Abstract
Objective
To investigate whether short-term annual declines of 5–10% in the incidence of tuberculosis (TB) can be sustained over the long term by maintaining high case detection rates (CDRs).
Methods
We constructed a compartmental difference-equation model of a TB epidemic in a hypothetical population of constant size with a treatment success rate of 85%. The impact of CDR on TB incidence was then investigated by generating an equilibrium population with no TB case detection and increasing the smear-positive CDR under two scenarios: (i) rapid expansion by 10% per year to a CDR of 80% after 8 years, and (ii) gradual expansion by 1% per year to a CDR of 90% after 90 years. The model was applied in two hypothetical populations: one without HIV and the other with a stable HIV incidence representative of the African Region. The CDR for smear-negative TB was assumed to be a constant fraction of the smear-positive CDR.
Findings
In the absence of a TB control programme, the projected annual incidence of TB was 513 cases per 100 000 population, with a point prevalence of 1233 per 100 000 and an annual TB-specific mortality rate of 182 per 100 000. Immediately increasing the TB CDR from 0% to 70% caused a 74% reduction in TB incidence within 10 years. However, once case detection stabilized at any constant level ≤ 80%, projected TB incidence also stabilized. Ten years after a CDR of 70% was reached, the annual decline in TB incidence was < 1.5%, regardless of how rapidly case detection was scaled up and despite wide variation of all model parameters.
Conclusion
While improved CDRs have a dramatic short-term effect on TB incidence, maintaining those rates, even at current target levels, may not reduce long-term incidence by more than 1–2% per year. TB control programmes and researchers should vigorously pursue improvements in case detection, regardless of current CDRs.
Résumé
Objectif
Etudier la possibilité de prolonger durablement la baisse annuelle à court terme de 5 à 10% de l’incidence de la tuberculose (TB) en maintenant un taux élevé de dépistage.
Méthodes
Nous avons construit un modèle à compartiments régi par des équations différentielles pour représenter une épidémie de tuberculose parmi une population hypothétique de taille constante, avec un taux de succès thérapeutique de 85 %. Nous avons ensuite étudié l’impact du taux de dépistage sur l’incidence ce la TB en générant une population d’équilibre dans laquelle le taux de dépistage était nul et en faisant augmenter le taux de dépistage des TB à frottis positifs selon deux scénarios : (i) augmentation rapide de 10 % par an aboutissant à un taux de dépistage de 80 % au bout de 8 ans et (ii) accroissement progressif de 1 % par an pour atteindre 90 % au bout de 90 ans. Le modèle a été appliqué à deux populations hypothétiques : l’une exempte de VIH et l’autre présentant une incidence stable du VIH, représentative de la Région Afrique. Nous avons supposé qu’il existait un rapport constant entre le taux de dépistage des tuberculoses à frottis négatifs et le taux de dépistage des cas frottis positifs.
Résultats
En l’absence de programme de lutte antituberculeuse, nous obtenions une valeur projetée pour l’incidence annuelle de la TB de 513 cas pour 100 000 habitants, avec une prévalence de pointe de 1233 cas pour 100 000 et un taux de mortalité annuel par tuberculose de 182 pour 100 000. Le passage quasi-immédiat du taux de dépistage de 0 à 70 % a provoqué une baisse de 74 % de l’incidence de la TB en l’espace de 10 ans. Cependant, une fois le dépistage stabilisé à un niveau constant ≤ 80 %, l’incidence projetée de la TB s’est également stabilisée. Dix ans après l’obtention d’un taux de dépistage de 70 %, la baisse annuelle de l’incidence de la TB était inférieure à 1,5 %, quel que soit le rythme d’augmentation du dépistage et même si l’on faisait varier fortement tous les paramètres du modèle.
Conclusion
Bien que l’amélioration des taux de dépistage ait un effet de courte durée considérable sur l’incidence de la TB, le maintien de taux élevés, même aux niveaux visés actuellement, peut ne pas faire baisser l’incidence de plus de 1 à 2 % par an. Les programmes de lutte antituberculeuse et les chercheurs doivent continuer à tout faire pour améliorer le dépistage de la TB, quels que soient les taux de dépistage actuels.
Resumen
Objetivo
Determinar si las disminuciones anuales a corto plazo de un 5%–10% de la incidencia de tuberculosis pueden sostenerse a largo plazo manteniendo unas altas tasas de detección de casos (TDC).
Métodos
Elaboramos un modelo de ecuaciones en diferencias con compartimentos para simular una epidemia de tuberculosis en una población hipotética de tamaño constante con una tasa de éxito terapéutico del 85%. A continuación se investigó el efecto de la TDC en la incidencia de tuberculosis generando una población de equilibrio sin detección de casos de tuberculosis y aumentando las tasas de detección de casos bacilíferos en dos escenarios: (i) una expansión rápida a razón de un 10% anual hasta una TDC del 80% al cabo de 8 años, y (ii) una ampliación gradual a razón de un 1% anual hasta una TDC del 90% al cabo de 90 años. El modelo se aplicó en dos poblaciones hipotéticas: una sin VIH y la otra con una incidencia de infección por VIH estable representativa de la región de África. Se asumió que la TDC correspondiente a los casos no bacilíferos era una fracción constante de la TDC de los casos con baciloscopia positiva.
