Abstract
Objective
To describe the prevalence of hypoxaemia in children admitted to a hospital in Kenya for the purpose of identifying clinical signs of hypoxaemia for emergency triage assessment, and to test the hypothesis that such signs lead to correct identification of hypoxaemia in children, irrespective of their diagnosis.
Methods
From 2002 to 2005 we prospectively collected clinical data and pulse oximetry measurements for all paediatric admissions to Kilifi District Hospital, Kenya, irrespective of diagnosis, and assessed the prevalence of hypoxaemia in relation to the WHO clinical syndromes of “pneumonia” on admission and the final diagnoses made at discharge. We used the data collected over the first three years to derive signs predictive of hypoxaemia, and data from the fourth year to validate those signs.
Findings
Hypoxemia was found in 977 of 15 289 (6.4%) of all admissions (5% to 19% depending on age group) and was strongly associated with inpatient mortality (age-adjusted risk ratio: 4.5; 95% confidence interval, CI: 3.8–5.3). Although most hypoxaemic children aged ≥ 60 days met the WHO criteria for a syndrome of “pneumonia” on admission, only 215 of the 693 (31%) such children had a final diagnosis of lower respiratory tract infection (LRTI). The most predictive signs for hypoxaemia included shock, a heart rate < 80 beats per minute, irregular breathing, a respiratory rate > 60 breaths per minute and impaired consciousness. However, 5–15% of the children who had hypoxaemia on admission were missed, and 18% of the children were incorrectly identified as hypoxaemic.
Conclusion
The syndromes of pneumonia make it possible to identify most hypoxaemic children, including those without LRTI. Shock, bradycardia and irregular breathing are important predictive signs, and severe malaria with respiratory distress is a common cause of hypoxaemia. Overall, however, clinical signs are poor predictors of hypoxaemia, and using pulse oximetry in resource-poor health facilities to target oxygen therapy is likely to save costs.
Résumé
Objectif
Décrire la prévalence de l’hypoxémie chez les enfants admis dans un hôpital kenyan en vue d’identifier les signes cliniques de l’hypoxémie utilisables pour l’évaluation dans le cadre du triage d’urgence et tester l’hypothèse selon laquelle ces signes permettraient une identification correcte des enfants atteints d’hypoxémie, indépendamment du diagnostic qui doit leur être affecté.
Méthodes
De 2002 à 2005, nous avons recueilli prospectivement des données cliniques et des mesures par oxymétrie pulsée pour l’ensemble des admissions pédiatriques à l’hôpital de district de Kilifi au Kenya, indépendamment du diagnostic concernant ces enfants, et évalué la prévalence de l’hypoxémie en relation avec la présence d’un syndrome clinique de type pneumonie selon l’OMS lors de l’admission et du diagnostic final de sortie. Nous avons utilisé les données collectées sur les trois premières années pour déterminer les signes prédictifs de l’hypoxémie et les données de la quatrième année pour valider ces signes.
Résultats
Nous avons relevé une hypoxémie chez 977 des 15289 enfants hospitalisés (soit 6,4 %, chiffre variant entre 5 à 19 % selon la tranche d’âge) et une association forte entre hypoxémie et mortalité des patients hospitalisés (rapport de risques ajusté selon l’âge : 4,5 ; intervalle de confiance à 95 %, IC : 3,8-5,3). Si la plupart des enfants hypoxémiques de 60 jours et plus remplissaient, lors de leur admission, les critères OMS de définition du syndrome de type pneumonie, seuls 215 des 693 enfants hypoxémiques de 60 jours et plus (31 %) ont finalement été diagnostiqués comme atteints d’une infection des voies respiratoires inférieures (IVRI). Parmi les signes les plus prédictifs de l’hypoxémie figuraient l’état de choc, une fréquence cardiaque inférieure à 80 battements par minute, une respiration irrégulière, une fréquence respiratoire supérieure à 60 respirations par minute et un état de conscience altérée. Néanmoins, 5 à 15 % des enfants hypoxémiques lors de leur admission n’ont pas été repérés et 18 % des enfants ont été identifiés à tort comme hypoxémiques.
Conclusion
Le recours au syndrome de type pneumonie permet d’identifier la plupart des enfants hypoxémiques, y compris ceux atteints d’une IVRI. L’état de choc, la bradycardie et l’irrégularité respiratoire sont des signes prédictifs importants et le paludisme grave, avec détresse respiratoire, est une cause courante d’hypoxémie. Globalement néanmoins, les signes cliniques n’ont pas une grande valeur prédictive pour l’hypoxémie et il est probable que l’utilisation de l’oxymétrie pulsée par les établissements de soins qui manquent de moyens pour identifier les patients devant bénéficier d’un traitement par l’oxygène permettra de réaliser des économies.
Resumen
Objetivo
Describir la prevalencia de hipoxemia en niños ingresados en un hospital de Kenya a fin de identificar los signos clínicos de la misma para un triaje de emergencia, y contrastar la hipótesis de que esos signos permiten detectar correctamente la hipoxemia en los niños, independientemente del diagnóstico.
Métodos
Entre 2002 y 2005 se reunieron prospectivamente datos clínicos y de oximetría de pulso para todos los ingresos pediátricos en el Hospital de Distrito de Kilifi, Kenya, con independencia del diagnóstico, determinándose la prevalencia de hipoxemia en relación con el síndrome clínico de «neumonía» definido por la OMS en el momento del ingreso y con el diagnóstico final en el momento del alta. Los datos recogidos durante los tres primeros años se usaron para determinar los signos predictivos de hipoxemia, y los datos reunidos a partir del cuarto año fueron utilizados para validar esos signos.
