Abstract
The trafficking of AMPA receptors (Rs) to and from synaptic membranes is a key component underlying synaptic plasticity mechanisms such as long-term potentiation (LTP) and long-term depression (LTD), and is likely important for synaptic development in embryonic organisms. However, some of the key biochemical components required for receptor trafficking in embryos are still unknown. Here, we report that in embryonic zebrafish, the activation of PKCγ by phorbol 12-myristate 13-acetate, strongly potentiates the amplitude of AMPAR-mediated miniature excitatory postsynaptic currents (AMPA-mEPSCs) via a N-ethylmaleimide-sensitive fusion (NSF) and protein interacting with C-kinase-1 (PICK1)-dependent process. We found that the mEPSC potentiation is DAG- and Ca2+-dependent, and occurs on application of active PKCγ. Peptides that prevent the association of NSF and PICK1 with the GluR2 subunit, and the actin-polymerization blocker, latrunculin B, prevented the increase in mEPSC amplitude. Also, application of tetanus toxin (TeTx), which cleaves SNARE proteins, also blocked the increase in mEPSC amplitude. Last, application of a 5 mM K+ medium led to an enhancement in mEPSC amplitude that was prevented by addition of the PKCγ and NSF-blocking peptides, and the NMDA receptor blocker, 2-amino-5-phosphonovaleric acid (APV). Thus, activation of PKCγ is necessary for the activity-dependent trafficking of AMPARs in embryonic zebrafish. This process is NMDA and SNARE-dependent and requires AMPARs to associate with both NSF and PICK1. The present data further our understanding of AMPAR trafficking, and have important implications for synaptic development and synaptic plasticity.
Keywords: Mauthner neuron, glutamate, NMDA, synapse, development
AMPA receptors (Rs) mediate fast excitatory synaptic transmission in the CNS, and have critical roles in neuronal formation and synaptic plasticity (1, 2). They are heterotetrameric cation channels composed of glutamate receptor subunits 1–4 (GluR 1–4) with varying stoichiometries (3). Synaptic transmission at central glutamatergic synapses is enhanced by several factors including modulation by enzymes such as protein kinase A (PKA), calcium-calmodulin kinase (CaMK), tyrosine kinase (TyK), and PKC (4–6). This enhancement in synaptic transmission occurs via modulation of postsynaptic AMPA receptor activity, but more recently has been shown to occur via trafficking of AMPARs to and from synaptic membranes (7–9).
AMPAR phosphorylation by PKC is involved in various forms of synaptic plasticity (10–12). For example, PKC phosphorylation of ser880 on the GluR2 subunit is critical for the expression of long-term synaptic depression (LTD) in hippocampal CA1 and pyramidal neurons (7, 13). Also, PKMζ phosphorylation of GluR2 has been shown to maintain a stable enhancement of synaptic transmission by increasing the number of functional postsynaptic AMPARs through trafficking mechanisms (8, 9). Regulation of the dynamic movement of AMPARs into and out of synaptic membranes requires interactions between AMPAR subunits and cytosolic scaffolding proteins such as glutamate receptor-interacting protein (GRIP), AMPAR-binding protein (ABP), protein interacting with C kinase 1 (PICK1) and N-ethyl-maleimide-sensitive fusion protein (NSF) (14, 15).
We have been investigating the development of glutamate synapses associated with Mauthner cells in embryonic zebrafish. Zebrafish possess a single pair of Mauthner cells that are tonically inhibited via glycine, but are transiently activated through glutamate excitatory synapses (16, 17). Our previous work suggested that Mauthner cells express GluR2 containing AMPA receptors early in development (18). Also, they express relatively high levels of PKC from early developmental stages (19, 20). Therefore, we hypothesized that PKC might modulate AMPAR function in embryonic zebrafish. In this study, we show that application of the PKC activators, phorbol 12-myristate 13-acetate (PMA) and 1,2-dioctanoyl-sn-glycerol (DOG), enhance the amplitude of AMPARs-mediated miniature excitatory postsynaptic currents (mEPSCs) in embryonic zebrafish Mauthner cells. This potentiation of AMPAR-mEPSCs is Ca2+-dependent, and requires activation of the PKCγ isoform. Also, we show that activating PKCγ leads to the movement and insertion of AMPARs into the synaptic membrane via a SNARE-dependent mechanism that requires the interaction of AMPARs with both NSF and PICK1.
