Abstract
Senile plaque-associated changes in neuronal connectivity such as altered neurite trajectory, dystrophic swellings, and synapse and dendritic spine loss are thought to contribute to cognitive dysfunction in Alzheimer’s disease and mouse models. Immunotherapy to remove amyloid beta is a promising therapy that causes recovery of neurite trajectory and dystrophic neurites over a period of days. The acute effects of immunotherapy on neurite morphology at a time point when soluble amyloid has been cleared but dense plaques are not yet affected are unknown. To examine whether removal of soluble amyloid β (Aβ) has a therapeutic effect on dendritic spines, we explored spine dynamics within one hour of applying a neutralizing anti Aβ antibody. This acute treatment caused a small but significant increase in dendritic spine formation in PDAPP brain far from plaques, without affecting spine plasticity near plaques or average dendritic spine density. These data support the hypothesis that removing toxic soluble forms of amyloid-beta rapidly increases structural plasticity possibly allowing functional recovery of neural circuits.
Keywords: Alzheimer, dendritic spine, immunotherapy, mouse model, structural plasticity
Introduction
Compelling evidence from rare familial cases of Alzheimer disease (AD) caused by alterations in the amyloid precursor protein or presenilin 1, which affects its cleavage, support the theory that amyloid-β (Aβ) processing and aggregation is central to the pathogenesis of AD [30]. Plaque deposition is an early event in the disease process, however plaque burden does not correlate well with cognitive decline unlike synapse loss and neurofibrillary tangle pathology [14, 36]. Thus there is a pressing search for the link between amyloid and cognitive decline. Recent data support the idea that synaptotoxicity is mediated by soluble, oligomeric forms of Aβ [5, 13, 34], which may provide such a link.
Work in several mouse models of plaque deposition has shown a decrease in dendritic spine density near plaques [20, 26, 37, 39]. This decrease is greatest near plaques and spine density increases to approximately 75% of control levels at distances of greater than 50 μm from the plaque surface [37, 38], likely reflecting the local concentrations of soluble, plaque-associated synaptotoxic molecules. We recently demonstrated that in the Tg2576 model, loss of spines is due to a decrease in stability of spines near plaques. Spine formation and elimination were observed over one hour and more spines were eliminated near plaques in Tg2576 mice than in control cortex or young Tg2576 animals [38]. Spine loss near plaques could potentially be associated with the presence of soluble forms of Aβ, or with other phenomena such as the presence of activated astrocytes and microglia. In organotypic culture, addition of oligomeric Aβ causes rapid spine loss involving activation of NMDA receptors, calcineurin, and cofilin [31]. Removal of both NMDA receptors and AMPA receptors has also been implicated in Aβ induced spine loss in culture [13, 34].
To examine whether soluble forms of Aβ contribute to plaque-associated spine loss in vivo, we observed spine dynamics acutely after application of a neutralizing antibody. It has been well established that anti Aβ antibodies can clear plaques [2, 23], restore neuritic architecture [3, 23], and prevent behavioral changes in APP transgenic mice [7], but these events occur over days to weeks. By contrast, our current data suggest an acute therapeutic effect of Aβ neutralizing antibodies, strongly implicating soluble Aβ species as toxic to dendritic spines in vivo.
Materials and Methods
Animals and surgery
PDAPP animals transgenic for human amyloid precursor protein with the familial Alzheimer disease associated V717F mutation [10] and non-transgenic littermate controls were used for this study. Mice were 15–19 months of age and had substantial plaque pathology. For surgeries and imaging, mice were anesthetized with avertin (1.3% 2,2,2-tribromoethanol, 0.8% tert-pentylalcohol; 250 mg/kg). Green fluorescent dextrans (3000mw Alexa-488, 50 μg/μL in PBS – molecular probes) were injected into primary somatosensory cortex. The animal recovered for 3–5 days to allow dextrans to fill neurons. The day before cranial window implantation, methoxy X-O4 was injected (4 mg/kg i.p., generously provided by Dr William Klunk, University of Pittsburg) to label amyloid plaques [16]. The next day, a temporary cranial window was installed as described previously [1, 23, 33, 37]. Texas Red dextran (70,000 Daltons molecular weight, 12.5 mg/mL in sterile PBS, Molecular Probes, Eugene OR) was injected into a lateral tail vein to provide a fluorescent angiogram. An initial set of images of dextran-filled dendrites and plaques were obtained, then the temporary coverslip was removed and a 1 mg/mL solution containing Alexa 594 labeled anti-Abeta antibody (3D6; n=3 control, 5 PDAPP mice) or anti human tau antibody (16B5; n=4 control, 5 PDAPP mice) was applied topically to the surface of the brain (dura resected) for 20 minutes (antibodies supplied by Elan, San Francisco, CA). The brain was then washed with PBS and a glass coverslip cemented in place before a second set of images were obtained on the multiphoton microscope. All animal work was approved by institutional committees and conformed to NIH guidelines.
