Abstract
An implantation-competent blastocyst, several hours prior to its attachment on the uterine wall, transmits signals to surrounding uterine cells and vice-versa to initiate a two-way interaction. The language of this precocious dialogue is versatile, taking advantage of secreted molecules for long-range interactions and membrane-bound molecules for more immediate interactions. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) was identified as an early messenger of implantation which uses both modes of communication. In this review, we discuss the footprint of HB-EGF as to how it was initially identified as a mediator of implantation and how it initiates embryo-uterine interactions during this process.
Keywords: HB-EGF, ErbB, blastocyst, implantation
Embryo-uterine interaction in implantation – A brief overview
One prerequisite for mammalian reproduction is an effective reciprocal interaction between an implantation-competent blastocyst and the receptive uterus. The blastocyst will implant only when this molecular dialogue is established. In women, the uterus becomes receptive for a short time, i.e., 7–9 days after ovulation (cycle days 21–23) during the mid luteal phase. After this time period, the uterus becomes nonreceptive (refractory) and remains refractory for the rest of the luteal phase. Many shortcomings of human infertility have been overcome by in vitro fertilization and embryo transfer (IVF-ET) techniques. Nevertheless, the implantation rate in IVF programs remains disappointingly low, with one cause being transfer of embryos into nonreceptive uteri, resulting in implantation failure [1,2].
The master regulators that specify uterine sensitivity related to implantation are the ovarian steroid hormones progesterone (P4) and estrogen. In most mammals studied, the uterus differentiates into an altered state when implantation-competent blastocysts are capable of effective two-way communication to initiate the process of implantation. This state is termed uterine receptivity for implantation and lasts for a limited period. The receptive uterine environment is conducive to blastocyst growth and implantation [3–6].
In mice, the initial attachment reaction between the blastocyst and the receptive uterus occurs on the evening (2000–2400 h) of day 4 of pregnancy (day 1= vaginal plug), and this attachment always occurs at the antimesometrial pole of the uterus. The attachment reaction is preceded by uterine luminal closure bringing the blastocyst in close apposition with the uterine luminal epithelium. The attachment of the blastocyst is coincident with increased endometrial vascular permeability at the site of blastocyst apposition and is then followed by decidualization of stromal cells surrounding the blastocyst. Failure to sustain uterine growth and differentiation after blastocyst attachment results in spontaneous abortion. Still, relatively little is known about the hierarchy of events that direct uterine receptivity, blastocyst attachment and uterine refractoriness [7–9].
In mice, the coordinated actions of P4 and estrogen regulate proliferation and/or differentiation of uterine cells in a spatiotemporal manner to establish the window of receptivity for implantation [10]. On days 1 and 2 of pregnancy, uterine epithelial cells undergo proliferation under the influence of preovulatory estrogen secretion. Rising levels of P4 secreted from newly formed corpora lutea then initiate stromal cell proliferation from day 3 onward which is further stimulated by a small amount of ovarian estrogen secretion on the morning of day 4. These coordinated effects of P4 and estrogen result in the cessation of uterine epithelial cell proliferation, initiating differentiation [11]. During normal pregnancy, the presence of an active blastocyst in the uterus is the stimulus for implantation. After the attachment reaction is initiated on the evening of day 4 (2000–2400h), stromal cells surrounding the implanting blastocyst undergo extensive proliferation and differentiate to decidual cells (decidualization) [3]. In pseudopregnant mice, the uterine steroid hormonal milieu is similar to pregnant mice during the periimplantation period due to the presence of newly formed corpora lutea. Thus, uterine sensitivity to implantation in pseudopregnant mice on days 1–5 is quite similar to normal pregnancy, and blastocyst transfer into the uterine lumen during the receptive phase (day 4) provokes normal implantation reactions and subsequent decidualization.
During normal pregnancy, the uterine sensitivity in the context of implantation is classified as prereceptive, receptive and refractory [3,4]. In pregnant or pseudopregnant mice, the uterus becomes receptive on day 4 (the day of implantation), while by late day 5 (examined by blastocyst transfer experiments), the uterus becomes refractory and implantation fails. These uterine phases can also be induced in ovariectomized mice by appropriate P4 and estrogen treatment and also in delayed implanting mice. The uterus becomes neutral when exposed to P4 alone similar to that which occurs during delayed implantation. Under this neutral condition, the uterus will respond to the presence of blastocysts for implantation only if exposed to estrogen after 24–48 h of P4 priming. Even so, the induced window of receptivity will only last for a limited period (about 24 h). The uterus then automatically proceeds to the refractory phase [3,4].