Resultados
Se detectó hipoxemia en 977 de 15 289 (6,4%) niños ingresados (5%-19% según el grupo de edad), muy estrechamente asociada a mortalidad hospitalaria (razón de riesgos ajustada por edad: 4,5; intervalo de confianza [IC] del 95%: 3,8 - 5,3). Aunque la mayoría de los niños hipoxémicos con ≥ 60 días de edad cumplían los criterios de la OMS para que pudiera diagnosticarse un síndrome de «neumonía» en el momento del ingreso, sólo 215 de los 693 (31%) niños hipoxémicos de esa edad recibieron un diagnóstico final de infección de las vías respiratorias inferiores (IVRI). Los signos más predictivos de hipoxemia fueron el choque, una frecuencia cardiaca < 80 latidos por minuto, una respiración irregular, una frecuencia respiratoria > 60 respiraciones por minuto y un deterioro de la conciencia. Sin embargo, no se detectó la hipoxemia en un 5%-15% de los niños que la sufrían en el momento del ingreso, y un 18% de los niños fueron considerados hipoxémicos incorrectamente.
Conclusión
Los síndromes de neumonía permiten identificar a la mayoría de los niños hipoxémicos, incluidos los que no presentan IVRI. Un cuadro de choque, la bradicardia y una respiración irregular son signos predictivos importantes, y la malaria grave con distrés respiratorio es una causa común de hipoxemia. Sin embargo, en general los signos clínicos son poco fiables como factores predictivos de la hipoxemia, y es probable que, en los servicios de salud con recursos escasos, el uso de la oximetría de pulso como criterio para aplicar oxigenoterapia permita ahorrar costos.
ملخص
الهدف
وصف نقص تأكسج الدم لدى الأطفال الذين يقبلون في أحد المستشفيات الكينية للتعرف على العلامات السريرية لنقص الأكسجة لتقيـيم فرز حالات الطوارئ ولاختبار الفرضية التي تقول أن مثل هذه العلامات تقود إلى التعرف الصحيح على نقص تأكسج الدم لدى الأطفال، بغض النظر عن التشخيص.
الطريقة
جمعنا في الفترة من عام 2002 إلى عام 2005 بطريقة استقبالية المعطيات السريرية وقياس التأكسج على دفعات لجميع الأطفال الذين قبلوا في مستشفى مقاطعة كيفيلي في كينيا، بغض النظر عن التشخيص، وقيَّمنا معدل انتشار نقص التأكسج فيما يتعلّق بالمتلازمات السريرية لمنظمة الصحة العالمية حول “الالتهاب الرئوي” وقت القبول في المستشفى وبالتشخيص النهائي الذي وضع عند التخريج من المستشفى. واستخدمنا ما جمعناه من معطيات خلال السنوات الثلاث الأولى لتحديد العلامات المنبئة بنقص الأكسجة، والمعطيات المستمدة من السنة الرابعة لتوثيق مصدوقية هذه العلامات.
الموجودات
وجدنا نقص التأكسج لدى 977 حالة من أصل 289 15 حالة (6.4%) لجميع المقبولين (5-19% وفقاً للمجموعة العمرية)، وقد ارتبطت ارتباطاً وثيقاً بمعدل الوفيات بين الحالات المقبولة داخل المستشفى (بلغ منسب الاختطار المصحح وفق العمر 4.5، بفاصل ثقة 95% إذ تراوح معدل الأرجحية بين 3.8 و5.3). ورغم أن معظم الأطفال المصابين بنقص التأكسج الذين تزيد أعمارهم عن 60 يوماً قد حقّقوا معايـير منظمة الصحة العالمية “للالتهاب الرئوي” عند قبولهم في المستشفى، فإن 215 فقط من بين 693 من الأطفال المصابين بنقص التأكسج والذين تزيد أعمارهم عن 60 يوماً (31%) كان تشخيصهم النهائي التهاب السبل التنفسية السفلية. أما أهم العلامات المثبتة لنقص التأكسج فهي الصدمة، وبطء القلب لأقل من 80 ضربة بالدقيقة، واضطراب نُظُم التنفس، وسرعة التنفس لأكثر من 60 نفساً بالدقيقة، وتأثر الوعي. ورغم ذلك فإننا فقدنا تشخيص 5-15% من الأطفال المصابين بنقص التأكسج عند قبولهم، كما أن 18% من الأطفال قد وصفو خطأ على أنهم مصابون بنقص التأكسج.
الاستنتاج
يمكن لأعراض الالتهاب الرئوي أن تبيّن معظم الأطفال المصابين بنقص التأكسج، ومنهم المصابون بالتهاب السبل التنفسية السفلية. أما الصدمة وبطء القلب واضطراب نظم التنفس فهي علامات منبئة هامة، كما أن الملاريا الوخيمة المترافقة بعسرة تنفسية سبب شائع لنقص التأكسج. والنتيجة بشكل عام أن العلامات السريرية منبئات سيئة على نقص التأكسج، وأن استخدام قياس التأكسج على دفعات في المرافق الصحية الفقيرة بالموارد لتوجيه المعالجة بالأكسجين قد يوفر التكاليف.