Multiphoton imaging and image analysis
Anesthetized animals with a cranial window were placed in a specialized stage on a BioRad 1024ES multiphoton microscope mounted on an Olympus Optical BX50WI upright microscope (Olympus, Tokyo, Japan). An Olympus 20x dipping objective with 0.95 numerical aperture was used to collect images. A mode-locked titanium-sapphire laser (Maitai; Spectra-physics, Freemont, CA) generated 800nm excitation, and three photomultiplier tubes (Hamamatsu; Ichinocho, Japan) collected emitted light in the following ranges: 380–480nm, 500–540nm, and 560–650nm. To avoid photodamage, the lowest laser power that could discern all spines was used. Each dendrite observed was imaged at an initial time point using a 10x optical zoom for a final magnification of 200x. A z-stack of images was acquired with an inter-slice interval of 0.5 μm. Dendrites were re-imaged under the same conditions one hour after antibody treatment and in some cases, 1 day after treatment.
To correct for artifacts introduced by breathing and heartbeat, image stacks from the multiphoton microscope were aligned and deconvolved using Autodeblur software (AutoQuant; Watervliet, NY). Folders were coded to blind the observer and dendrites were analyzed in green channel images. Dendrites that could be followed for more than 20 microns and had more than 3 spines were defined as spiny and counted manually using Image J (free software from the NIH http://rsb.info.nih.gov/ij/download.html). For illustration, Neuron Studio (free software from the Computational Neurobiology and Imaging Center at Mount Sinai School of Medicine was used to semi-automatically detect neurites and spines for reconstruction (http://www.mssm.edu/cnic/tools.html).
Postmortem analysis of Axonal alterations and plaque burden
Animals were euthanized at least 1 day following antibody treatment with an overdose of avertin (400 mg/kg i.p.) and brains collected into 4% paraformaldehyde containing 15% glycerol as a cryoprotectant. Brains were sectioned coronally at 50 μm on a freezing microtome (Leica, Germany) and every 10th section used for immunostaining. Axons were labeled by staining with primary antibody SMI312 (1:1000 in 1 % NGS in Tris-buffered saline, Covance Research Products, Inc., Berkely, CA) and secondary antibody anti-mouse IgG conjugated to Cy3 dye (1:200 in 1 % NGS in Tris-buffered saline, Jackson ImmunoResearch, West Grove, PA). Plaques were labeled on these sections with Thioflavine-S (0.05% in 50% ethanol).
For axon analysis, the outer 200 μm of cortex in the area treated with antibody (the area under the window) was sampled at 100% on every 10th section and photomicrographs of axons and plaques taken using an upright Olympus BX51 (Olympus, Denmark) fluorescence microscope with a DP70 camera. Axonal curvature and plaque-associated dystrophic axons were analyzed using Image J software. The curvature ratio was calculated for axonal segments > 20 μm in length by dividing the curvilinear length of the axon by the straight line length of the process [6, 11, 18, 23]. Distance from the measured axon segment to the closest senile plaque (if present) or a phantom plaque for the non transgenic group was measured at three points – from each end and the midpoint of the segment and the average distance was taken from these three measurements. Axons that were closer than 50 μm to the plaque were defined as being close to the plaque and others as far from the plaque. To count dystrophies per plaque, SMI312 positive swellings within the plaque area larger than 6 μm2 were counted as dystrophic axons and the average number of dystrophies per plaque calculated.