Results
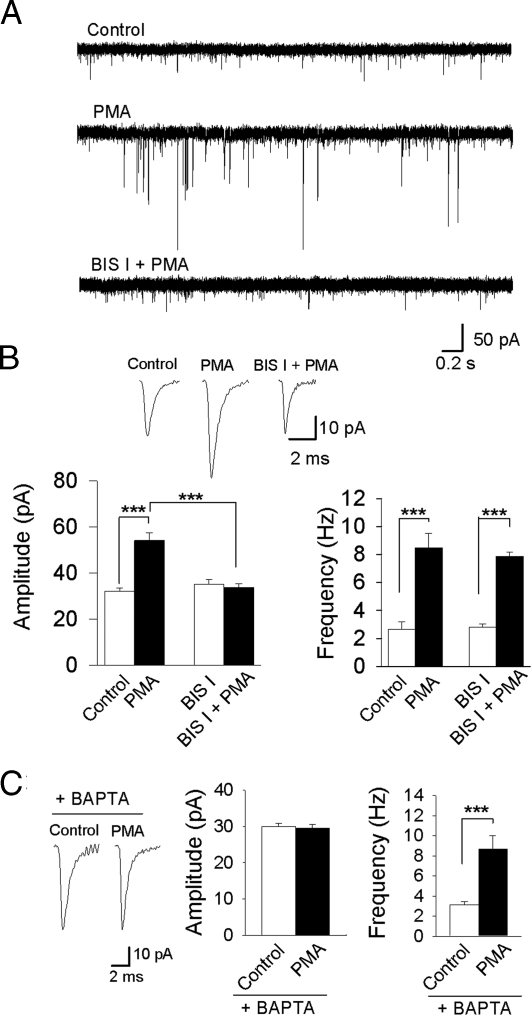
To determine whether activation of PKC resulted in modulation of AMPAR activity in embryonic (2 days post fertilization; dpf) zebrafish, we recorded AMPA mEPSCs in the presence of the PKC activator, PMA and found that both the amplitude and frequency increased significantly over control levels (amplitude, from 32.0 ± 1.5 to 54.1 ± 3.3 pA; frequency, from 2.6 ± 1.2 to 8.5 ± 1.0 Hz; n = 7, P < 0.001) (Fig. 1 A and B). The kinetics of the AMPA currents were not affected (Fig. S1). To confirm that PMA activated PKC, we included the specific PKC blocker, BIS-1 in the pipette, which prevented the increase in amplitude (n = 6, P = 0.676), but which had no effect on the frequency (n = 6, P < 0.001) (Fig. 1 A and B). These results were consistent with a presynaptic effect of PMA on mEPSC frequency, and a postsynaptic effect on amplitude. Application of the diacylglycerol (DAG) analog, DOG, resulted in an increase in mEPSC amplitude (from 29.1 ± 1.3 to 42.9 ± 2.2 pA) and frequency (from 3.2 ± 0.3 to 8.3 ± 0.8 Hz) (n = 5, P < 0.001) (Fig. S2A), but there was no effect on mEPSC kinetics (Fig. S2B). Also, intracellular application of the calcium-chelating agent 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), blocked the effect of PMA (Fig. 1C) (and DOG; Fig. S2C) on amplitude (n = 6 and n = 5 respectively, P = 0.783 and P = 0.864, respectively), but not frequency. These results suggest that activation of a DAG and Ca2+-dependent PKC isoform may be necessary for the increase in AMPA mEPSC amplitude.
Fig. 1.
