NAcSh (19). In this article, we report another role for the MCH system related to its receptor expression in the NAcSh: namely, a modulatory role on dopamine-related responses and, in particular, on cocaine addiction.
First, we report that practically all dopamine receptor-positive neurons of the NAcSh also express MCH1R. This data is in agreement with the data of Georgescu et al. (19), who showed that MCH1R is colocalized with both dynorphin- and enkephalin-containing cells in the NAcSh. Although the high degree of overlap found in both studies points toward coexpression of D1R and D2R in medium spiny neurons, it should be mentioned that this is still a matter of controversy (28), and that using MCH1R, D1R, and D2R triple in situ hybridization analyses could resolve this issue. But the existence of MCH1R on DAR-expressing neurons suggests that the MCH system may modulate dopamine responses that rely on both DAR activations.
In vivo data have shown that several motivated behaviors require both D1 and D2 receptor activation in the NAc (29). For example, dopamine-induced spike firing in NAcSh neurons requires activation of both D1 and D2 receptors (20). We show here that MCH can modulate this response, and that its effects support the need for D1 and D2 coactivation. MCH had no significant effect on spike firing in NAcSh neurons, whether alone or together with one of the D1 or D2 agonists, although it had been previously shown to decrease D1R induced GluR1 phosphorylation (19). This difference may be due to differences in the methodologies (GluR1 phosphorylation vs. spike firing) or in the agonist doses used in the studies. However, from our data, only when D1 and D2 are coactivated is observed the potentiating effect of MCH in spike firing.
The modulatory role of MCH on the dopamine system was further investigated at the cellular level. DARPP-32 is known to be a central target of dopamine-induced intracellular responses (30). In vivo, acute cocaine injection increases Thr 34 DARPP-32 phosphorylation, whereas chronic cocaine administration increases Thr 75 DARPP-32 phosphorylation (23, 31). In vitro, in neostriatal brain slices, dopamine increases DARPP-32 phosphorylation at Thr 34 (21). When we tested whether MCH is able to affect DARPP-32 phosphorylation in NAcSh brain slices, we found that MCH is able to increase Thr 34 DARPP-32 phosphorylation induced by coincubation of D1R and D2R agonists. This potentiating effect of MCH is consistent with the one discovered through our electrophysiological observations. In vivo, we analyzed Thr 75 DARPP-32 phosphorylation in MCH1R KO and WT mice after 5 days of cocaine administration. We found that, although WT mice exhibit an increase in DARPP-32 phosphorylation at Thr 75 in NAcSh, MCH1RKO mice do not. Our data support the view that DARPP-32 phosphorylation at Thr 75 has a role in mediating the development of cocaine sensitization, as already shown by the fact that Thr75Ala DARPP-32 mutant mice do not exhibit cocaine sensitization (32). Therefore, these results show that MCH system activation has a potentiating effect on the responses that dopamine induces in the NAcSh.
Next, we explored whether MCH system activation can potentiate dopamine effects in vivo. We tested whether MCH injections can enhance cocaine-induced hyperactivity. We found that MCH enhances locomotor activity at 6 and 12 mg/kg of cocaine. Thus, injection of exogenous MCH can potentiate a cocaine effect known to be induced by increased dopamine concentration in the nerve terminals. These in vivo data are consistent with our in vitro data, and show that the MCH system can potentiate dopamine effects.