Introduction
Recognizing and correcting poor oxygenation early is an essential aspect of paediatric emergency care. The management of hypoxaemia includes establishing an open airway and alveolar ventilation, providing supplemental oxygen, restoring circulation and treating the underlying cause. Hypoxaemia correlates with mortality,1 and early assessment and prompt oxygen therapy probably improve survival.2,3
In developing countries, bottled oxygen is expensive and commonly in short supply. However, most hospitals providing secondary care cannot perform pulse oximetry or measure arterial oxygen concentration to properly target oxygen delivery. Furthermore, clinical assessment is often carried out by relatively inexperienced health workers, as a result of which simple algorithms for identifying hypoxaemia have been developed and studied. However, the studies have been conducted almost entirely among children with lower respiratory tract infection (LRTI),4–9 and little prospective validation has been performed.
Certain clinical signs have been identified as predictive of hypoxaemia: a fast respiratory rate for age, lower chest wall indrawing, grunting, head nodding, cyanosis, the absence of crying during the examination, and the inability to breastfeed or drink. No single sign is a reliable predictor, and sensitivity is generally low for any single sign.10 WHO currently recommends giving oxygen urgently to children with cyanosis, signs of shock, severe respiratory distress, the syndrome of “very severe pneumonia” or a respiratory rate of 70 breaths per minute (min) or more.11
Given the paucity of data outside the context of LRTI, we first aimed to describe the prevalence of hypoxaemia in relation to the WHO clinical syndromes of “pneumonia” at admission and the final diagnoses made at discharge. We then tested the hypothesis that hypoxaemia could be identified on the basis of clinical signs by studying a large population of children consecutively admitted to a rural, Kenyan district hospital, irrespective of their diagnosis. We report findings from 15 289 children and neonates admitted during a 3-year period and validate our findings using data from 4695 admissions over a fourth year.
Methods
Study setting
Since 1998 we have conducted continuous inpatient surveillance to describe the causes and clinical features of common illnesses among children admitted to Kilifi District Hospital in Kenya.12,13 The hospital is located at sea level in an area with endemic malaria. Government-employed clinical officers not involved in research refer children to the paediatric ward (40 beds) or the high-dependency unit (6 beds). On admission, discharge or death, standardized clinical and laboratory data are collected by clinical officers, who in Kenya receive 3 years of basic medical training, or by fully trained medical officers with less than 5 years of paediatric experience.12,13 For this analysis, we used data collected at admission from January 2002 to December 2005 and final discharge diagnosis for each consecutive admission over the same period of time. The Kenyan National Scientific and Ethical Review Boards and the Coventry Research Ethics Committee approved the study.
Clinical definitions
WHO defines a set of clinical syndromes of “pneumonia” on the basis of clinical history and clinical signs at presentation for the purpose of determining the need for admission and the type of antibiotic treatment required.11,14 The definitions have high sensitivity with respect to LRTI, but they lack specificity. The “syndrome of pneumonia” is defined as a history of cough or difficulty breathing plus an elevated respiratory rate for age (if < 2 months, ≥ 60 breaths per min; if 2–11 months, ≥ 50 breaths per min; and if 1–5 years, ≥ 40 breaths per min), and no signs of severe pneumonia syndrome. “Severe pneumonia syndrome” is defined as a history of cough or difficulty breathing plus lower chest wall indrawing or nasal flaring and no signs of very severe pneumonia syndrome. “Very severe pneumonia syndrome” is defined as cough or difficulty breathing plus any of the following signs: cyanosis, inability to drink or breastfeed, convulsions, lethargy or unconsciousness.
Regarding signs not included in the above definitions, prostration was defined as the inability to sit unsupported for a child ≥ 9 months of age, or the inability to breastfeed or drink for a child < 9 months of age. Level of consciousness was assessed using the Blantyre coma scale.15 Shock was defined as the presence of any one of the following signs: capillary refill delayed for > 3 seconds (s), a noticeable temperature gradient or a weak pulse volume. For this analysis, we defined severe anaemia as a haemoglobin concentration ≤ 4 g/dl. Malaria screening was performed on every admission with thick and thin blood films stained and examined by standard techniques.
Oxygen saturation (SaO2) was measured at admission by trained clinical assistants using fingertip pulse oximetry (Nellcor Puritan Bennett NPB-40, United States of America). We defined hypoxaemia as an SaO2 < 90%. Bottled oxygen was always available for hypoxaemic children. LRTI and other conditions were managed according to current WHO guidelines.11 All final diagnoses, including LRTI, were made prospectively by the discharging clinician after a review of admission history, inpatient management notes and all available laboratory and radiologic tests.
For this analysis, we first used the WHO pneumonia syndromes to classify children according to the clinical findings observed at admission. We then examined up to two final diagnoses recorded at discharge or death using all available clinical, laboratory and radiological information. Thus, the sum of all final diagnoses may exceed 100%.
Statistical methods
At the point of contact, clinical data were entered into a FileMaker Pro database 5.5v1 (FileMaker Inc., USA). Laboratory data were double entered and verified using FoxPro 2.5b for Windows (Microsoft Corporation, Seattle, WA, USA). Stata version 9.2 (Stata Corp, College Station, TX, USA) was used for the final analysis.