Resultados
Sin un programa de control de la tuberculosis, la incidencia anual prevista de tuberculosis fue de 513 casos por 100 000 habitantes, con una prevalencia instantánea de 1233 por 100 000 y una tasa de mortalidad anual específica por tuberculosis de 182 por 100 000. Un aumento inmediato de la TDC de tuberculosis de 0% a 70% causaba una reducción de la incidencia de tuberculosis del 74% en el término de 10 años. Sin embargo, una vez que la detección de casos se estabilizaba a un nivel constante cualquiera ≤ 80%, la incidencia de tuberculosis prevista también se estabilizaba. Transcurridos diez años tras alcanzarse una TDC del 70%, la disminución anual de la incidencia de tuberculosis era inferior al 1,5%, con independencia de lo rápidamente que se hubiera expandido la detección de casos y pese a hacer variar ampliamente todos los parámetros del modelo.
Conclusión
Aunque la mejora de la TDC tiene un enorme efecto a corto plazo en la incidencia de tuberculosis, el mantenimiento de esas tasas, incluso a los niveles actualmente fijados como meta, podría no reducir la incidencia a largo plazo en más de un 1%–2% anual. Los programas y los investigadores dedicados al control de la tuberculosis deberían proseguir enérgicamente sus actividades de mejora de la detección de casos, independientemente de las TDC actuales.
ملخص
الهدف
استكشاف فيما إذا كان التناقص السنوي في معدل وقوع السل على المدى القصير والبالغ 5-10% سيتواصل على المدى الطويل نتيجة المحافظة على معدلات مرتفعة لاكتشاف الحالات.
الطريقة
بنينا نموذجاً لمعادلة للتفريق بين الأقسام حول وباء للسل في جمهرة مفترضة ذات حجم ثابت وذات معدل نجاح للمعالجة يصل إلى 85%. ثم اكتشفنا الأثر الذي يحدثه معدل اكتشاف الحالات على معدل وقوع السل، وذلك بإحداث جمهرة متعادلة تخلو من اكتشاف حالات السل، ثم قمنا بزيادة معدل اكتشاف الحالات الإيجابية اللطاخة، متّبعين سيناريوهين: السيناريو الأول توسُّع سريع في معدل اكتشاف الحالات مقداره 10% كل عام ليصل إلى 80% خلال 8 سنوات، والسيناريو الثاني توسـُّع تدريجي مقداره 1% في معـدل اكتشاف الحالات ليصـل إلى 90% بعـد 90 عاماً. وقد طبقنا النموذج على جمهرتين مفترضتين؛ الجمهرة الأولى تخلو من فيروس العوز المناعي البشري والجمهرة الثانية يمثل فيها معدل وقوع فيروس العوز المناعي البشري الثابت في الإقليم الإفريقي. وقد افترضنا أن معدل اكتشاف حالات السل السلبية اللطاخة جزءاً ثابتاً من معدل اكتشاف الحالات الإيجابية اللطاخة.
الموجودات
في غياب برنامج مكافحة السل كان معدل الوقوع السنوي المتوقَّع للسل 513 حالة لكل مئة ألف من الجمهرة، فيما كان معدل الانتشار في نقطة ما 1233 لكل مئة ألف، ومعدل الوفيات الخاصة بالسل 182 لكل مئة ألف. وقد أدت الزيادة الفورية في معدل اكتشاف الحالات من صفر بالمئة إلى 70 بالمئة إلى انخفاض مقداره 74% في معدل وقوع السل خلال 10 سنوات؛ إلا أن معدل وقوع السل المتوقع سرعان ما أصبح ثابتاً بمجرد تثبيت قيمة اكتشاف الحالات ضمن أي مستوىٍ ثابت يعادل أو يقل عن 80%. وبعد بلوغ معدل اكتشاف الحالات 70% بعشر سنوات أصبح التناقص السنوي في معدل وقوع السل أقل من 1.5% بغض النظر عن مدى التصعيد في سرعة اكتشاف الحالات، ورغم التفاوت الواسع المجال في جميع المتثابتات الخاصة بالنموذج.
الاستنتاج
في حين أن تحسن معدلات اكتشاف الحالات أدى إلى تأثيرٍ باهرٍ قصير الأمد على معدل وقوع السل، فإن المحافظة على تلك المعدلات، حتى في المستويات المستهدفة حالياً، قد لا يؤدي إلى إنقاص طويل الأمد يزيد على 1-2% كل عام. وينبغي على برامج مكافحة السل والباحثين فيها مواصلة إحراز تحسينات هامة في اكتشاف الحالات بغض النظر عن المعدلات الحالية لاكتشاف الحالات.