During delayed implantation, the uterus remains in a quiescent state and blastocysts undergo dormancy. Delayed implantation occurs naturally in many mammals, but the factors that direct this process vary [12]. For instance, delayed implantation occurs naturally (facultative) during lactation after postpartum ovulation and fertilization in mice and rats [13], with implantation ensuing rapidly after termination of the suckling stimulus. This lactational delay occurs due to insufficient ovarian estrogen secretion. Whether this phenomenon occurs in humans is not yet known. Delayed implantation can also be induced experimentally. For example, in mice, ovariectomy on the morning of day 4 of pregnancy before preimplantation ovarian estrogen secretion results in implantation failure with blastocyst dormancy [13,14]. This condition of delayed implantation can be maintained for many days by continued treatment with P4 and is analogous to the neutral phase, since blastocyst activation with initiation of implantation is rapidly initiated by a single injection of estrogen [13,14]. Mechanisms by which estrogen mediates the processes of blastocyst activation and implantation in the P4-primed uterus are poorly understood. This delayed implantation mouse model is widely used to better understand the molecular signaling that emanates from the embryo and influences uterine biology and vice versa.
HB-EGF in the mouse uterus: where the story began
The blastocyst, upon encountering the maternal interface, initiates a two-way communication several hours before the attachment reaction occurs. HB-EGF first appears in epithelial cells juxtaposed with blastocysts around 1600 h on day 4 of pregnancy [15]. As stated earlier, the attachment reaction ensues in the evening of the same day. This early expression of HB-EGF in the uterus is not hormone-dependent, as it is not seen in pseudopregnant mouse uteri when hormonal milieu is very similar to that of normal pregnancy. Embryonic induction of HB-EGF provided insights for the first time that blastocysts send signals for the preparation of the maternal environment conducive to subsequent attachment reaction.
Why is HB-EGF turned on during early embryo-uterine interactions? Two in vitro experiments offer clues to this question. First, 8-cell mouse embryos exposed to HB-EGF in culture showed increased rates of hatching and growth [15]. EGF was not as effective as HB-EGF in promoting hatching and growth of embryos. Second, HB-EGF promotes the outgrowth of trophoblasts in vitro, which supports its role in trophoblast invasion during the process of implantation.
HB-EGF was identified as a mitogen for fibroblasts and smooth muscle cells [16]. HB-EGF, like other EGF-like growth factors, is initially expressed as a transmembrane form (HB-EGF™) with multiple domains such as signal peptide, heparin-binding, EGF-like, juxtamembrane, transmembrane, and cytoplasmic domains [16]. HB-EGF™ then can be enzymatically processed further for shedding and the secreted mature HB-EGF acts as a paracrine factor. While the mature form is a potent mitogen for various cell types, HB-EGF™, because of its physical containment within the membrane, plays a role as an adhesion molecule by interacting with juxtaposed receptors. In the uterine luminal epithelium, HB-EGF is expressed as transmembrane and in soluble forms [17], implying its role as a juxtacrine and paracrine signaling mediator. At the time of HB-EGF™ expression, the blastocyst is closely apposed to the luminal epithelium due to the generalized uterine edema and uterine luminal closure induced by P4 and estrogen. Thus, it was speculated that a closely apposed blastocyst expresses receptors for HB-EGF™ for juxtacrine interactions. Raab and others devised an in vitro experimental system to show the juxtacrine interaction of blastocysts and HB-EGF™ [17]. They engineered 32D cells to express HB-EGF™ and co-incubated these cells with blastocysts. When dormant blastocysts were used in the experiment, HB-EGF™-expressing 32D cells did not adhere to the embryonic surface. One of the factors that are downregulated in dormant blastocysts is ErbB1 (EGF-R) and ErbB4 [18,19]. Thus, HB-EGF™-mediated adherence of 32D cells is likely to use ErbB1 and/or ErbB4 on implantation-competent blastocysts. In addition, HB-EGF™ may also utilize various heparin sulfate proteoglycans (HSPGs) synthesized by blastocysts. When blastocysts were pretreated with heparinase to remove cell surface heparin sulfate, the number of adhering 32D cells was reduced by 50% [17].