Mice lacking MCH1R were used to study whether the lack of MCH signaling attenuates cocaine effects in vivo. These mice exhibited attenuated cocaine-induced CPP and a blunted development of cocaine sensitization, which suggests that MCH system inhibition decreases the rewarding effects of cocaine. Tyhon et al. (22) already have reported that MCH1RKO mice do not develop cocaine sensitization. Our results confirm these data, but conflict with their more recent study, which reports that MCH1RKO mice show no deficit in the cocaine CPP paradigm (33). This discrepancy may be explained by differences in procedures. Tyhon et al. (33) used a biased design, in which cocaine is paired with the compartment where animals do not prefer to stay (aversive/anxious compartment). CPP under this biased design requires the animal to also overcome the aversion (or anxiety) toward the nonpreferred compartment (34–36). Therefore, the CPP score under a biased CPP procedure represents not only the motivation for cocaine, but also the overcoming of the anxiety experienced in the conditioning compartment. However, the unbiased design used in our CPP experiments excludes the anxiety factor, an important factor, because the MCH system has been reported to regulate anxiety (37, 38), and MCH1RKO mice tend to be less anxious (38). Therefore, we suggest that the differences in CPP results derive from differences in the designs of the assays. Our data also could be seen to conflict with studies that have reported that MCH1R KO mice are hypersensitive to amphetamine (39, 40), which is thought to act via the same mechanism as cocaine. However, some evidence suggests that cocaine and amphetamine induce hyperactivity via different mechanisms. The effect of cocaine on dopamine accumulation depends on the firing of dopaminergic neurons, whereas the effects of amphetamine do not (41, 42). Other KO mice strains, such as the NMDA receptor-deficient mice (NR1-KD), did not exhibit hyperactivity on acute cocaine administration (43) while their amphetamine response was not altered. Therefore, it is possible that the effects of cocaine on behavior are distinct from those of amphetamines.
KO mice represent a chronically inhibited model that does not allow one to study the effects that acute blockade of a neuronal system has on drug self-administration and drug relapse. Using an MCH1R antagonist, we show that acute blockade of the MCH system reduces cocaine self-administration in a dose-dependent manner. This effect is seen when the antagonist is administered in the shell, but not in the core of the NAc. However, this effect may be due to either an increase in cocaine reinforcing effect or a decrease in motivation for cocaine (25, 44). To differentiate between these 2 possibilities, we tested whether acute blockade of the MCH system can affect cocaine self-administration under a PR schedule. We found a dose-dependent decrease of break point on MCH1R blockade by TPI 1361-17. Although this decrease is smaller than that found for other compounds that are able to inhibit cocaine reward (45–47), it is significant, which indicates that blockade of the MCH system does not increase the reinforcing effect of cocaine. Therefore, TPI 1361-17 decreases motivation to self-administer cocaine. These data strongly support the results we obtained with mice lacking MCH1R. The inhibitory effect of TPI 1361-17 on cocaine self-administration, a dopamine-induced response, may seem contrary to the increased performance that another MCH1R antagonist, peptide 30 has in the forced swim test (19), a model where reduced dopamine levels correlate with increased depression-like state. However, it should be noted that the physiology of depression, more than that of cocaine addiction, involves the activities of neurotransmitter systems others than dopamine (48), and that, for unknown reasons, different MCH1R antagonists exert variable performance in antidepressant-like assays (49).
There is considerable interest in the cellular mechanisms of reinstatement of drug-seeking. Our data add another player in these mechanisms by showing that acute blockade of the MCH system significantly reduces relapse to cocaine-seeking that is induced by cocaine or by a conditioned cue, but not by a stressor. Previous studies have shown that the relapse induced by a conditioned cue or by cocaine activates the mesolimbic dopamine system, whereas the relapse induced by stress involves activities of the corticotrophin-releasing factor (CRF) and of the noradrenergic (NE) system (50, 51). Therefore, our data indicate that the mesolimbic dopamine system is most directly related to the role of the MCH system in reinstatement.
Together, our data allow us to conclude that blockade of the endogenous MCH system inhibits cocaine taking and seeking, in agreement with our discovery that activation of the MCH system potentiates the dopamine system to induce cellular responses in NAcSh. Thus, a specific MCH1R antagonist may represent a previously undescribed therapeutic tool for treating cocaine addiction or other disorders that result from dopamine imbalances in the NAcSh.
Materials and Methods
Detailed methods are described in the SI Materials and Methods.