Introduction
In 1991, Styblo & Bumgarner quantified the expected effect of detecting and treating cases of tuberculosis (TB) and found that TB incidence could be reduced by 5–10% per year by detecting at least 70% of TB cases and successfully treating 85% of the cases detected.1 Based largely on these initial projections – which were subsequently corroborated by more complex models2 – WHO adopted a 70% case detection rate (CDR) for new sputum-smear positive cases and a treatment success rate of 85% as its primary targets for TB control.3 These targets have remained a constant benchmark for over 15 years and are currently the basis of the TB control strategy outlined in the international Stop TB strategy4 and the United Nations’ Millennium Development Goals.5 However, despite dramatic progress in TB case detection and treatment under the DOTS strategy,6 estimated global TB incidence has remained relatively stable over the past decade.7 Among the 22 countries with the highest TB burden worldwide, only two (Kenya and Zimbabwe) achieved a 5% reduction in TB incidence (as estimated by WHO) between 2005 and 2006.8 Furthermore, although four high-burden countries (China, Myanmar, the Philippines and Viet Nam) were declared to have met the global targets for case detection and treatment in 2005, the estimated TB incidence in each of these countries declined by ≤ 1.0% in the following year.8
Although numerous factors – including the co-epidemic of HIV infection,9,10 TB drug resistance,11,12 diabetes,13 smoking14 and urban crowding15 – may explain the unchanged TB incidence in the face of improving control efforts, these factors are not markedly more prominent in countries where TB incidence is stable compared with those where it has rapidly declined. An alternative explanation is that models of rapid expansion in TB control do not apply more generally to situations where TB case detection and treatment improve slowly or stabilize at target levels. Such considerations have important policy implications in an era when 93% of the world’s population is covered by DOTS8 and further gains in case detection and treatment are likely to be incremental. Thus, we developed a novel mathematical model to further investigate the relationship between TB CDRs and incidence.
Methods
Baseline model
We developed a compartmental difference-equation model of a TB epidemic as depicted in Fig. 1, with parameter estimates shown in Table 1 and model equations given in Appendix A (available at: http://www.tbhiv-create.org). Our treatment of basic TB disease states is similar to that applied in prior TB models2,34, although important differences are described in the appendix. We assume a hypothetical population of constant size in which the treatment success rate has reached 85%. This population was brought to equilibrium (defined as a change in total TB incidence of < 0.001 case per million from one year to the next) with a CDR of 0%, which represents the period before effective TB treatment became available. TB case detection was then increased under two scenarios: (i) rapid expansion from 0% (absolute increase of 10% per year for up to 8 years), followed by stabilization at a constant CDR; and (ii) gradual expansion from 0% over a prolonged period (absolute increase of 1% per year over 90 years). The former scenario may more accurately represent the “best possible” response to DOTS implementation, whereas the latter better fulfils model assumptions of a steady state.35
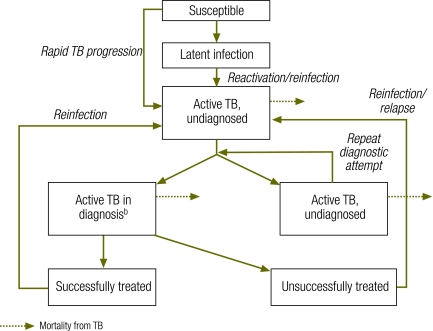
Fig. 1.
Compartmental difference-equation model of a TB epidemica
TB, tuberculosis.
a Compartments represent individuals with TB at different disease stages. The model was run both in the presence of and in the absence of HIV infection. Active TB compartments are also subdivided on the basis of sputum smear status. The dotted lines correspond to mortality from TB; non-TB mortality is experienced in all compartments. Although two boxes of undiagnosed active TB are shown here to illustrate repeat diagnostic attempts, they correspond to a single compartment in the model structure.
b Includes patients who have presented for diagnosis but have not yet completed two weeks of TB therapy.
Table 1. Parameter values for a compartmental difference-equation model of a TB epidemic1.
| Parameter | Value | Sensitivity range | Reference | |
|---|---|---|---|---|
| TB dynamics | ||||
| Secondary TB infections per smear-positive person-yeara (No.) | 11.9 | 6–15 | ||
| Proportion of incident TB cases that are smear-positive | ||||
| If HIV– | 0.45 | 0.3–0.6 | 16 | |
| If HIV+ | 0.35 | 0.2–0.5 | 16 | |
| Infectivity of patients with pulmonary smear-negative TB (relative to that of smear‑positive TB patients) | 0.22 | 0.1–0.3 | 17 | |
| Proportion of smear-negative TB cases that are extrapulmonary (i.e. non-infectious) | 0.33 | 0.2–0.5 | 18 | |
| Proportion of new TB infections progressing to active TB within 1 year | ||||
| If HIV– | 0.14 | 0.05–0.25 | 19 | |
| If HIV+ | 0.25 | 0.1–0.4 | 20 | |
| Efficacy of latent infection in preventing reinfection | ||||
| If HIV– | 0.72 | 0.6–1.0 | 21 | |
| If HIV+ | 0.25 | 0–0.5 | 22,23 | |
| Yearly rate of endogenous reactivation (%) | ||||
| If HIV– | 0.11 | 0.05–0.25 | 24 | |
| If HIV+ | 4.86 | 2.0–10.0 | 25 | |
| Yearly spontaneous conversions from smear-negative to smear-positive (%) | 2.0 | 1.0–3.0 | 24 | |
| Mortality | ||||
| Mean life expectancy without HIV infection (years)b | 55 | 45–70 | 26 | |
| Mean life expectancy after HIV infection (years) | 11 | 8–14 | 27 | |