More concrete evidence establishing the role for HB-EGF in implantation came later with the application of in vitro model of implantation and the availability of knockout mouse models. Growth factor-soaked beads are widely used in the field of developmental biology to study cellular and molecular responses to morphogens. This approach was adopted in implantation by substituting embryos to growth factor-soaked beads. Beads of about the size of blastocysts were chosen and soaked with various growth factors. They were transferred into the uteri of pseudopregnant mice similar to transfer of normal blastocysts. Among many factors, HB-EGF and IGF-1 efficiently elicited discrete local implantation-like responses, such as increased vascular permeability, decidualization and expression of implantation marker genes [20]. HB-EGF-soaked beads also induced expression of HB-EGF itself in the luminal epithelium, suggesting an auto-induction loop in regulating HB-EGF expression during implantation [21]. Since implantation-competent blastocysts themselves express HB-EGF, it is thought that HB-EGF serves as a two-way signaling bridge between the embryo and uterus during implantation [21].
Genetic studies have shown that Hbegf−/− female mice are sub-fertile with reduced litter size. Further scrutiny of these mice revealed that HB-EGF plays a dual role in reproduction by regulating both ovarian and uterine functions [22]. As stated above, HB-EGF is also expressed both in the blastocyst and the uterus during implantation [21]. Reciprocal embryo transfer experiments, however, showed that uterine HB-EGF deficiency causes deferral in implantation, leading to compromised pregnancy outcome, since Hbegf−/− blastocysts can implant when transferred into uteri of wild-type mice [22]. This work also demonstrated that prolonged amphiregulin expression partially compensates for the loss of HB-EGF in Hbegf−/− uteri. Interestingly, this compensation was achieved by the reduction in ovarian estrogen secretion caused by ovarian HB-EGF deficiency. Overall, these studies show that HB-EGF is crucial for embryo-uterine interactions during implantation in mice.
Tracking down the receptors for HB-EGF in implantation
One interesting feature of HB-EGF™ is that it utilizes various molecules as its “receptors”. The primary receptors of HB-EGF are the ErbB system, especially ErbB1 and ErbB4. Mature HB-EGF induces dimerization of ErbB receptors, autophosphorylation, and activation of MAPK pathway [16]. HB-EGF can also use HSPGs as receptors and the heparin-binding domain is required for this interaction.
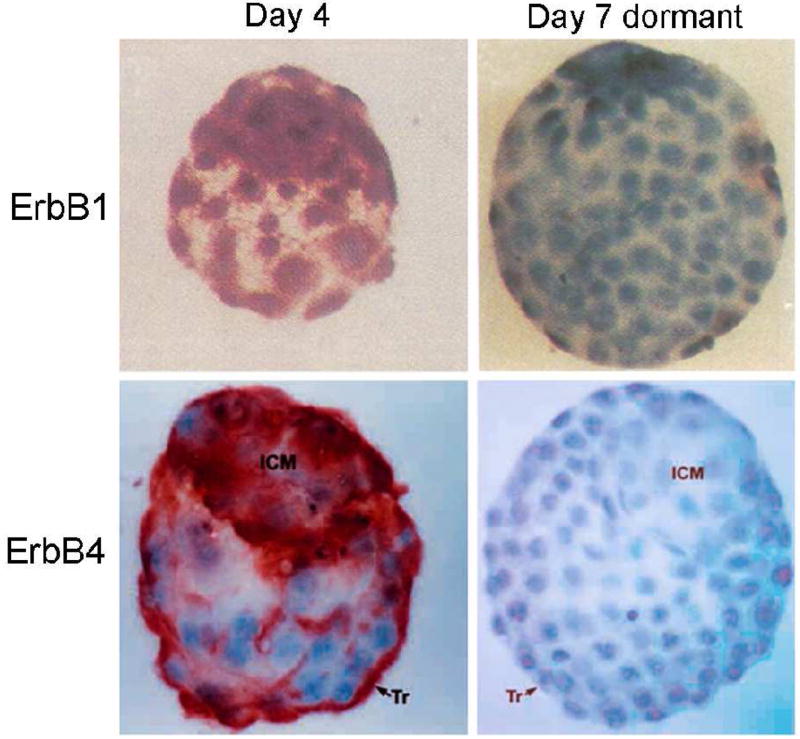
When HB-EGF is expressed in the luminal epithelium during early implantation phase, the classical ErbB system and HSPGs are both available for its interaction. To delineate which ErbB is used for blastocyst activation for implantation, the chimeric toxins composed of HB-EGF or TGF-α coupled to Pseudomonas exotoxin (PE) were used as measures of ligand preference and receptor expression [19]. In this experiment, HB-EGF-PE was much more efficient in killing implantation-competent (active) blastocysts than TGF-α-PE. HB-EGF-PE also killed egfr−/− blastocysts, suggesting a presence of yet another receptor mediating HB-EGF binding. The presence of ErbB4 in active, but not in dormant blastocysts, and HB-EGF’s strong affinity towards ErbB4 suggest that ErbB4 is a high affinity receptor for HB-EGF in embryo-uterine interactions (Fig. 1).