Animals.
MCH1R KO mice (backcrossed to a C57BL/6 backgrounds; age-matched males; obtained from Merck) (52), or male C57BL/6 mice (National Cancer Institute) were used. Male Sprague–Dawley (Charles River) rats were used for all of the other experiments.
Biochemical and Physiological Analyses.
Double in situ hybridization was carried out on brain coronal sections that were hybridized with 35S-labeled MCH1R riboprobe and DIG-labeled riboprobes of D1R or D2R (provided by Dr. Stanley Watson) as described previously (53) with modifications (see SI Materials and Methods). Electrophysiology experiments were performed by using whole-cell recording as described previously (20). Phospho DARPP-32 assays were carried out as descried previously (21) with slight modifications (see SI Materials and Methods) by using phospho-Thr 34 DARPP-32 antibody or phospho-Thr 75 DARPP-32 antibody.
Behavioral Analyses.
Mice were habituated to the activity monitor (VERSAMEX system) for 1 h, then injected i.c.v. either with vehicle or MCH (total volume 2 μL) as described before (53). After 5 min, they were injected either with saline or cocaine (6, 12 mg/kg i.p.), and their locomotion was monitored for 60 min. CPP was conducted in 2-chambered boxes (containing visual, olfactory, and tactile cues) with the unbiased method for drug conditioning (see SI Materials and Methods). The time spent in each compartment during pre and postconditioning tests (20 min) was measured.
In cocaine self-administration experiments, rats were trained to administer cocaine (500 μg/kg per injection) under an FR1 schedule for 2-h daily sessions. Response in the active hole resulted in illumination of the signal light (5.6 s) above the reinforced nose poke and the infusion of cocaine, after which the house light shut off for a 20-s time-out (a maximum of 100 cocaine infusions per session). Response in the inactive hole was counted, but had no consequences (see SI Materials and Methods).
Supplementary Material
Acknowledgments.
We thank Dr. Paul Greengard, Dr. Angus Nairn, Elizabeth Cedars (The Rockefeller University, New York), and Dr. Akinori Nishi (Kurume University, Fukuoka, Japan) for the DARPP-32 antibodies; Dr. Frances Leslie and Susan McQuown for the help with surgeries, cocaine self-administration, and CPP; Tim Wong, Jessica Lin, and Armen Chivichyan for technical assistance; and our colleagues for discussions about the studies. The MCH1R KO mouse strain was a generous gift from Dr. Su Qian of Merck. O.C. was supported by National Institutes of Health Grants MH60231, DK63001, and BIO05–10485.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811331106/DCSupplemental.
References
- 1.Di Chiara G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;14:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 4.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 6.Bittencourt JC, et al. The melanin-concentrating hormone system of the rat brain: An immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier G, et al. Melanin-concentrating hormone (MCH) is colocalized with alpha-melanocyte-stimulating hormone (alpha-MSH) in the rat but not in the human hypothalamus. Brain Res. 1987;423:247–253. doi: 10.1016/0006-8993(87)90846-8. [DOI] [PubMed] [Google Scholar]
- 8.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 9.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 10.Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- 11.Mouri T, et al. Melanin-concentrating hormone in the human brain. Peptides. 1993;14:643–646. doi: 10.1016/0196-9781(93)90158-d. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan JM, Fischer WH, Hoeger C, Rivier J, Vale W. Characterization of melanin-concentrating hormone from rat hypothalamus. Endocrinology. 1989;125:1660–1665. doi: 10.1210/endo-125-3-1660. [DOI] [PubMed] [Google Scholar]
- 13.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 14.Bachner D, Kreienkamp H, Weise C, Buck F, Richter D. Identification of melanin concentrating hormone (MCH) as the natural ligand for the orphan somatostatin-like receptor 1 (SLC-1) FEBS Lett. 1999;457:522–524. doi: 10.1016/s0014-5793(99)01092-3. [DOI] [PubMed] [Google Scholar]