| Untreated TB patients who will die (%) | ||||
| If HIV–, smear-positive | 50 | 40–70 | 19,28 | |
| If HIV+ | 100 | 80–100 | 16 | |
| Mean duration of untreated TB disease (years) | ||||
| If HIV–, smear-positive | 2.0 | 1.0–3.0 | 16,28 | |
| If HIV+ | 1.0 | 0.5–2.0 | 29 | |
| Mortality in smear-negative TB patients (relative to smear-positive TB patients) | ||||
| If HIV– | 0.33 | 0.2–0.4 | 16 | |
| If HIV+ | 1.0 | 0.75–1.25 | 16 | |
| TB diagnosis and treatment | ||||
| Mean duration of successful diagnostic attempt (weeks)c | 5 | 2–10 | ||
| Diagnostic sensitivity for smear-negative TB patients (relative to that for smear-positive TB patients)d | 0.67 | 0.5–1.0 | 30 | |
| Treatment success rate | 0.85 | 0.7–0.9 | Assumed | |
| Proportion of TB patients registered as deceasede | 0.07 | 0.02–0.10 | 8 | |
| Annual relapse rate among patients treated unsuccessfullyf | 0.065 | 0.05–0.15 | 31 | |
CDR, case detection rate; TB, tuberculosis. a The source gives an annual rate of TB infection of 2.0% in a population representative of sub-Saharan Africa (51% CDR for smear-positive TB, 45% overall CDR, and 22% HIV infection prevalence among incident TB cases).18 b Representative of the projected South African population in 2007. c The source assumes 3 weeks from presentation to diagnosis32 plus 2 weeks of therapy to render the patient non-infectious and at baseline mortality risk.33 d The source assumes that a sputum smear is obtained from 63% of suspected TB cases and that others receive a combination of chest X-ray (with 76% sensitivity) and a trial of broad-spectrum antibiotics (with 60% sensitivity). e The source gives data for the WHO African Region in 2006. f Calculated in the source as the rate of TB recurrence after default, minus the rate of confirmed reinfection, in two urban populations near Cape Town, South Africa.
The model runs were performed in two hypothetical populations. The first was assumed to be entirely HIV-negative; the second was assumed to have a constant incidence of HIV infection sufficient to generate a stable 22% prevalence of such infection among incident TB cases (representative of the WHO African Region) at a TB CDR of 61%.27 HIV-infected and uninfected individuals have different rates of TB progression, reactivation, smear positivity and mortality (Table 1). For simplicity, we assumed only two HIV states, without further parameterization according to CD4+ cell counts and/or antiretroviral therapy status.
Definitions and case detection rate
The TB CDR is actually a ratio defined as follows:
| (number of diagnosed TB cases) / (number of total incident TB cases) × | 100 |
By convention, we used “case detection rate” to denote the sputum smear-positive CDR; the sputum smear-negative CDR was calculated by assuming that diagnostic sensitivity for smear-negative TB was a constant fraction of the diagnostic sensitivity for smear-positive disease (Table 1). The CDR is reported by WHO as the number of registered TB cases divided by an estimated denominator. Thus, occasionally the result is an estimated CDR of > 100%.8 Because in the present model we measured incidence directly, we were able to calculate the CDR exactly without an estimated denominator.
We defined a diagnostic attempt as any independent diagnostic effort by a health-care worker who can register and treat a patient with TB; such an attempt will often span multiple clinic visits. Diagnostic attempts may either result in appropriate diagnosis and initiation of TB treatment (“successful” attempt) or failure to diagnose TB (“unsuccessful” attempt). The diagnostic sensitivity, defined as the proportion of successful diagnostic attempts, encompasses the physician’s index of suspicion for TB, the sensitivity of available laboratory techniques (e.g. sputum smear microscopy), and the losses to follow-up before treatment initiation. Since patients with more severe disease (e.g. HIV-positive patients) are likely to present for diagnosis more often than those with less severe disease, we defined the quantity r as the number of diagnostic attempts per death from TB. In the present model, we solved for the CDR numerically by varying the value of r in increments of 0.01.
Effect of case detection on TB incidence
To estimate the effect of changes in case detection on TB incidence, we created decision points at six pre-defined levels of baseline case detection: 0%, 20%, 40%, 60%, 70% and 80%. At each decision point, the CDR was immediately stabilized and held constant for 10 years, and TB incidence was measured annually. For the scenario of gradual expansion in case detection, we also evaluated the effect of continuing to increase the TB CDR by 1% per year and of accelerating this to 2% per year. Finally, we considered the scenario in which the CDR increased immediately from 0% to 70% and remained constant thereafter.
Model validation and uncertainty analysis
To verify that the baseline population provided reasonable estimates of TB incidence, we compared model output to the 2005 WHO estimates of TB burden in 22 high-burden countries.18 Uncertainty analysis was performed by varying each parameter across the range specified in Table 1 and stabilizing the CDR at 70% for 10 years. At the end of those 10 years we measured the per cent annual change in TB incidence between years 10 and 11. We also considered a “best-case” and “worst-case” scenario by setting all parameters to the values that gave the highest and lowest annual reduction in TB incidence at that time.