Fig. 1.
Expression of ErbB1 and ErbB4 in day 4 and day 7 delayed blastocysts. Day 4 pregnant mice were ovariectomized and received daily P4 injections from day 4 to day 6. On day 7, dormant blastocysts were obtained by uterine flushing. Immunoreactive ErbB1 and ErbB4 show red deposits mainly in the trophectoderm of day 4 normal blastocysts. In dormant blastocysts, both ErbB1 and ErbB4 are downregulated (reproduced from [18, 19]).
While HSPG can interact with HB-EGF, the proposed function of HSPG is somewhat broader than that of HB-EGF. HSPG is not only present in extracellular scaffolds and basement membranes as a structural component, but also sequesters various heparin-binding growth factors including HB-EGF [23]. Heparinase or metalloproteases are required for releasing sequestered growth factors for further paracrine interaction. Heparan sulfate (HS) is found on the surface of trophectoderm cells at the time of attachment reaction [24] and may provide an efficient mechanism for delivery of uterine HB-EGF for interaction with its receptor on blastocysts.
Cross-talk: reciprocal signal transmission
Above studies have shown that HB-EGF™ in the uterine luminal epithelium interacts with ErbB1 and/or ErbB4 of blastocysts. Now the questions are how the signal for HB-EGF-ErbB interaction is propagated and what cellular changes this interaction induces in furthering the implantation process.
There is evidence that HB-EGF activates intracellular Ca2+ signaling and promotes trophoblast development to an adhesion-competent stage [25]. This effect of HB-EGF on trophoblast development first requires the translocation of ErbB4 to the apical surface of trophoblast cells and then eliciting a Ca2+ influx through N type Ca2+ channels. Accumulated Ca2+ within trophoblast cells then activates calmodulin and protein kinase C pathway [25]. Ca2+ influx by HB-EGF is further regulated by cross-talk with lysophosphatidic acid (LPA) pathway and ErbB receptors. LPA apparently induces the transient accumulation of HB-EGF on the embryo surface, thereby promoting autocrine signaling of HB-EGF followed by Ca2+ influx [26]. These results suggest that HB-EGF-ErbB interactions converge upon other signaling pathways for intricate intracellular signaling cascades.
HB-EGF also has a unique role in regulating certain uterine functions. In vitro cultured primary uterine stromal cells respond to HB-EGF and show heightened DNA synthesis with increased polyploidy [27]. In these cells, HB-EGF induces expression of cyclin D3, which is a distinct D-type cyclin involved in decidualization [27,28]
EGF-like ligands in embryo-uterine interactions
Transforming growth factor-α (TGF-α), amphiregulin (Ar), betacellulin (BTC), epiregulin (Ereg), neu differentiating factor (NDF) and neuregulins (NRGs) are other members of EGF-like ligands that can generate ErbB signaling. Interestingly, many of these growth factors exhibit unique yet overlapping expression patterns during the periimplantation period in mice. As previously reviewed [29], overlapping expression of HB-EGF, Ar, BTC, EPI, and NDF is transiently achieved at the time of attachment reaction around midnight of day 4 of pregnancy [29,30]. Thus, it is assumed that a compensatory mechanism rescues implantation in the absence of one or more members of the EGF family of ligands.