We split the data into a 3-year derivation set and a 1-year validation set. We divided admissions into three age groups because we hypothesized that the prevalence, causes and predictors of hypoxaemia could differ among neonates, young infants and older children. We first determined the prevalence of hypoxaemia in all age groups. We then examined hypoxaemia in relation to the WHO clinical syndromes of pneumonia at admission and final diagnosis at discharge. After excluding children in whom oximetry was not performed or with a failed oximeter reading or who had known cardiac disease or asthma, we developed predictive models of hypoxaemia in three stages by means of likelihood ratios (LRs). The LR indicates the degree to which a positive or negative result increases or lowers, respectively, the odds of having the disease in question (hypoxaemia in this case). LRs change less under the influence of disease prevalence than sensitivity and specificity, and they may be calculated for multiple test results.16 For each putative clinical sign used to predict hypoxaemia, we first calculated the crude LR for a positive result and for a negative result. Signs with crude LR ≥ 2.0 or ≤ 0.5 were considered clinically useful. We then used the method of Speigelhalter and Knill-Jones to adjust for the confounding effects of related variables, as determined by multivariate analyses, in four separate groups: respiratory, cardiovascular, neurological and general signs.13,17,18 In the third step, we constructed practical prediction rules for hypoxaemia using signs with adjusted likelihood ratios of ≥ 2.0 or ≤ 0.5, prioritized by strength of prediction and clinical practicability. We finally evaluated the final rules using the area under the receiver operating characteristic (ROC) curves using the validation data set. Distributions of age were compared by means of the Wilcoxon rank sum test.
Results
Between 2002 and 2004, 15 401 children were admitted (derivation set) to the hospital. Of these children, 13 183 were aged ≥ 60 days; 991 were aged 7–59 days, and 1115 were aged < 7 days. Excluded from the study were 112 children: 35 without pulse oximetry results; 59 with known cardiac disease and 18 with known asthma.
Characteristics
Admissions aged ≥ 60 days
Among the 13 183 children admitted, the proportion of males was 56% and the median age was 32 months (interquartile range: 11–42). The most common final diagnoses were malaria (4982, or 38%), LRTI (2869, or 22%), gastroenteritis (2026, or 15%), malnutrition (1261, or 10%) and severe anaemia (717, or 5%).
Hypoxaemia was found in 693 (5.3%) of the children admitted. The median age of the children with and without hypoxaemia was 18.9 and 22.9 months, respectively (P = 0.036). The most frequent final diagnoses among hypoxaemic children were malaria (244, or 35%), LRTI (221, or 32%), malnutrition (68, or 10%) and gastroenteritis (49, or 7%). Severe anaemia was found in 30 children (< 1%). Overall, 753 (6%) children died, including 150 of the 693 (22%) with hypoxaemia. Irrespective of the final clinical diagnosis, hypoxaemia was strongly associated with inpatient death (age-adjusted risk ratio, RR: 4.5; 95% confidence interval, CI: 3.8–5.3).
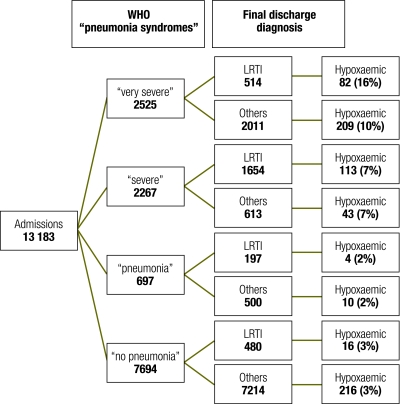
Very severe pneumonia syndrome
Of the children admitted, 2525 (19%) satisfied the WHO criteria for very severe pneumonia: 291 (12%) of these children were hypoxaemic and 287 (11%) died. The final diagnoses included severe malaria (1268, or 50%), LRTI (514, or 20%), gastroenteritis (185, 7%), acute febrile convulsion of unknown cause (without malaria, meningitis or epilepsy) (181, or 7%), malnutrition (173, or 7%), meningitis (75, or 3%) and severe anaemia (56, or 2%). In 86 (3%) children, both malaria and LRTI were present. Of the 291 hypoxaemic children, 119 (41%) had a final diagnosis of malaria and 82 (28%), of LRTI. The case fatality rate among hypoxaemic children was 30% (89/291).
Severe pneumonia syndrome
Of the children admitted, 2267 (17%) satisfied the WHO criteria for severe pneumonia, 156 (7%) were hypoxaemic and 107 (5%) died. The final diagnoses included LRTI (1654, or 73%), malaria (349, or 15%), malnutrition (180, or 8%), gastroenteritis (49, or 2%) and severe anaemia (43, or 2%). A further 155 (7%) children had both malaria and LRTI. Of the 156 hypoxaemic children, 113 (72%) had a final diagnosis of LRTI and 18 (12%), of malaria. The case fatality rate was 16% (25/156) among children who were hypoxaemic.
Pneumonia syndrome
Of the children admitted, 697 (5%) satisfied the WHO criteria for pneumonia, and of this group, 14 (2%) were hypoxaemic and 33 (5%) died. Frequent final diagnoses among these children were malaria (245, or 35%), LRTI (197, or 28%), malnutrition (93, or 13%), gastroenteritis (76, or 11%) and severe anaemia (26, or 4%). Of the 14 hypoxaemic children, 8 (57%) had a final diagnosis of malaria and 4 (29%), of LRTI. The case fatality rate was 21% (3/14) among children who were hypoxaemic.