Results
Projected TB epidemic at baseline
Among HIV-negative individuals, the projected annual TB incidence in the study population at baseline (no TB control programme) was 513 per 100 000, with a point prevalence of 1233 per 100 000 and a TB-specific annual mortality rate of 182 per 100 000. Among those with HIV infection, the projected annual TB incidence (705 cases per 100 000) and TB-specific annual mortality rate (317 per 100 000) were 37% and 43% higher, respectively; the increase in point prevalence (1518 per 100 000) was less marked (23%). By immediately scaling up TB control efforts to a case detection rate of 70% and a treatment success rate of 85%, we observed the TB incidence in the HIV-negative population drop by 42% (38% in the HIV+ population) in the first year and by 74% (67%) after 10 years, an effect equivalent to an annual decline of 13.5% (11.2%) per year over 10 years.
Effect of CDR on TB incidence
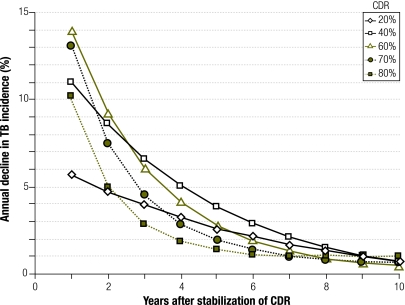
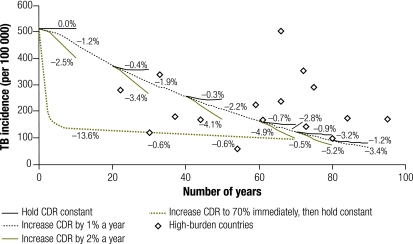
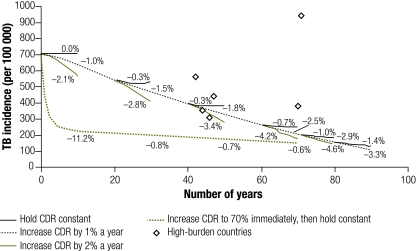
When TB case detection – at an assumed treatment success rate of 85% – was increased from 0% to 70% over 7 years (an absolute 10% increase per year) and then held constant in the absence of HIV infection, the effect on TB incidence was similar: a 72% decrease after 10 years was noted. However, once case detection stabilized at a constant level, projected TB incidence also stabilized. After holding case detection constant for 10 years, TB incidence rates fell by no more than 1.0% per additional year, regardless of the CDR at which stabilization occurred (Fig. 2). A similar result was found when the TB CDR was increased at a more gradual rate of 1% per year to a target rate and then held constant for 10 years (Fig. 3). However, if the case detection rate increased to 70% and then continued to increase by 1% annually, TB incidence continued to fall by 2.9–3.2% per year over 10 years; if the annual increase in the case detection rate was accelerated to 2%, the fall in TB incidence was 4.6–5.2% per year (Fig. 3). Although HIV co-infection increased TB incidence, it had relatively little effect on case detection (Fig. 3 and Fig. 4).
Fig. 2.
Annual decline in TB incidence under stable case detectiona
CDR, case detection rate; TB, tuberculosis.
a Points depict the relative decline in TB incidence, compared with the previous year, during the first 10 years after transitioning from rapid expansion in TB case detection (absolute 10% increase in CDR annually, starting from zero) to maintenance of case detection at a steady level. Solid markers indicate populations in which the goal of 70% case detection has been met (i.e. at least 7 prior years of rapid expansion). By year 10, no scenario had an annual decline in TB incidence of 1.0% or higher.
Fig. 3.
Projected TB incidence in a population without HIV infection when CDR is gradually increaseda
CDR, case detection rate; TB, tuberculosis.
a Percentages show the annual decline in TB incidence, averaged over 10 years, for modelled results in the absence of HIV. Diamond-shaped markers show corresponding data from high-burden countries8 with < 20% seropositivity among adult TB cases.
Fig. 4.
Projected TB incidence in a population with co-endemic HIV infection when CDR is gradually increaseda
CDR, case detection rate; TB, tuberculosis.
a Percentages show the annual decline in TB incidence, averaged over 10 years, for modelled results in a population with co-endemic HIV infection. Diamond-shaped markers show corresponding data from high-burden countries8 with > 20% seropositivity among adult TB cases.
A CDR ≥ 72% (≥ 74% in the presence of HIV co-infection) was required for TB elimination, defined as an equilibrium TB incidence of less than 10 cases per 100 000 population per year. This state corresponds to a basic reproductive rate (the epidemiological parameter R0) of < 1. At a CDR of 70%, R0 was 1.1. The case-detection threshold for TB elimination was highly sensitive to the effective contact rate, defined as the number of secondary TB infections per smear-positive person-year. When the effective contact rate was reduced to 6.0, a CDR of ≥ 30% was required for TB elimination; increasing the effective contact rate to 15.0 raised the CDR threshold for elimination to ≥ 80%. When the base-case contact rate of 11.9 was used, a constant CDR of 80% resulted in an equilibrium annual decline in TB incidence of 1.2% per year.