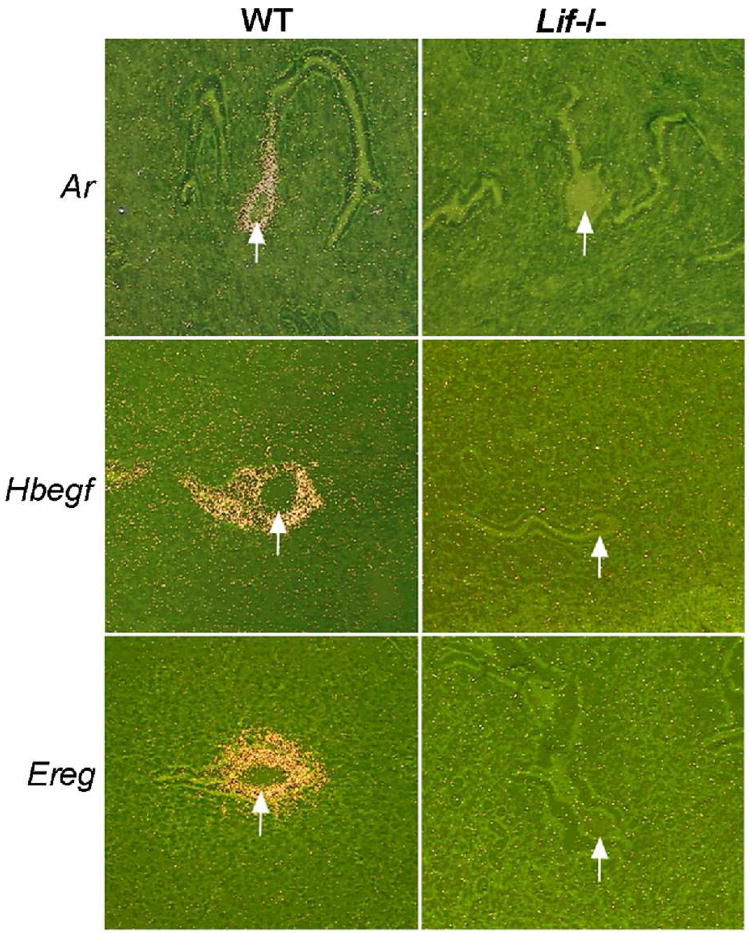
Among these ligands, Ar is the only P4-regulated gene which disappears shortly after the attachment reaction. Although Ar has these unique features over other EGF-like ligands, Ar deficient mice do not show any overt reproductive abnormalities [31], suggesting that it is dispensable for implantation or that a compensatory mechanism is in operation. Ereg deficient mice also do not exhibit implantation defects [32]. Because of the distinct attribute of HB-EGF as a mediator of implantation, studies in other species centered on this molecule and potential involvement of other EGF-like ligands in embryonic development and implantation has not been pursued extensively. Leukemia inhibitory factor (LIF) is a cytokine critical for blastocyst implantation [33]. In Lif−/− female uteri, even the normal blastocysts cannot initiate implantation due to the failure of the uterus to achieve the receptive state. In uteri of these mice, many of the EGF-like growth factors are not expressed at the sites of blastocyst apposition. Ar, HB-EGF and Ereg are all absent in the luminal epithelium where the blastocysts maintain close contact before and during the anticipated time of implantation [34] (Fig. 2). This suggests that induction of EGF-like growth factors including HB-EGF during early implantation phase requires normal LIF signaling.
Fig. 2.
Altered uterine expression of Ar, HB-EGF and Ereg around the time of implantation in wildtype and Lif−/− mice. Photomicrographs of representative uterine sections showing in situ hybridization of Areg, Hbegf, and Ereg mRNAs on day 5 of pregnancy are shown at 100X. Arrows indicate the location of blastocysts (reproduced from [34]).
Since a variety of EGF-like ligands was identified at implantation sites, the availability of ErbBs within the uterine compartment and in the embryo has been scrutinized. In the mouse uterus, ErbB1, ErbB2, ErbB3 and ErbB4 exhibit differential expression pattern. In epithelial cells, ErbB2 and ErbB3 are co-expressed, enabling signaling by BTC and NDF. In the uterine stroma, ErbB1, ErbB2, and ErbB4 are expressed [35,36]. Thus, at least three combinations of ErbB dimers can form. However, judging from the expression of HB-EGF and other factors in the luminal epithelium at the time of attachment reaction, roles for ErbBs in the trophectoderm appear important for implantation. In the mouse blastocyst, all four ErbBs are expressed in the trophectoderm, suggesting direct interaction with uterine ligands [18,19,37].
Why is HB-EGF special in implantation? – The conservation across species
Expression of HB-EGF and cognate ErbB receptors during implantation is described in other species as well. In rabbits, day 7 of pregnancy is considered the time of blastocyst attachment. HB-EGF mRNA is induced at the antimesometrial side of the uterus where the attachment reaction occurs on day 7 [38]. ErbBs exhibit differential expression patterns in trophoblast cells, but understanding roles of HB-EGF and ErbBs in rabbit endometria and embryos will require further investigation. HB-EGF is also expressed in the baboon endometrium and is regulated by progesterone and trophoblastic chorionic gonadotropin [39]. In pigs, uterine luminal flushings contain multiple forms of HB-EGF, suggesting the secretion of this factor to the lumen [40]. HB-EGF is also detected in the elongated bovine blastocysts on day 13 of pregnancy along with EGF and TGF-α [41].