Activation of PKC enhances the amplitude and frequency of AMPA mEPSCs. (A) Representative recordings of mEPSCs in an embryonic zebrafish Mauthner-cell before and after application of PMA (100 nM) and the PKC inhibitor BIS 1 (500 nM). (B) PMA increased the mean mEPSC amplitude and frequency (n = 7, P < 0.001). Intracellular application of Bisindolymaleimide I (BIS I, 500 nM; n = 6), before PMA application, blocked the increase in amplitude (n = 6, P = 0.676), but had no effect on the frequency. (C) Inclusion of the Ca2+-chelating agent, BAPTA (5 mM), in the patch pipette blocked the PMA-induced increase in amplitude (n = 6, P < 0.783), but had no effect on the mEPSC frequency. ***, significantly different, P < 0.001.
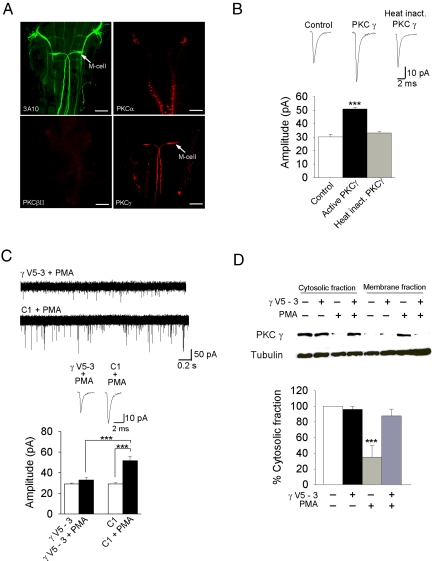
To determine which conventional PKC isoform might be responsible for mediating the increase in mEPSC amplitude, we performed immunohistochemistry on whole larvae with antibodies directed against PKCα, βII, and γ, and used the anti-3A10 antibody, a neurofilament marker that identifies Mauthner cells, as a positive control. Our results indicated that Mauthner cells express PKCγ, but not PKCα or βII (n = 5) (Fig. 2A). Therefore, we attempted to mimic the effect of PMA by applying active PKCγ directly to the cytosol. A 10-min application of active PKCγ via the recording pipette resulted in a gradual increase in mEPSC amplitude, from 29.3 ± 1.1 to 50.9 ± 1.2 pA (n = 5, P < 0.001) (Fig. 2B; Fig. S3A), but there was no effect on mEPSC kinetics (Fig. S3). Application of heat-inactivated PKCγ had no effect on mEPSC amplitude or kinetics (n = 4, P = 0.227) (Fig. 2B; Fig. S3). Next, we applied a specific PKCγ inhibiting peptide (γV5-3) to the Mauthner cell cytosol before PMA application, and found that γV5-3 completely blocked the PMA-induced increase in amplitude (n = 6, P < 0.001), whereas the control peptide (C1) had no effect (Fig. 2C) (n = 4, P = 0.667). Application of γV5-3 or C1 alone had no effect on basal mEPSC amplitude. To confirm that the blocking peptide prevented the activation, and translocation of PKCγ to the membrane, we immunoblotted zebrafish CNS tissue with anti-PKCγ in the presence and absence of γV5-3 and PMA. Inactive PKCγ was largely limited to the cytosol, and activation by PMA led to the movement of PKCγ from the cytosol to the membrane, as expected (n = 4, P < 0.001) (Fig. 2D). Addition of γV5-3 completely blocked the translocation of PKCγ from the cytosol to the membrane. Together, our results suggest that PKCγ is responsible for the increase in mEPSC amplitude.
Fig. 2.
PKCγ is expressed in the Mauthner cell, and its activation leads to an increase in mEPSC amplitude. (A) Anti-3A10, anti-PKCα, anti-βII, and anti-γ immunoreactivity in zebrafish (n = 5). Anti-3A10 is a neurofilament marker that identifies Mauthner cells and is used as a positive control. Only anti-PKCγ labels the Mauthner cell. (Scale bar, 50 μM.) (B) Ten-minutes application of the active form of PKCγ to the Mauthner cell cytosol caused an increase in mEPSC amplitude (n = 5, P < 0.001); 10-min application of heat inactivated PKCγ had no effect (n = 4). Controls represent a recording of AMPA mEPSCs at the 10-min time point in normal intra and extracellular solutions. (C) Intracellular application of γV5-3 (10 nM) blocked the PMA-induced increase in the amplitude (n = 6), whereas the control peptide (C1; 10 nM) had no effect (n = 4). (D) Immunoblot analysis of cytosolic and membrane fractions from zebrafish brain after incubation with or without γV5-3 and PMA (5 nM); γV5-3 inhibited the PMA-induced loss of PKCγ from the cytosolic fraction (n = 4, P < 0.001). ***, significantly different, P < 0.001.