Uncertainty analysis revealed that, 10 years after stabilizing the CDR at 70%, the annual decline in TB incidence was always between 0.25% and 1.4%, regardless of the variation in any single parameter across the range specified in Table 1. The decline in TB incidence at 10 years was most sensitive to the effective contact rate (0.4–1.4%) and the proportion of new TB infections progressing to active TB among HIV-negative individuals (0.3–1.3%). When we set all parameters to their most favourable values, we made TB incidence decline by 2.7% during year 10 after CDR stabilization; the corresponding “worst-case scenario” caused a 2.6% increase.
Effect of diagnostic interventions on the CDR
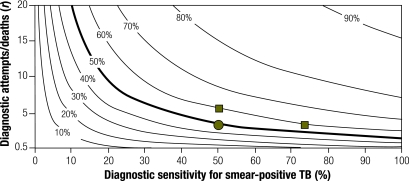
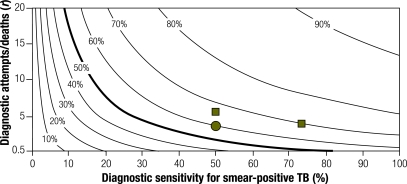
Fig. 5 shows the CDR as a function of three parameters: diagnostic sensitivity, diagnostic frequency (expressed as r, the ratio of diagnostic attempts to TB deaths), and presence or absence of continuous active TB case-finding (modelled as one additional diagnostic attempt every 2 years for each individual in the population). The effect of active case finding on CDR is greatest at low diagnostic frequency (i.e. the lower sections of Fig. 5 and Fig. 6 differ more than the upper sections). CDRs > 70% are more easily achievable by increasing diagnostic frequency than sensitivity. For example, at a smear-positive diagnostic sensitivity of 65% and an r of 7 (for a CDR of 70.0%), increasing diagnostic sensitivity to 100% yields a CDR of 78.6%, while a 50% increase in baseline diagnostic frequency plus active case finding yields a CDR of 81.0%. However, interventions that increase diagnostic frequency and those that increase diagnostic sensitivity have a greater effect on the CDR when applied in combination (e.g. movement from the circle in Fig. 5 to the right-hand square in Fig. 6) than sequential interventions that increase only diagnostic frequency or sensitivity alone (e.g. movement from the circle in Fig. 5 to the upper square in Fig. 6).
Fig. 5.
TB CDR, according to diagnostic sensitivity and ratio of diagnostic attempts to deaths, under the assumption of no active case-finding efforts for TBa
CDR, case detection rate; TB, tuberculosis.
a Contour lines depict the series of all points with the same CDR (displayed as a percentage) in a hypothetical HIV‑negative population. The circle indicates that, for a population with a TB diagnostic sensitivity of 50% and an r of 4, the CDR is 50%. This can be increased to 60% by any of the following: adopting active case-finding (circle in Fig. 6), increasing diagnostic sensitivity to 74%, or increasing r to 6 (squares). If done in concert with active case‑finding, increasing diagnostic sensitivity to 74% results in a CDR of 69%, versus 67% when r is increased to 6 (squares in Fig. 6).
Fig. 6.
TB CDR, according to diagnostic sensitivity and ratio of diagnostic attempts to deaths, under the assumption of ongoing active TB case-finding covering the population once every 2 yearsa
CDR, case detection rate; TB, tuberculosis.
a Contour lines depict the series of all points with the same CDR (displayed as a percentage) in a hypothetical HIV-negative population. The circle in Fig. 5 indicates that, for a population with a TB diagnostic sensitivity of 50% and an r of 4, the CDR is 50%. This can be increased to 60% by any of the following: adopting active case-finding (circle), increasing diagnostic sensitivity to 74%, or increasing r to 6 (squares in Fig. 5). If done in concert with active case-finding, increasing diagnostic sensitivity to 74% results in a CDR of 69%, versus 67% when r is increased to 6 (squares).
Discussion
This mathematical model of a TB epidemic in a hypothetical population corroborates earlier projections1,36 that rapid scale-up of TB control efforts to a case detection rate of 70% and a treatment success rate of 85% can reduce TB incidence by an average of 10% per year for 10 years (equal to a 65% decrease over 10 years). However, gradual improvement in case detection leads to more gradual declines in TB incidence (a 3% annual decline in TB incidence when the TB case detection rate increases by 1% per year). Furthermore, once target levels of case detection are achieved, TB incidence stabilizes within 10 years unless case detection continues to improve. CDRs > 70% are most attainable by increasing the frequency of diagnostic attempts. Our findings suggest that current goals for TB control are unlikely to be met without continued improvements in case detection beyond current target levels. Such improvements should be pursued vigorously.
The present model explains two empirical observations. First, in areas with relatively low HIV infection prevalence and high CDRs (e.g. the WHO Western Pacific Region), TB incidence is declining at approximately 1% per year,8 a rate consistent with model projections. Second, among countries with a high TB burden, the annual change in TB incidence is not correlated with absolute CDR (r²= 0.03; P = 0.45, among 22 high-burden countries in 2006),8 in keeping with the contents of Fig. 2. By contrast, this model predicts a substantial decline in TB incidence during any period in which TB CDRs are increasing. However, TB incidence increased between 1990 and 20067,8 despite the achievement of a 61% smear-positive case detection rate under DOTS.6,8 This discrepancy may reflect offsetting increases in TB incidence from other causes (e.g. HIV infection), improved reporting without concomitant improvements in actual diagnosis, or overly optimistic model assumptions about the effect of DOTS. In all of these cases, however, TB incidence is still predicted to stabilize if continued improvements in case detection are not made.