In hamsters, the process of implantation is unique in that it occurs in the presence of P4 alone, not requiring the presence of ovarian estrogen. HB-EGF in the hamster uterus is solely expressed in the luminal epithelium surrounding the blastocyst prior to and during the initial phase of implantation [42]. This is reminiscent of the typical embryonic induction pattern of HB-EGF observed in mice [15]. However, in the ovariectomized hamster uterus, E2, via its nuclear receptor ER, induces expression of HB-EGF rapidly in the luminal epithelium within 2 hrs of injection. This offers an interesting hypothesis that embryonic induction of HB-EGF locally in the implantation site differs from rapid estrogenic induction seen in the ovariectomized hamster uteri. In the mouse uterus, HB-EGF expression is briefly seen on day 1 of pregnancy when estrogen level is high due to preovulatory estrogen surge [15]. Thus, HB-EGF expression in the uterus is likely to be regulated by multiple factors depending on cellular and physiological contexts.
How work in mice translates to humans
As with many studies on implantation, the importance of a molecule in embryo-uterine interactions is first defined in mouse models and other species. In humans, it is virtually impossible to obtain the actual site of implantation due to ethical and technical issues. Therefore, studies using human samples are generally confined to tissue biopsies and limited number of embryos that are discarded with the consent of patients who undergo in vitro fertilization.
In the human endometrium, HB-EGF expression is the highest in the apical surface of the luminal epithelium immediately prior to the implantation window (day 19–21 of the human menstrual cycle) [43]. In the archived pathology specimens from pregnancy terminations between 6–8 week, HB-EGF expression was noted in both cytotrophoblast and syncytiotrophoblasts of the chorionic villi during early pregnancy [44]. These observations suggested that HB-EGF in the human endometrium is associated with the promotion of trophoblast invasion and mitogenesis. HB-EGF expression in the epithelium is the highest when fully developed pinopodes are present, suggesting its involvement in the attachment and penetration steps [45]. The mitogenic potential of HB-EGF on the human endometrium was further demonstrated in an in vitro experiment using cultured endometrial stromal cells. These cells express ERBB1, ERBB4, and HB-EGF™, and addition of HB-EGF in culture media induces heightened DNA synthesis [46]. The endometrial expression of the human HB-EGF seems to be under hormonal regulation, as addition of P4 and E2 not only increases HB-EGF expression but also the ability of HB-EGF to induce epithelial expression of leukemia inhibitory factor (LIF), HOXA-10, and β3 integrin subunit, all of which are also potential mediators of implantation [47].
More direct evidence showing the role for HB-EGF in the attachment reaction in implantation in humans was generated using a cleverly designed in vitro experimental model. The ectodomain of HB-EGF was fused to the Fc region of human IgG (HB-EGF-Fc) and applied onto protein A-coated cover slips via the Fc tag. When hatched human blastocysts were incubated on the coated surface, most of the blastocysts adhered to the surface. This binding was abrogated by adding soluble HB-EGF in the incubation media. Similar results were obtained when HB-EGF™-expressing CHO cells were used. In human blastocysts, ERBB1 is localized in the inner cell mass while ERBB4 is abundant in the trophectoderm. This observation suggests that HB-EGF™ expressed in the luminal epithelium of the endometrium interacts with blastocyst ERBB4 to mediate implantation in humans [48]. HB-EGF also has a stimulatory effect on human embryo development from the 8-cell stage and on hatching [49].
Role for HB-EGF as a key regulator of implantation is further provided in a study on its association with pre-eclampsia. Pre-eclampsia is a disorder of pregnancy with poor cytotrophoblast invasion and patients generally suffer from hypertension and proteinuria. In placental tissues obtained from pre-eclamptic patients, dramatic reduction of HB-EGF immunostaining was noted [50]. This result suggests that defective HB-EGF signaling is responsible for poor trophoblast invasion and flawed placentation leading to pre-eclampsia.
Conclusion
For the last several decades, a variety of molecules were identified as potential mediators of embryo-uterine interactions during implantation. These studies, usually started by describing an expression map of a molecule, were followed by more mechanistic approaches such as using gene-targeted mouse models and in vitro systems. While the list of “implantation” regulators is expanding, the role of HB-EGF as the earliest indicator of embryonic signals is unique (Fig. 3). HB-EGF-ErbB signaling for the attachment reaction seems to be conserved across several species. Further investigations focusing on the propagation of HB-EGF signaling for trophoblast invasion and identification of target genes are warranted.