We then sought to determine the mechanism whereby activation of PKCγ led to an increase in AMPAR mEPSCs amplitude. First, we performed nonstationary fluctuation analysis (NSFA) on AMPA mEPSCs to determine whether the single channel conductance increased, or whether the number of synaptic AMPARs changed after application of PMA. NSFA indicated that the channel conductance of AMPARs did not change after bath application of PMA (n = 7, P = 0.865) (Fig. S4 A and B). However, there was a significant increase in the number of synaptic AMPA receptors, from 33.7 ± 1.3 to 63.3 ± 4.6 (n = 7, P < 0.001), which was prevented by including BIS 1 in the recording pipette (P = 0.788, n = 6) (Fig. S4B). This data suggested that the PMA-induced increase in mEPSC amplitude was probably due to receptor trafficking rather than an increase in channel conductance. Therefore, we tested this hypothesis by bath applying a general actin polymerization blocker, latrunculin B, which is known to prevent receptor trafficking (21). We found that latrunculin B completely inhibited the PMA-induced increase in amplitude (n = 5, P < 0.001) (Fig. S4C), which is consistent with a trafficking mechanism.
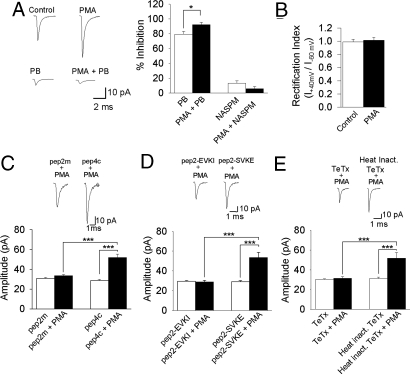
To determine the subunit composition of the AMPARs, we tested the ability of the GluR2 blocker, pentobarbital (PB), and the non-GluR2 blocker, NASPM, to block mEPSC amplitude before and after PMA application. PB (100 μm) blocked mEPSC amplitude by 79 ± 4% in control recordings, and by 92 ± 3% after PMA application (n = 6, P < 0.05) (Fig. 3A), suggesting that the majority of AMPARs contain the GluR2 subunit. Also, the non-GluR2 blocker, NASPM (10 μm) blocked the mEPSC amplitude by 13 ± 3% in control recordings, and by 6 ± 3% after addition of PMA (n = 5, P < 0.05) (Fig. 3A). Last, the presence of GluR2 subunits may be inferred from a linear or outward rectification of AMPA currents (22). Therefore, we examined the mEPSC rectification index (I+40 mV/I−60 mV) before and after addition of PMA, and found there to be no difference (0.99 ± 0.03 before vs. 1.01 ± 0.04 after application of PMA; n = 6; Fig. 3B). Together, these data suggest that zebrafish Mauthner cells express, and up-regulate, GluR2 containing AMPARs via receptor trafficking.
Fig. 3.
GluR2-containing AMPAR trafficking depends on NSF and PICK1, and requires assembly of a SNARE complex. (A) Application of the GluR2 blocker, PB (100 μM, n = 6) reduces mEPSC amplitude in the absence and presence of PMA, whereas the non-GluR2 blocker, NASPM (10 μM, n = 5) had little effect. (B) The mEPSC rectification index (I+40 mV/I−60 mV) did not change on application of PMA (n = 6). (C) Intracellular application of pep2m (200 μM, n = 4) blocked the effect of PMA on amplitude, whereas the control peptide, pep4c (200 μM, n = 4) had no effect. (D) Inclusion of pep2-EVKI (200 μM, n = 4) in the patch pipette prevented the PMA-induced increase in mEPSC amplitude, whereas the control peptide pep2-SVKE (200 μM, n = 4) had no effect. (E) Intracellular application of TeTx (200 nM; n = 6) prevented the PMA-induced increase in mEPSCs, whereas application of heat-inactivated TeTx (n = 3) had no effect. ***, significantly different, P < 0.001; *, significantly different, P < 0.05.