The concept that a sustained, high CDR will result in a constant annual decline in TB incidence is intuitively appealing but implies mathematically that TB will ultimately be eradicated if current targets for case detection and treatment success are maintained. A seminal model by Blower et al.37 demonstrated that > 75% of cases must be treated for eradication to occur, even for a “mild” TB epidemic. We found a similar threshold at a 72% CDR in the absence of HIV infection. Below this threshold for TB elimination, a constant annual decline in TB incidence is a mathematical impossibility. Even at a CDR of 80% (which is likely to be above the threshold for elimination), our model suggests that long-term annual declines in incidence of greater than 2.0% are unlikely in the absence of further interventions or other external forces (e.g. the decline in the effective contact rate in western Europe during the 20th century19). Initially these results appear to conflict with those of Dye et al.,2 who estimated that achieving a 70% CDR with a DOTS success rate of 85% could reduce TB incidence by 11% per year. However, this apparent discrepancy reflects the fact that Dye et al. used a 20-year time horizon to evaluate the immediate effect of DOTS, whereas the present model studied the effect of DOTS over a longer time scale. Our model projects a 13.6% annual reduction in TB incidence over 10 years after full-scale DOTS expansion (Fig. 3), or a 7.1% annual reduction if averaged over 20 years. These estimates do not fall far outside the corresponding uncertainty range (8–12%) quoted in the initial model by Dye et al.2; the small additional discrepancy is largely due to our model’s provision of partial infectivity in smear-negative patients, who are difficult to diagnose if under DOTS.
Our findings have important practical implications. They highlight the importance of case detection in TB control and suggest that DOTS, combined with a case detection rate of 70% and a treatment success rate of 85%, can reduce TB incidence by 70% by comparison with no TB control. However, they also suggest that maintaining stable case detection levels may not meaningfully reduce TB incidence further where these targets have already been achieved. Case detection targets above 70% must be pursued if elimination of TB is to be attained with existing tools. Such targets are achievable by improving access to care, which in turn increases the frequency of diagnostic attempts.38 Additional efforts to increase diagnostic frequency (e.g. active case finding39) are enhanced by simultaneous improvements in diagnostic sensitivity (e.g. improved diagnostic testing40). Further research into new diagnostic tools and mechanisms to increase diagnostic frequency is sorely needed.
The present model also has important limitations. It uses a simplified TB epidemic structure in a hypothetical population and thus cannot fully represent the epidemiologic situation in any given region. As such, this model provides proof of principle under reasonable assumptions and elucidates the longer-term implications of similar models that generate many key hypotheses (e.g. an annual 5–10% reduction in TB incidence at target case detection and treatment success rates); it does not make explicit predictions about future TB control efforts in any specific population. Furthermore, while uncertainty analysis can estimate the effect of uncertain parameter estimates on key model outputs, it cannot fully account for uncertainty inherent in the model structure or in the ability of a mathematical representation to characterize actual TB control efforts. Moreover, to maintain a parsimonious model, we did not incorporate elements such as drug resistance or alternative treatment regimens, which may strongly influence the effect of DOTS in certain populations. Finally, by focusing on case detection, this model fails to evaluate other essential elements of TB control, including treatment success rates and DOTS coverage.
In conclusion, this novel mathematical model suggests that improved CDRs may have a dramatic effect on TB incidence in the short-term, but that maintaining those rates – even at currently-defined target levels – may not reduce TB incidence by more than 1–2% per year in the long term. CDRs greater than 70% may be achievable by increasing the frequency of diagnostic attempts and exploiting the additive effects of diagnostic frequency and sensitivity. TB control programmes and researchers should vigorously pursue improvements in case detection, regardless of current CDRs. ■
Acknowledgments
We thank Christopher Dye for his helpful comments on an earlier draft of this manuscript.
Footnotes
Funding: This research is supported by the National Institutes of Health – Medical Scientist Training Program Award (T32 GMO7309 to DWD) and K24 Award (AI 01637 to REC) – and by the Bill and Melinda Gates Foundation through the Consortium to Respond Effectively to the AIDS-TB Epidemic (CREATE).
Competing interests: None declared.