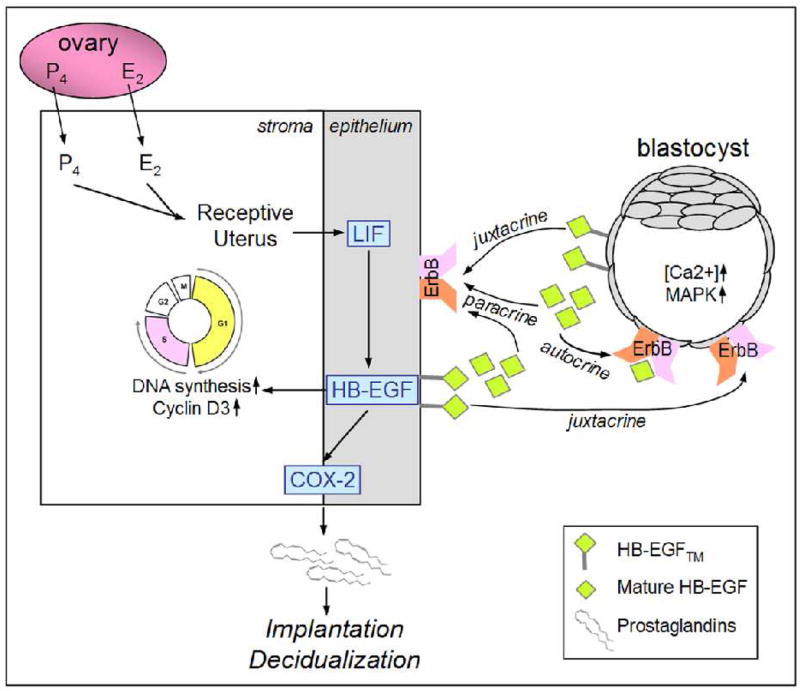
Fig. 3.
A schematic landscape of signaling by HB-EGF™ and mature HB-EGF. HB-EGF takes advantage of autocrine, paracrine, and juxtracrine modes of signaling to regulate both embryonic and uterine functions. HB-EGF affects trophoblast growth and adhesion during implantation. In the uterine stroma, HB-EGF increases DNA synthesis and cell cycle progression. This versatility of HB-EGF synergizes upon the process of successful implantation.
Acknowledgments
The work described in this review article is partially supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-313-C00607) and by the National Institute of Child Health & Human Development grant (HD12304).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 2.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 3.Dey SK. Implantation. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery and Technology. Lippincott-Raven; New York, NY: 1996. pp. 421–434. [Google Scholar]
- 4.Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973;31:201–256. doi: 10.1016/s0083-6729(08)60999-1. [DOI] [PubMed] [Google Scholar]
- 5.Paria BC, Huet H, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshinaga K. Uterine receptivity for blastocyst implantation. Ann N Y Acad Sci. 1988;541:424–431. doi: 10.1111/j.1749-6632.1988.tb22279.x. [DOI] [PubMed] [Google Scholar]
- 7.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 8.Gardner RL, Papaioannou VE. Differentiation in the trophectoderm and inner cell mass. In: Balls M, Wild AE, editors. The early development of mammals. Cambridge University Press; London: 1975. pp. 107–132. [Google Scholar]
- 9.Renfree MB. Implantation and placentation. In: Austin CR, Short RV, editors. Reproduction in mammals. Cambridge University Press; Cambridge, UK: 1982. pp. 26–69. [Google Scholar]
- 10.Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 11.Lundkvist O, Nilsson BO. Endometrial ultrastructure in the early uterine response to blastocysts and artificial deciduogenic stimuli in rats. Cell Tissue Res. 1982;225:355–364. doi: 10.1007/BF00214688. [DOI] [PubMed] [Google Scholar]
- 12.McLaren A. A study of balstocysts during delay and subsequent implantation in lactating mice. J Endocrinol. 1968;42:453–463. doi: 10.1677/joe.0.0420453. [DOI] [PubMed] [Google Scholar]
- 13.Psychoyos A. Endocrine control of egg implantation. In: Greep RO, Astwood EG, Geiger SR, editors. Handbook of physiology. American Physiology Society; Washington, DC: 1973. pp. 187–215. [Google Scholar]
- 14.Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil. 1966;12:593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- 15.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 16.Nishi E, Klagsbrun M. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a mediator of multiple physiological and pathological pathways. Growth Factors. 2004;22:253–260. doi: 10.1080/08977190400008448. [DOI] [PubMed] [Google Scholar]
- 17.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 18.Paria BC, Das SK, Andrews GK, Dey SK. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci USA. 1993;90:55–59. doi: 10.1073/pnas.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- 20.Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci USA. 2004;101:10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA. 2007;104:18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farach-Carson MC, Carson DD. Perlecan--a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17:897–905. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- 24.Carson DD, Tang JP, Julian J. Heparan sulfate proteoglycan (perlecan) expression by mouse embryos during acquisition of attachment competence. Dev Biol. 1993;155:97–106. doi: 10.1006/dbio.1993.1010. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Mayernik L, Schultz JF, Armant DR. Acceleration of trophoblast differentiation by heparin-binding EGF-like growth factor is dependent on the stage-specific activation of calcium influx by ErbB receptors in developing mouse blastocysts. Development. 2000;127:33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp Cell Res. 2004;296:317–326. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das SK, Lim H, Paria BC, Dey SK. Cyclin D3 in the mouse uterus is associated with the decidualization process during early pregnancy. J Mol Endocrinol. 1999;22:91–101. doi: 10.1677/jme.0.0220091. [DOI] [PubMed] [Google Scholar]