To gain more insight into the mechanism of AMPAR trafficking, we tested whether a peptide (pep2m), which interferes with the interaction between NSF and the GluR2 subunit (Fig. S5), would prevent the effect of PMA. Pep2m (200 μM) completely prevented the increase in mEPSC amplitude (n = 4, P < 0.001) (Fig. 3C), whereas the inactive control peptide, pep4c, had no effect (n = 4, P = 0.173) (Fig. 3C). The AMPAR interacting protein PICK1 is also known to regulate the surface expression of GluR2-containing AMPARs (13). Therefore, we blocked the interaction between PICK1 and GluR2 with the peptide pep2-EVKI (200 μM), and found that it prevented the effect of PMA (n = 4, P < 0.001). The control peptide, pep2-SVKE (200 μM), had no effect (n = 4, P = 0.378) (Fig. 3D). Together, these results suggest that the effect of PKCγ can be fully accounted for via trafficking of GluR2-containing AMPARs to the synapse in a NSF and PICK1-dependent manner. To determine whether a SNARE complex was involved in the insertion of AMPARs into the membrane, we applied the tetanus toxin (TeTx) light chain to the Mauthner cell cytosol before PMA application. TeTx (200 nM) completely prevented the increase in mEPSC amplitude (n = 6, P < 0.001) (Fig. 3E), but had no effect on frequency (Fig. S6A). Miniature EPSC amplitude during the TeTx wash, before application of PMA, was not significantly different from control recordings (Fig. 1B). Application of heat-inactivated TeTx had no significant effect on either the amplitude or frequency of mEPSCs (n = 3, P = 0.474) (Fig. 3E; Fig. S6A).
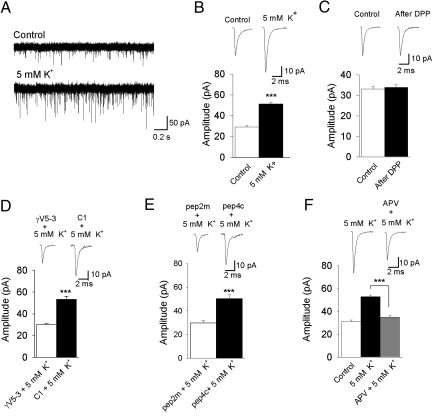
Last, to determine whether endogenous PKCγ can induce the trafficking of AMPARs via a more physiological mechanism, we mimicked depolarization of the Mauthner cells via 2 separate methods. First, we bath applied a 5 mM K+ medium for 10 min to depolarize the cell and its synaptic afferents. Second, we induced a direct depolarization of the Mauthner cell using a depolarization protocol (DPP) (23). The 5 mM K+ treatment caused an enhancement in mEPSC amplitude (from 29.3 ± 1.0 to 51.3 ± 1.4 pA) and frequency that was similar to that of PMA (n = 6, P < 0.001) (Fig. 4 A and B; Fig. S7). However, direct depolarization of the Mauthner cell using the DPP had no effect on mEPSC amplitude (n = 5, P = 0.675) (Fig. 4C). Together, these results suggest that depolarization of the afferents onto Mauthner cells leads to an increase in AMPA mEPSC amplitude.
Fig. 4.
Activity-induced trafficking of AMPAR requires activation of PKCγ. (A) Representative recordings of mEPSC before and after application of 5 mM K+. (B) Application of 5 mM K+ for 10 min significantly increased mEPSC amplitude (n = 5). (C) Induction of postsynaptic depolarization of the Mauthner cell via a DPP had no effect on the mEPSC amplitude (n = 5). (D) Intracellular application of γV5-3 (10 nM) prevented the potentiation in amplitude after the 5 mM K+ bath (n = 5), whereas the control peptide (C1; 10 nM) had no effect (n = 5). (E) Inclusion of pep2m (200 μM, n = 5) in the recording solution completely prevented the 5 mM K+ induced increase in mEPSC amplitude, whereas the control peptide, pep4c (200 μM, n = 5) had no effect. (F) Application of APV (50 μM, n = 7) completely blocked the 5 mM K+ induced increase in mEPSC amplitude. ***, significantly different, P < 0.001.