Overall, 461 of 693 (67%) hypoxaemic children who were admitted met the clinical criteria for one of the WHO pneumonia syndromes, and 215 of 693 (31%) of the hypoxaemic children admitted had a final discharge diagnosis of LRTI (Fig. 1).
Fig. 1.
Distribution of LRTI and hypoxaemic cases across the WHO “pneumonia” syndromes
LRTI, lower respiratory tract infection.
Admissions aged 7–59 days
Of the 991 children aged 7–59 days who were admitted (57% males) 78 (8%) were hypoxaemic and 82 (8%) died. The main final diagnoses were neonatal sepsis (497, or 50%); LRTI (301, or 30%) and neonatal jaundice (37, or 4%). Of the hypoxaemic children, 37 (47%) had a final diagnosis of LRTI and 25 (32%), of neonatal sepsis. Among 78 hypoxaemic children, 22 (28%) died. Hypoxaemic children had higher mortality (age-adjusted RR: 4.3; 95% CI: 2.8–6.6).
Admissions in the first week of life
Of 1105 children (58% males) admitted during the first week of life, 206 (19%) were hypoxaemic on admission, and 336 (30%) died. Common final diagnoses were neonatal sepsis (600, or 54%); prematurity (216, or 20%); birth asphyxia (153, or 14%) and uncomplicated neonatal jaundice 98 (9%). Among those with hypoxaemia, neonatal sepsis (80, or 39%), birth asphyxia (62, or 30%), prematurity (49, or 24%) and neonatal jaundice (7, or 3%) were common. Among 206 children admitted with hypoxaemia, 118 (57%) died. This represented 35% of all deaths. Hypoxaemia was associated with inpatient death (age-adjusted RR: 2.4; 95% CI: 2.0–2.8).
Clinical indicators of hypoxaemia
Admissions aged ≥ 60 days
Clinical signs that appeared predictive on univariate analysis (LR ≥ 2.0 or ≤ 0.5) were lower chest wall indrawing, nasal flaring, central cyanosis, irregular breathing, deep breathing, stridor, auscultatory crackles, dullness on percussion, respiratory rate ≥ 60 breaths per min, weak pulse volume, delayed capillary refill (≥ 3 s), heart rate < 80 beats per min, impaired consciousness with a Blantyre coma scale < 3, prostration, convulsion on admission, restlessness and hypothermia (Table A1 in Appendix A, available at: http://www.kemri-wellcome.org/images/appendixa.pdf). On multivariate analysis within each group, central cyanosis, heart rate < 80 beats per min, irregular breathing, impaired consciousness with a Blantyre coma scale < 3, respiratory rate ≥ 60 breaths per min, delayed capillary refill (≥ 3 s) and weak pulse volume remained predictive of hypoxaemia (Table A2 in Appendix A).
The overall sensitivity and specificity of these signs in the validation data set are shown in (Table 1). Although lower chest wall indrawing did not meet our criteria as a useful predictor when included with other signs in the respiratory group, we re-tested it with the final list of predictive signs because it is easy to recognize clinically and WHO recommends it as an indication for oxygen therapy when severe. Although including lower chest wall indrawing improved sensitivity, significant specificity was lost (Table 1). The area under the ROC curve of these signs in the validation set was 0.80 (95% CI: 0.77–0.84) overall, 0.77 (95% CI: 0.73–0.82) among children who fulfilled the clinical criteria for a pneumonia syndrome, and 0.81 (95% CI: 0.75–0.89) among children who did not fulfil them.
Table 1. Performance of clinical predictors of hypoxaemia in children ≥ 60 days of agea admitted to Kilifi District Hospital, Kenya.
| Clinical signs | No. with sign | No. without sign | True positives | False positives | True negatives | False negatives | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyanosis, heart rate < 80 beats/min or irregular breathing | 110 | 3733 | 30 | 80 | 3583 | 148 | 17 | 98 | 27 | 96 |
| Capillary refill ≥ 3 seconds, weak pulse, or any of the signs listed above in this column | 413 | 3430 | 62 | 351 | 3314 | 116 | 35 | 90 | 15 | 97 |
| Respiratory rate > 60 breaths/min or any of the signs listed above in this column | 643 | 3200 | 89 | 554 | 3111 | 89 | 50 | 85 | 14 | 97 |
| Impaired consciousness (BCS < 3), or any of the signs listed above in this column | 772 | 3071 | 113 | 659 | 3006 | 65 | 64 | 82 | 15 | 98 |
| Lower chest wall indrawing, or any of the signs listed above in this column | 1410 | 2433 | 151 | 1259 | 2406 | 27 | 89 | 67 | 11 | 99 |
BCS, Blantyre coma scale; NPV, negative predictive value; PPV, positive predictive value. a n = 3843; 178 (4.6%) of the children admitted were hypoxaemic.
Admissions aged 7–59 days
The clinical signs that appeared predictive on univariate analysis were cyanosis, irregular breathing, nasal flaring, crackles on auscultation, respiratory rate > 80 per min, weak pulse, delayed capillary refill (≥ 3 s), heart rate < 100 beats per min, absent cry, unresponsiveness to painful stimuli, convulsions present on admission, restlessness, a bulging fontanelle and axillary temperature < 36°C (Table A3 in Appendix A). Cyanosis, heart rate < 100 beats per min, respiratory rate > 80 breaths per min, irregular breathing, unresponsiveness to painful stimuli, restlessness, inability to breastfeed and delayed capillary refill (≥ 3 s) remained predictive after controlling for other signs (Table A4 in Appendix A). For the reasons given above, we also re-tested lower chest wall indrawing in the final list of signs. The sensitivity and specificity of the signs are shown in Table 2. The area under the ROC curve for the prospective validation data set was 0.77 (95% CI: 0.71–0.84).