For plaque burden estimation, another series of every 10th section was immunostained with anti abeta antibody (10D5, Elan) and an HRP conjugated secondary developed with DAB precipitation. The outer 200 μm of cortex in the area treated with antibody was sampled at 100% and plaque staining automatically detected using BioQuant software (BioQuant, Nashville, TN) to give percent area occupied by plaques (plaque burden) and individual plaque sizes.
Statistics
Normality of datasets was assessed using a Kolmogorov-Smirnov test. Normal data (spine density) were analyzed using 2-way ANOVAs. For axonal dystrophy/plaque data and dendrite and axonal curvature (which were not normally distributed), a Mann-Whitney test was used to compare groups. To analyze spine formation and elimination (also not normal distributions), each dendrite was grouped by whether the percent elimination (and separately formation) of spines was above or below the upper quartile of the control distribution. Contingency table analysis was then used to compare transgenic dendrite segments to the control group as in a previous study [38].
Results
Plaque-associated dendritic pathology in PDAPP cortex
Dendritic spine density is reduced near plaques in several mouse models [20, 26, 37, 39]. Here we measured linear spine density in layer II–III of primary somatosensory cortex (apical branches of layer 3 and 5 pyramidal neurons filled with fluorescent dextrans) in PDAPP and control animals. We found that at baseline (before antibody treatment) PDAPP mice have reduced spine density in cortex compared to control animals (n=1201 spines on 126 dendrites in all groups: ANOVA with genotype as independent variable F[1,122]=12.214, p=0.0007). This loss is exacerbated near plaques; PDAPP dendrites within 50 μm of a plaque edge exhibit a 27% reduction in linear spine density compared to control dendrites and a 17% decrease compared to dendrites further than 50 μm from a plaque in PDAPP brain (ANOVA with genotype as independent variable categorized by distance from the nearest plaque < or > 50 μm, cell <50 F[1,58]=5.346, p=0.0243, Fig 1).
Figure 1.
PDAPP animals exhibit dendritic curvature and dendritic spine loss near plaques. Before antibody treatment, spiny dendrites (green) were observed in control (a) and PDAPP animals (b,c). Near plaques (blue), spiny dendrites were significantly curvier than those farther from plaques and curvier than dendrites in control animals (Mann-Whitney test control vs PDAPP and PDAPP <50 μm from plaque vs >50 μm from plaque, both p<0.05). Curvature ratios of individual spiny dendrite segments are plotted in (d). The arrow in (b) shows a particularly curved dendrite near a plaque. Dendritic spines are also lost near plaques in PDAPP mice (e * p<0.01 ANOVA). The arrowhead in (c) points to a dendrite that becomes less spiny as it approaches the plaque. Scale bar 10 μm.
Previous work has shown that both axons and dendrites curve around plaques in mouse models and in AD brain [6, 17, 18, 21, 23] and that passive immunization can lead to normalization of dendritic morphologies within several days [23]. We therefore also examined the baseline (pre-treatment) trajectory of spiny dendrites (filled with fluorescent dextrans) in control and PDAPP cortex both near and far from dense plaques (labeled with methoxy X-O4). We confirmed that spiny dendrites are significantly more curved in plaque bearing PDAPP mice than control animals and in PDAPP cortex, dendrites are more curved within 50 μm of a plaque edge (Fig 1, control vs transgenic Mann-Whitney test p<0.05, PDAPP near vs far from plaque Mann-Whitney test p<0.05).