To determine whether PKCγ was activated after 5 mM K+ treatment, we applied the PKCγ blocking peptide γV5-3 (n = 5, P < 0.001), which blocked the 5 mM K+-induced increase in amplitude, whereas the control peptide (C1) had no effect (n = 5, P = 0.219) (Fig. 4D). We tested whether trafficking mechanisms were involved in the 5 mM K+ effect applying pep2m to the cytosol of the Mauthner cell before application of the 5 mM K+ medium. We found that pep2m (n = 5, P < 0.001) prevented the enhancement of mEPSC amplitude, whereas the control peptide pep4c had no effect (n = 5, P = 0.089) (Fig. 4E). Both γV5-3 and pep2m had no effect on mEPSC frequency (Fig. S6 B and C). Together, these results suggest that depolarization of the afferents onto Mauthner cells leads to activation of endogenous PKCγ and the subsequent trafficking of GluR2-containing AMPARs to the synapse via a NSF-dependent mechanism. Last, we tested whether the trafficking process was NMDA-dependent by blocking NMDA receptor activation with 2-amino-5-phosphonovaleric acid (APV; 50 μM). We found that APV abolished the 5 mM K+ induced increase in mEPSC amplitude (n = 7, P < 0.001) (Fig. 4F).
Discussion
The present results show that activation of PKCγ enhances AMPAR-mEPSC amplitude in an embryonic organism by inducing the insertion of GluR2-containing AMPA receptors into synaptic membranes. Also, the trafficking mechanism requires AMPARs to associate with the scaffolding proteins NSF and PICK1, and occurs through NMDA- and SNARE-dependent processes.
To our knowledge, this is the first study to report the PKCγ-induced trafficking of GluR2-containing AMPARs in an embryonic organism, and one of the few to implicate a combined role for both NSF and PICK1 in the movement and insertion of AMPARs into synaptic membranes. Several lines of evidence support our conclusions. First, PKCγ is highly expressed in Mauthner cells. Second, application of active PKCγ increases AMPAR-mEPSC amplitude. Third, the PKCγ blocking peptide γV5-3 completely prevented the effect of PMA. Fourth, peptides that specifically block the association of NSF and PICK1 with the AMPA GluR2 subunit prevent the PMA-induced increase in mEPSC amplitude. Fifth, the GluR2 blocker PB blocks >90% of the AMPA current after application of PMA. Thus, we conclude that PKCγ is the principal PKC isoform in zebrafish Mauthner cells that is required for the trafficking of GluR2-containing AMPAR into synaptic membranes. We cannot discount the possibility that other PKC isoforms may be partially involved in the process, but the ability of γV5-3 to completely prevent the 5 mM K+-induced increase in mEPSC amplitude indicates that PKCγ is the principal isoform. How is PKCγ activated after an increase in cellular activity? We do not yet know, but the ability of APV to prevent the effects of 5 mM K+ indicates a crucial role for NMDA receptor activation similar to that shown in adult systems (21).
Both PMA and DOG caused large increases in mEPSC frequency, presumably via acting presynaptically on transmitter release. These findings are consistent with other studies (24, 25), and may be due to an increase in the vesicle recycling rate, an increase in the size of the readily releasable pool (26), an increase in presynaptic Ca2+ influx (27), or the induction of new release sites.
Our results suggest that the PKCγ-induced delivery of AMPARs to embryonic synapses depends on an interaction with NSF. PICK1 is also known to regulate the surface expression of GluR2-containing AMPARs (32). Here, we report that PICK1 is involved in the PKCγ-driven insertion of AMPARs into the membrane. Our findings are supported by a recent study on rat hippocampal neurons, where it was shown that PKMζ interacts with PICK1 to direct AMPARs to the synaptic membrane (9). Thus, our results show the requirement of both NSF and PICK1 for the trafficking of AMPARs to the synaptic membrane.