Table 2. Performance of clinical predictors of hypoxaemia in children 7-59 days of agea admitted to the Kilifi District Hospital, Kenya.
| Clinical signs | No. with sign | No. without sign | True positives | False positives | True negatives | False negatives | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyanosis, heart rate < 100 beats/min, irregular breathing | 27 | 362 | 6 | 21 | 331 | 31 | 16 | 94 | 22 | 91 |
| Respiratory rate > 80 breaths/min, or any of the signs listed above in this column | 39 | 350 | 8 | 31 | 321 | 29 | 22 | 92 | 21 | 92 |
| Capillary refill ≥ 3 seconds, or any of the signs listed above in this column | 48 | 341 | 10 | 38 | 314 | 27 | 27 | 90 | 21 | 92 |
| Inability to breastfeed, or any of the signs listed above in this column | 94 | 295 | 22 | 72 | 280 | 15 | 60 | 80 | 26 | 95 |
| Unresponsiveness to painful stimuli, restlessness, or any of the signs listed above in this column | 104 | 285 | 23 | 81 | 271 | 14 | 62 | 77 | 10 | 95 |
| Lower chest wall indrawing, or any of the signs listed above in this column | 212 | 177 | 35 | 177 | 175 | 2 | 95 | 50 | 10 | 98 |
NPV, negative predictive value; PPV, positive predictive value. a n = 389; 37 (10%) of the children admitted were hypoxaemic.
Admissions the first week of life
In this group of children, predictive signs on univariate analysis were cyanosis, irregular breathing, deep breathing, respiratory rate > 80 breaths per min, auscultatory crackles, indrawing, nasal flaring, heart rate < 100 beats per min, weak pulse, delayed capillary refill (≥ 3 s), absent cry, unresponsiveness to painful stimuli, history of birth asphyxia and birth trauma (Table A5 in Appendix A). Signs that remained significantly associated on multivariate analysis were cyanosis, heart rate < 100 beats per min, irregular breathing, absent cry, unresponsiveness to painful stimuli, respiratory rate > 80 breaths per min, history of birth asphyxia, delayed capillary refill (≥ 3 s) and inability to breastfeed (Table A6 in Appendix A). The sensitivity and specificity of the signs are shown in (Table 3). For these signs, the area under the ROC in the validation set was 0.76 (95% CI: 0.72–0.81).
Table 3. Performance of clinical predictors of hypoxaemia in children less than 1 week of agea admitted to Kilifi District Hospital, Kenya.
| Clinical signs | No. with sign | No. without sign | True positives | False positives | True negatives | False negatives | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyanosis, heart rate < 100 beats/min, irregular breathing | 107 | 356 | 47 | 60 | 298 | 58 | 45 | 83 | 44 | 84 |
| Respiratory rate > 80 breaths/min, or any of the signs listed above in this column | 122 | 341 | 49 | 73 | 285 | 56 | 47 | 80 | 40 | 84 |
| Unresponsiveness to painful stimuli, absent cry, or any of the signs listed above in this column | 143 | 320 | 58 | 85 | 273 | 47 | 55 | 76 | 41 | 88 |
| Capillary refill ≥ 3 seconds, or any of the signs listed above in this column | 159 | 304 | 64 | 95 | 263 | 41 | 63 | 74 | 40 | 90 |
| History of birth asphyxia, or any of the signs listed above in this column | 194 | 269 | 81 | 113 | 245 | 24 | 79 | 66 | 42 | 91 |
| Inability to breastfeed, or any of the signs listed above in this column | 286 | 177 | 97 | 189 | 169 | 8 | 94 | 45 | 34 | 96 |
NPV, negative predictive value; PPV, positive predictive value. a n = 463; 105 (23%) of the children admitted were hypoxaemic.
Discussion
Most studies of the prevalence and clinical signs of hypoxaemia have focused on children with LRTI. We therefore chose to examine unselected paediatric admissions. Hypoxaemia was present in 5%–19% of all children admitted to a Kenyan district hospital, depending on age, and this concurs with recent data from the Gambia.19 Although two-thirds of hypoxaemic children aged ≥ 60 days presented with one of the clinical syndromes of pneumonia, only one-third had a final diagnosis of LRTI. Most children meeting the WHO criteria for very severe pneumonia were finally diagnosed with severe illnesses other than LRTI, most commonly severe malaria or shock. Children who have metabolic acidosis in severe malaria may present with respiratory distress (Kussmaul’s respiration) and could thus overlap with the syndrome of very severe pneumonia.11,20,21 The situation may differ in geographical regions where malaria is not endemic.
Clinical signs were relatively poor predictors of hypoxaemia. The most sensitive rules we developed still missed 5–15% of hypoxaemic children, depending on age. As in other studies, specificity posed a significant problem, as it was markedly reduced when we included lower chest wall indrawing (82–67% among children ≥ 60 days of age and 77–50% among infants aged 1 week to 59 days) and the inability to breastfeed among infants in the first week of life (66–45%). Had we followed these rules, even after excluding lower chest wall indrawing at all ages and an inability to breastfeed in the first week of life, we would have given bottled oxygen to a total of 862 of 4695 (18%) children in the validation set who were not hypoxaemic by pulse oximetry. Although the false positive and false negative assessment results were fewer than reported recently from Papua New Guinea, they still represent a significant financial burden.22 At an estimated daily cost of US$ 6–14 per child on oxygen at 1 litre per min (depending on the size of cylinder available), in our setting this would have translated into US$ 5172 to US$ 12 068 in 1 year had each child not requiring oxygen (862) received it for 1 day.2 A hand-held pulse oxymeter costs less than US$ 1000.