- 29.Das SK, Das N, Wang J, Lim H, Schryver B, Plowman GD, Dey SK. Expression of betacellulin and epiregulin genes in the mouse uterus temporally by the blastocyst solely at the site of its apposition is coincident with the “window” of implantation. Dev Biol. 1997;190:178–190. doi: 10.1006/dbio.1997.8694. [DOI] [PubMed] [Google Scholar]
- 30.Reese J, Brown N, Das SK, Dey SK. Expression of neu differentiation factor during the periimplantation period in the mouse uterus. Biol Reprod. 1998;58:719–727. doi: 10.1095/biolreprod58.3.719. [DOI] [PubMed] [Google Scholar]
- 31.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 32.Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol. 2004;24:8907–8916. doi: 10.1128/MCB.24.20.8907-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 34.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 35.Lim H, Dey SK, Das SK. Differential expression of the erbB2 gene in the periimplantation mouse uterus: potential mediator of signaling by epidermal growth factor-like growth factors. Endocrinology. 1997;138:1328–1337. doi: 10.1210/endo.138.3.4991. [DOI] [PubMed] [Google Scholar]
- 36.Lim H, Das SK, Dey SK. erbB genes in the mouse uterus: cell-specific signaling by epidermal growth factor (EGF) family of growth factors during implantation. Dev Biol. 1998;204:97–110. doi: 10.1006/dbio.1998.9072. [DOI] [PubMed] [Google Scholar]
- 37.Brown N, Deb K, Paria BC, Das SK, Reese J. Embryo-uterine interactions via the neuregulin family of growth factors during implantation in the mouse. Biol Reprod. 2004;71:2003–2011. doi: 10.1095/biolreprod.104.031864. [DOI] [PubMed] [Google Scholar]
- 38.Klonisch T, Wolf P, Hombach-Klonisch S, Vogt S, Kuechenhoff A, Tetens F, Fischer B. Epidermal growth factor-like ligands and erbB genes in the peri-implantation rabbit uterus and blastocyst. Biol Reprod. 2001;64:1835–1844. doi: 10.1095/biolreprod64.6.1835. [DOI] [PubMed] [Google Scholar]
- 39.Leach RE, Khalifa R, Armant DR, Brudney A, Das SK, Dey SK, Fazleabas AT. Heparin-binding EGF-like growth factor modulation by antiprogestin and CG in the baboon (Papio anubis) J Clin Endocrinol Metab. 2001;86:4520–4528. doi: 10.1210/jcem.86.9.7835. [DOI] [PubMed] [Google Scholar]
- 40.Kim GY, Besner GE, Steffen CL, McCarthy DW, Downing MT, Luquette MH, Abad MS, Brigstock DR. Purification of heparin-binding epidermal growth factor-like growth factor from pig uterine luminal flushings, and its production by endometrial tissues. Biol Reprod. 1995;52:561–571. doi: 10.1095/biolreprod52.3.561. [DOI] [PubMed] [Google Scholar]
- 41.Kliem A, Tetens F, Klonisch T, Grealy M, Fischer B. Epidermal growth factor receptor and ligands in elongating bovine blastocysts. Mol Reprod Dev. 1998;51:402–412. doi: 10.1002/(SICI)1098-2795(199812)51:4<402::AID-MRD7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Wang H, Matsumoto H, Roy SK, Das SK, Paria BC. Dual source and target of heparin-binding EGF-like growth factor during the onset of implantation in the hamster. Development. 2002;129:4125–4134. doi: 10.1242/dev.129.17.4125. [DOI] [PubMed] [Google Scholar]
- 43.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 44.Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84:3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- 45.Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Coexistence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8:765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- 46.Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, Barlow DH, Mardon HJ. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002;87:5769–5777. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J. Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- 48.Chobotova K, Spyropoulou I, Carver J, Manek S, Heath JK, Gullick WJ, Barlow DH, Sargent IL, Mardon HJ. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech Dev. 2002;119:137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 49.Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- 50.Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, Dey SK, Johnson A, Qureshi F, Jacques S, Armant DR. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet. 2002;360:1215–1219. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]