References
- 1.Styblo K, Bumgarner R. Tuberculosis can be controlled with existing technologies: evidence The Hague: Tuberculosis Surveillance Research Unit; 1991. [Google Scholar]
- 2.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352:1886–91. doi: 10.1016/S0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 3.Forty-forth World Health Assembly. Resolution WHA 44.8 Geneva: World Health Organization; 1991. [Google Scholar]
- 4.The Global Plan to Stop TB 2006-2015 Geneva: World Health Organization and Stop TB Partnership; 2006. [DOI] [PMC free article] [PubMed]
- 5.Dye C, Maher D, Weil D, Espinal M, Raviglione M. Targets for global tuberculosis control. Int J Tuberc Lung Dis. 2006;10:460–2. [PubMed] [Google Scholar]
- 6.Dye C, Hosseini M, Watt C. Did we reach the 2005 targets for tuberculosis control? Bull World Health Organ. 2007;85:364–9. doi: 10.2471/BLT.06.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–75. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 8.Global tuberculosis control 2008: surveillance, planning, financing Geneva: World Health Organization; 2008. [Google Scholar]
- 9.Page KR, Godfrey-Faussett P, Chaisson RE. Tuberculosis-HIV coinfection: epidemiology, clinical aspects, and interventions. In: Raviglione M, ed. Reichman and Hershfeld’s tuberculosis: a comprehensive international approach New York, NY: Informa Healthcare; 2006. pp. 371-416. [Google Scholar]
- 10.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis. 1999;3:457–65. [PubMed] [Google Scholar]
- 11.Aziz MA, Wright A, Laszlo A, De Muynck A, Portaels F, Van Deun A, et al. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet. 2006;368:2142–54. doi: 10.1016/S0140-6736(06)69863-2. [DOI] [PubMed] [Google Scholar]
- 12.Blondal K. Barriers to reaching the targets for tuberculosis control: multidrug-resistant tuberculosis. Bull World Health Organ. 2007;85:387–90. doi: 10.2471/BLT.06.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jha P, Jacob B, Gajalakshmi V, Gupta PC, Dhingra N, Kumar R, et al. A nationally representative case-control study of smoking and death in India. N Engl J Med. 2008;358:1137–47. doi: 10.1056/NEJMsa0707719. [DOI] [PubMed] [Google Scholar]
- 15.Davies PD. The world-wide increase in tuberculosis: how demographic changes, HIV infection and increasing numbers in poverty are increasing tuberculosis. Ann Med. 2003;35:235–43. doi: 10.1080/07853890310005713. [DOI] [PubMed] [Google Scholar]
- 16.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 17.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–9. doi: 10.1016/S0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 18.Global tuberculosis control 2007: surveillance, planning, financing Geneva: World Health Organization; 2007. [Google Scholar]
- 19.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/S0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes CB, Wood R, Badri M, Zilber S, Wang B, Maartens G, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland I, Svandova E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli. 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle. 1982;63:255–68. doi: 10.1016/S0041-3879(82)80013-5. [DOI] [PubMed] [Google Scholar]
- 22.Currie CS, Williams BG, Cheng RC, Dye C. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS. 2003;17:2501–8. doi: 10.1097/00002030-200311210-00013. [DOI] [PubMed] [Google Scholar]
- 23.Cohen T, Lipsitch M, Walensky RP, Murray M. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV-tuberculosis coinfected populations. Proc Natl Acad Sci USA. 2006;103:7042–7. doi: 10.1073/pnas.0600349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 25.Gilks CF, Godfrey-Faussett P, Batchelor BI, Ojoo JC, Ojoo SJ, Brindle RJ, et al. Recent transmission of tuberculosis in a cohort of HIV-1-infected female sex workers in Nairobi, Kenya. AIDS. 1997;11:911–8. doi: 10.1097/00002030-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Actuarial Society of South Africa. ASSA2003 AIDS and demographic model Available from: http://www.assa.org.za/aids/content.asp?id=1000000449 [accessed on 30 March 2007].
- 27.2007 AIDS epidemic update Geneva: Joint United Nations Programme on HIV/AIDS; 2007.
- 28.Styblo K. Epidemiology of tuberculosis The Hague: Royal Netherlands Tuberculosis Association (KNCV); 1991. [Google Scholar]
- 29.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keeler E, Perkins MD, Small P, Hanson C, Reed S, Cunningham J, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 31.Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171:1430–5. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 32.Pronyk RM, Makhubele MB, Hargreaves JR, Tollman SM, Hausler HP. Assessing health seeking behaviour among tuberculosis patients in rural South Africa. Int J Tuberc Lung Dis. 2001;5:619–27. [PubMed] [Google Scholar]
- 33.Menzies D. Effect of treatment on contagiousness of patients with active pulmonary tuberculosis. Infect Control Hosp Epidemiol. 1997;18:582–6. doi: 10.1086/647678. [DOI] [PubMed] [Google Scholar]
- 34.Murray CJ, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA. 1998;95:13881–6. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson RM, May RM. Infectious diseases of humans: dynamics and control Oxford: Oxford University Press; 1991. [Google Scholar]
- 36.Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low- and middle-income countries. Bull World Health Organ. 2002;80:217–27. [PMC free article] [PubMed] [Google Scholar]
- 37.Blower SM, Small PM, Hopewell PC. Control strategies for tuberculosis epidemics: new models for old problems. Science. 1996;273:497–500. doi: 10.1126/science.273.5274.497. [DOI] [PubMed] [Google Scholar]
- 38.Khatri GR, Frieden TR. Controlling tuberculosis in India. N Engl J Med. 2002;347:1420–5. doi: 10.1056/NEJMsa020098. [DOI] [PubMed] [Google Scholar]
- 39.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9:1183–203. [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl 1):S15–27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]