Where supplies of oxygen are limited and pulse oximetry is unavailable, as in most health facilities that treat severely ill children in resource-limited settings, prioritization depends on clinical signs. The signs we found to be most strongly predictive of hypoxaemia among admissions aged ≥ 60 days correspond with those given in the combined chapters of “triage and emergency conditions” and the “cough and difficult breathing” in the current WHO guidelines.11 The signs of shock (delayed capillary refill and a weak pulse volume) included in the triage guidelines have not been evaluated in previous studies of hypoxaemia in this setting, and including them in the WHO emergency care guidelines appears to be justified as part of the assessment of the airway, breathing and circulation. Importantly, hypoxaemia detected by transcutaneous measurement may be due in part to poor tissue perfusion (hence poor peripheral oxygenation), and oxygen may therefore be needed only in the initial stabilization phase. Although a slow heart rate and irregular breathing are not included in the currently recommended triage assessment, they were among the strongest predictors of hypoxaemia in our study. These signs, together with the signs of shock, led to identification of 50% of the cases of hypoxaemia and had reasonable specificity (85%). Importantly, although cyanosis alone was a very strong positive predictor (LR: 24), few of the children admitted had this sign, and the absence of cyanosis was not predictive of normal oxygenation (LR: 0.94).
Lower chest wall indrawing by itself was a weak predictor of hypoxaemia, and signs such as pallor, dullness on chest percussion, wheeze or crackles were not predictive. We did not include laboratory results in the final models because they are seldom available when an initial decision regarding oxygen therapy has to be made.
We performed separate analyses for different age groups on the hypothesis that predictive signs may differ in each. However, we found the signs to be remarkably similar. Few studies have looked at hypoxaemia in neonates and young infants in this setting. One study of 132 neonates in Papua New Guinea showed that a model of cyanosis, respiratory rate > 70 breaths per min, or respiratory rate < 30 breaths per min and reduced activity could predict hypoxaemia with a sensitivity of 84% and a specificity of 55%.23 A recent study from the Gambia showed that the prevalence of hypoxaemia in neonates was about 16.5%.19 However, in that study 40% of all neonatal admissions were missed. Importantly, no neonates were recruited in the first week of life.
Our study has several weaknesses. We did not collect data on head nodding and grunting, which have yielded variable findings in previous studies.4,9 Pulse oximetry was performed routinely during admission, and the admitting clinicians were not blinded to the SaO2 results during their initial clinical assessment. However, the low sensitivity of signs such as cyanosis, commonly regarded as a cardinal sign of hypoxaemia, and the fact that the predictive value of the clinical signs was similar to that reported in other studies, suggest that clinicians were not unduly influenced when recording their findings.1,8 A generalized problem is that among children admitted with overt shock and signs of poor circulation, peripheral pulse oximetry results may not always accurately reflect core hypoxaemia.24
We defined hypoxaemia as an SaO2 < 90% by pulse oximetry.11 However, children aged ≥ 60 days whose SaO2 was 90–94% had a higher case fatality proportion (102/1339, or 7.6%) than those whose SaO2 was ≥ 95% (503/11 203, or 4.5%) (P < 0.001). Some children with an SaO2 of 90–95% may benefit from oxygen supplementation; further studies are needed to elucidate if oxygen affects the outcome at this level.
Our findings strongly support current WHO recommendations surrounding triage for the detection of hypoxaemia. This is important because almost all prior studies have only included children thought to have pneumonia, and none has evaluated the triage signs of shock. Based on our findings, we advise against excessive emphasis on LRTI, which may potentially result in the underdiagnosis of other conditions associated with respiratory distress and hypoxaemia. Rather, we recommend a structured approach to assessment and triage that will target children likely to have hypoxaemia irrespective of the working diagnosis. Finally, pulse oximetry is essential for detecting hypoxaemia and is likely to save costs. ■
Acknowledgements
We thank the Kenya Medical Research institute (KEMRI) and The Wellcome Trust (Grants codes 061584, 076278, 081186) for their support. We also thank the Medical Officer of Health, the Medical Superintendent and the paediatric staff of Kilifi District Hospital. This article is submitted for publication with the permission of the Director of the KEMRI.
Footnotes
Competing interests: None declared.
References
- 1.Onyango FE, Steinhoff MC, Wafula EM, Wariua S, Musia J, Kitonyi J. Hypoxemia in young Kenyan children with acute lower respiratory infection. BMJ. 1993;306:612–5. doi: 10.1136/bmj.306.6878.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oxygen therapy for acute respiratory infections in young children in developing countries Geneva: World Healh Organization; 1993 (ARI/93.28). Available from: http://www.who.int/child-adolescent health/New Publications/CHILD_HEALTH/WHO_ARI_93.28.htm [accessed on 23 February 2007].