What is the developmental significance of AMPAR trafficking? Some of the earliest inputs onto Mauthner cells appear to be formed just before 24 h pf (36), around the time that embryos acquire a touch/startle response (37). However, synaptic development continues, and at ≈2 dpf, embryos start to hatch out of the egg casing, at which point they can swim away from a stimulus. Our previous work suggested that AMPARs switch subunits (and exhibit faster kinetics) between 33 and 48 h, immediately before hatching (18). Thus, the generation of faster AMPA currents, combined with the ability to up-regulate AMPA currents in an activity-dependent manner, would likely lead to a more efficient and a stronger startle response.
In summary, we show that activation of PKCγ leads to the trafficking of GluR2-containing AMPARs into excitatory synapses on embryonic Mauthner cells (Fig. S8). This process depends on an interaction with NSF, PICK1, and SNARE proteins, and occurs in an NMDAR- and activity-dependent manner. Our study provides insights into the role of PKCγ and the movement and insertion of AMPARs into synaptic membranes of an embryonic organism, and indicates that PKCγ is a necessary intermediate in the trafficking of GluR2-containing AMPA receptors.
Materials and Methods
Preparation.
Wild-type Zebrafish (Danio rerio) embryos were raised at 28.5 °C, and collected and staged as previously described (SI Materials and Methods) (18).
Electrophysiology.
Preparations were recorded as previously described (18). The extracellular recording solution contained (in mM) 134 NaCl, 2.9 KCl, 2.1 CaCl2, 1.2 MgCl2, 10 Hepes, and 10 glucose, osmolarity adjusted to 280 mOsm, pH 7.8. The Cs-gluconate intracellular patch clamp solution was composed of (in mM) 115 Cs-gluconate, 15 CsCl, 2 MgCl2, 10 Hepes, 10 EGTA, and 4 Na2ATP, osmolarity adjusted to 290 mOsm, pH 7.2. Chemical depolarization of the Mauthner cell was induced by a 10-min bath application of a 5 mM K+, low Mg2+ depolarizing medium that contained (in mM): 130 NaCl, 5 KCl, 2.1 CaCl2, 0.3 MgCl2, 10 Hepes, 10 glucose, and 1 TEA, osmolarity adjusted to 280 mOsm, pH 7.8. Postsynaptic depolarization was induced by a DPP (SI Materials and Methods) (23).
Analysis of mEPSCs.
Synaptic activity was monitored by using pClamp 8.1 software (MDS Analytical Technologies), and analyzed with Axograph X as previously described (SI Materials and Methods) (18).
NSFA.
We performed NSFA to estimate the single-channel current (i) and the available number of channels (N) as previously described (SI Materials and Methods) (18, 38).
Immunohistochemical Procedures.
Zebrafish 2 dpf embryos were processed for immunohistochemistry by using anti-3A10, anti-PKCα, anti-PKCβII, and anti-PKCγ antibodies as previously described (SI Materials and Methods) (20).
Western Blot Analysis.
Zebrafish brains were immunoblotted for PKCγ in the absence and presence of PMA, with or without the PKCγ inhibitor peptide (γV5-3) (SI Materials and Methods).
Supplementary Material
Acknowledgments.
We thank KAI Pharmaceuticals for supplying the following peptides: γV5-3 (γPKC antagonist) and the control peptide (C1). The monoclonal antibody 3A10 developed by Thomas M. Jessell and Jane Dodd was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). D.W.A. was supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Canadian Foundation for Innovation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811171106/DCSupplemental.