- 15.Chambers J, et al. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- 16.Lembo PM, et al. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat Cell Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, et al. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- 18.Shimomura Y, et al. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- 19.Georgescu D, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyhon A, et al. Mice lacking the melanin-concentrating hormone receptor-1 exhibit an atypical psychomotor susceptibility to cocaine and no conditioned cocaine response. Behav Brain Res. 2006;173:94–103. doi: 10.1016/j.bbr.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Bibb JA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 24.Nagasaki H, et al. The pharmacological properties of a novel MCH(1) receptor antagonist isolated from combinatorial libraries. Eur J Pharmacol. 2008;602:194–202. doi: 10.1016/j.ejphar.2008.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 26.Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- 27.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- 28.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 31.Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zachariou V, et al. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- 33.Tyhon A, Lakaye B, Adamantidis A, Tirelli E. Amphetamine- and cocaine-induced conditioned place preference and concomitant psychomotor sensitization in mice with genetically inactivated melatonin-concentrating hormone MCH(1) receptors. Eur J Pharmacol. 2008;599:72–80. doi: 10.1016/j.ejphar.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Schenk S, Ellison F, Hunt T, Amit Z. An examination of heroin conditioning in preferred and nonpreferred environments and in differentially housed mature and immature rats. Pharmacol Biochem Behav. 1985;22:215–220. doi: 10.1016/0091-3057(85)90380-6. [DOI] [PubMed] [Google Scholar]
- 35.Carr GD, Fibiger HC, Philips AG. Neuropharmacological Basis of Reward. New York: Oxford; 1989. pp. 264–319. [Google Scholar]
- 36.Swerdlow NR, Gilbert D, Koob GF. Neuromethods. Vol 13. Clifton, NJ: Human Press; 1989. pp. 399–446. [Google Scholar]
- 37.Borowsky B, et al. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 38.Smith DG, et al. Melanin-concentrating hormone-1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in mice. Neuropsychopharmacology. 2006;31:1135–1145. doi: 10.1038/sj.npp.1300913. [DOI] [PubMed] [Google Scholar]
- 39.Smith DG, et al. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25:914–922. doi: 10.1523/JNEUROSCI.4079-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyhon A, Lakaye B, Grisar T, Tirelli E. Deletion of Melanin-Concentrating Hormone Receptor-1 gene accentuates D-amphetamine-induced psychomotor activation but neither the subsequent development of sensitization nor the expression of conditioned activity in mice. Pharmacol Biochem Behav. 2008;88:446–455. doi: 10.1016/j.pbb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 41.White FJ. Electrophysiological basis of the reinforcing effects of cocaine. Behav Pharmacol. 1990;1:303–315. doi: 10.1097/00008877-199000140-00004. [DOI] [PubMed] [Google Scholar]
- 42.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsey AJ, et al. Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacology. 2008;33:2701–2714. doi: 10.1038/sj.npp.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: Human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Smith MA, et al. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology. 2004;173:105–111. doi: 10.1007/s00213-003-1706-5. [DOI] [PubMed] [Google Scholar]
- 46.Ward SJ, Roberts DC. Microinjection of the delta-opioid receptor selective antagonist naltrindole 5′-isothiocyanate site specifically affects cocaine self-administration in rats responding under a progressive ratio schedule of reinforcement. Behav Brain Res. 2007;182:140–144. doi: 10.1016/j.bbr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xi ZX, et al. Cannabinoid CB1 Receptor Antagonists Attenuate Cocaine's Rewarding Effects: Experiments with Self-Administration and Brain-Stimulation Reward in Rats. Neuropsychopharmacology. 2007;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- 48.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiat. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 49.David DJ, et al. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphen yl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321:237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- 50.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: A review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 51.Stewart J. Pathways to relapse: The neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 52.Marsh DJ, et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu YL, et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.