- 3.Blot SI, Rodriguez A, Solé-Violán J, Blanquer J, Almirall J, Rello J. Effects of delayed oxygenation assessment on time to antibiotic delivery and mortality in patients with severe community-acquired pneumonia. Crit Care Med. 2007;35:2509–14. doi: 10.1097/01.CCM.0000287587.43801.9C. [DOI] [PubMed] [Google Scholar]
- 4.Laman M, Ripa P, Vince J, Tefuarani N. Can clinical signs predict hypoxemia in Papua New Guinean children with moderate and severe pneumonia? Ann Trop Paediatr. 2005;25:23–7. doi: 10.1179/146532805X23317. [DOI] [PubMed] [Google Scholar]
- 5.Lodha R, Bhadauria PS, Kuttikat AV, Puranik M, Gupta S, Pandey RM, et al. Can clinical symptoms or signs accurately predict hypoxemia in children with acute lower respiratory tract infections? Indian Pediatr. 2004;41:129–35. [PubMed] [Google Scholar]
- 6.Reuland DS, Steinhoff MC, Gilman RH, Bara M, Olivares EG, Jabra A, et al. Prevalence and prediction of hypoxemia in children with respiratory infections in the Peruvian Andes. J Pediatr. 1991;119:900–6. doi: 10.1016/S0022-3476(05)83040-9. [DOI] [PubMed] [Google Scholar]
- 7.Smyth A, Carty H, Hart CA. Clinical predictors of hypoxemia in children with pneumonia. Ann Trop Paediatr. 1998;18:31–40. doi: 10.1080/02724936.1998.11747923. [DOI] [PubMed] [Google Scholar]
- 8.Usen S, Weber M, Mulholland K, Jaffar S, Oparaugo A, Omosigho C, et al. Clinical predictors of hypoxemia in Gambian children with acute lower respiratory tract infection: prospective cohort study. BMJ. 1999;318:86–91. doi: 10.1136/bmj.318.7176.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber MW, Usen S, Palmer A, Jaffar S, Mulholland EK. Predictors of hypoxemia in hospital admissions with acute lower respiratory tract infection in a developing country. Arch Dis Child. 1997;76:310–4. doi: 10.1136/adc.76.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayieko P, English M. In children aged 2-59 months with pneumonia, which clinical signs best predict hypoxemia? J Trop Pediatr. 2006;52:307–10. doi: 10.1093/tropej/fml036. [DOI] [PubMed] [Google Scholar]
- 11.Pocket book for hospital care of children: guidelines for the management of common illness with limited resources Geneva: World Health Organization; 2005. [PubMed] [Google Scholar]
- 12.Berkley JA, Maitland K, Mwangi I, Ngetsa C, Mwarumba S, Lowe BS, et al. Use of clinical syndromes to target antibiotic prescribing in seriously ill children in malaria endemic area: observational study. BMJ. 2005;330:995. doi: 10.1136/bmj.38408.471991.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkley JA, Ross A, Mwangi I, Osier FH, Mohammed M, Shebbe M, et al. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ. 2003;326:361. doi: 10.1136/bmj.326.7385.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shann F, Hart K, Thomas D. Acute lower respiratory tract infections in children: possible criteria for selection of patients for antibiotic therapy and hospital admission. Bull World Health Organ. 2003;81:301–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Management of severe malaria: a practical hand book Geneva: World Health Organization; 2000. ISBN 92 4 154523 2.
- 16.Centre for Evidence-Based Medicine. Likelihood ratios Available from: http://www.cebm.net/index.aspx?o=1043 [accessed on 1 February 2009].
- 17.Spiegelhalter DJ, Knill-Jones RP. Statistical and knowledge-based approaches to clinical decision-support systems, with an application in gastroenterology. J R Stat Soc [Ser A] 1984;147:35–77. doi: 10.2307/2981737. [Ser A] [DOI] [Google Scholar]
- 18.Seymour DG, Green M, Vaz FG. Making better decisions: construction of clinical scoring systems by the Spiegelhalter-Knill-Jones approach. BMJ. 1990;300:223–6. doi: 10.1136/bmj.300.6719.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junge S, Palmer A, Greenwood BM, Kim Mulholland E, Weber MW. The spectrum of hypoxemia in children admitted to hospital in The Gambia, West Africa. Trop Med Int Health. 2006;11:367–72. doi: 10.1111/j.1365-3156.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- 20.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 21.English M, Punt J, Mwangi I, McHugh K, Marsh K. Clinical overlap between malaria and severe pneumonia in Africa children in hospital. Trans R Soc Trop Med Hyg. 1996;90:658–62. doi: 10.1016/S0035-9203(96)90423-X. [DOI] [PubMed] [Google Scholar]
- 22.Wandi F, Peel D, Duke T. Hypoxemia among children in rural hospitals in Papua New Guinea: epidemiology and resource availability − a study to support a national oxygen programme. Ann Trop Paediatr. 2006;26:277–84. doi: 10.1179/146532806X152791. [DOI] [PubMed] [Google Scholar]
- 23.Duke T, Blaschke AJ, Sialis S, Bonkowsky JL. Hypoxemia in acute respiratory and non-respiratory illnesses in neonates and children in a developing country. Arch Dis Child. 2002;86:108–12. doi: 10.1136/adc.86.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooth G, Huch A, Huch R. Transcutaneous oxygen monitors are reliable indicators of arterial oxygen tension (if used correctly). Pediatrics. 1987;79:283–6. [PubMed] [Google Scholar]