References
- 1.Zhang L, et al. Role of GluR1 in activity-dependent motor system development. J Neurosci. 2008;28:9953–9968. doi: 10.1523/JNEUROSCI.0880-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Paola V, Arber S, Caroni P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci. 2003;6:491–500. doi: 10.1038/nn1046. [DOI] [PubMed] [Google Scholar]
- 3.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 4.Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 5.Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J Neurophysiol. 1998;80:2797–2800. doi: 10.1152/jn.1998.80.5.2797. [DOI] [PubMed] [Google Scholar]
- 6.Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 7.Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- 9.Yao Y, et al. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banke TG, et al. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 12.Wang JQ, et al. Phosphorylation of AMPA receptors: Mechanisms and synaptic plasticity. Mol Neurobiol. 2005;32:237–249. doi: 10.1385/MN:32:3:237. [DOI] [PubMed] [Google Scholar]
- 13.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Ali DW, Buss RR, Drapeau P. Properties of miniature glutamatergic EPSCs in neurons of the locomotor regions of the developing zebrafish. J Neurophysiol. 2000;83:181–191. doi: 10.1152/jn.2000.83.1.181. [DOI] [PubMed] [Google Scholar]
- 17.Ali DW, Drapeau P, Legendre P. Development of spontaneous glycinergic currents in the Mauthner neuron of the zebrafish embryo. J Neurophysiol. 2000;84:1726–1736. doi: 10.1152/jn.2000.84.4.1726. [DOI] [PubMed] [Google Scholar]
- 18.Patten SA, Ali DW. AMPA receptors associated with zebrafish Mauthner cells switch subunits during development. J Physiol. 2007;581:1043–1056. doi: 10.1113/jphysiol.2007.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slatter CA, Kanji H, Coutts CA, Ali DW. Expression of PKC in the developing zebrafish, Danio rerio. J Neurobiol. 2005;62:425–438. doi: 10.1002/neu.20110. [DOI] [PubMed] [Google Scholar]
- 20.Patten SA, Sihra RK, Dhami KS, Coutts CA, Ali DW. Differential expression of PKC isoforms in developing zebrafish. Int J Dev Neurosci. 2007;25:155–164. doi: 10.1016/j.ijdevneu.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Korkotian E, Segal M. Morphological constraints on calcium dependent glutamate receptor trafficking into individual dendritic spine. Cell Calcium. 2007;42:41–57. doi: 10.1016/j.ceca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 23.Baxter AW, Wyllie DJ. Phosphatidylinositol 3 kinase activation and AMPA receptor subunit trafficking underlie the potentiation of miniature EPSC amplitudes triggered by the activation of L-type calcium channels. J Neurosci. 2006;26:5456–5469. doi: 10.1523/JNEUROSCI.4101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen DH, et al. Missense mutations in the regulatory domain of PKC gamma: A new mechanism for dominant nonepisodic cerebellar ataxia. Am J Hum Genet. 2003;72:839–849. doi: 10.1086/373883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaba T, Neher E. Preferential potentiation of fast-releasing synaptic vesicles by cAMP at the calyx of Held. Proc Natl Acad Sci USA. 2001;98:331–336. doi: 10.1073/pnas.021541098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshihara M, Suzuki K, Kidokoro Y. Two independent pathways mediated by cAMP and protein kinase A enhance spontaneous transmitter release at Drosophila neuromuscular junctions. J Neurosci. 2000;20:8315–8322. doi: 10.1523/JNEUROSCI.20-22-08315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- 29.Boehm J, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Li P, et al. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nat Neurosci. 1999;2:972–977. doi: 10.1038/14771. [DOI] [PubMed] [Google Scholar]
- 31.Leitges M, Kovac J, Plomann M, Linden DJ. A unique PDZ ligand in PKCalpha confers induction of cerebellar long-term synaptic depression. Neuron. 2004;44:585–594. doi: 10.1016/j.neuron.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Perez JL, et al. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abeliovich A, et al. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 34.Abeliovich A, et al. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- 35.Saito N, Shirai Y. Protein kinase C gamma (PKC gamma): Function of neuron specific isotype. J Biochem. 2002;132:683–687. doi: 10.1093/oxfordjournals.jbchem.a003274. [DOI] [PubMed] [Google Scholar]
- 36.Kimmel CB, Hatta K, Metcalfe WK. Early axonal contacts during development of an identified dendrite in the brain of the zebrafish. Neuron. 1990;4:535–545. doi: 10.1016/0896-6273(90)90111-r. [DOI] [PubMed] [Google Scholar]
- 37.Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 38.Sigworth FJ. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.