Structural plasticity increases rapidly in response to antibody treatment
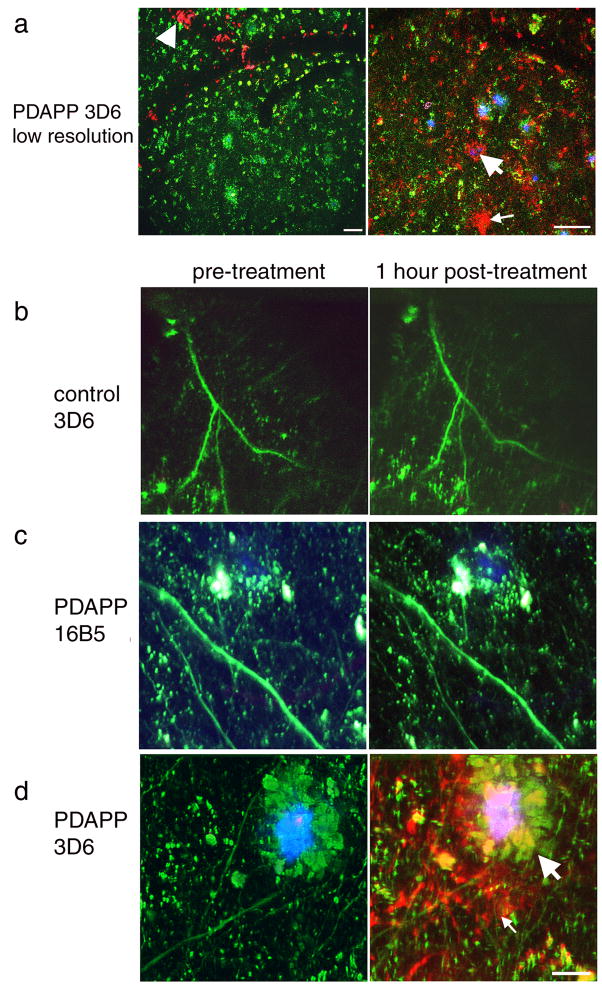
The above results confirm that plaque-associated dendritic spine loss occurs in aged PDAPP cortex. Previous work in the Tg2576 mouse model showed an impairment in spine stability near plaques underlying the loss of spines [38]. To test whether removing soluble Aβ can rapidly rescue abnormalities in spine plasticity in PDAPP mice, we treated animals with topical application of either an anti-Aβ antibody or an anti human tau antibody (as a negative control) and observed structural plasticity of dendritic spines over one hour. Application of anti-Aβ antibody 3D6 conjugated to alexa fluor 594 shows labeling of dense plaques, diffuse plaques, and cerebral amyloid angiopathy within the superficial layers of cortex (labeled antibody could be detected at least 100 μm deep in all cases). Application of anti human tau antibody 16B5 also labeled with alexa fluor 594 did not result in any red labeling of structures after one hour, indicating that the negative control antibody does not bind amyloid, as expected (Fig 2).
Figure 2.
Treatment with AlexaFlour 594 conjugated 3D6 labels amyloid pathology in PDAPP cortex. Low resolution images of PDAPP brain treated with labeled 3D6 (a) show cerebral amyloid angiopathy (arrowhead) dense plaques (large arrows), and diffuse amyloid (small arrows) are labeled red after treatment. No labeling was observed in either non-transgenic control animals treated with labeled 3D6 (b) or in PDAPP animals treated with labeled 16B5, an antibody to human tau which should not be present in mouse brain (c). High resolution images of PDAPP brain treated with AlexaFlour 594 conjugated 3D6 shows that plaques are labeled 1 hour after treatment but not before antibody is applied (d). Neurites are filled with dextrans (green) and dense plaques are labeled with methoxy XO4 (blue). Scale bars 50 μm (a), 10 μm (b–d)
Structural plasticity of individual spines was assessed over one hour in control and PDAPP animals treated with either 3D6 or 16B5 antibody (758 spines on 79 dendrite segments followed over 1 hour). In non-transgenic animals, a small fraction of spines (less than 5% of the total spines examined) were either formed or eliminated over one hour. When soluble Aβ was neutralized using 3D6 antibody in PDAPP animals, spine formation far from plaques was significantly increased compared to control levels (Chi squared p=0.0177, Fig 3). This indicates that structural plasticity increases in response to antibody treatment and demonstrates that immunotherapy has remarkably rapid effects on dendritic spine plasticity.
Figure 3.
Structural plasticity increases with antibody treatment. Individual dendritic spines (n=758) were followed before treatment and one hour after antibody application. New spines were occasionally observed after one hour (arrow, a), and some spines were eliminated within one hour (arrow, b). Reconstruction of dendrites (blue) and spine heads (green) highlights the formation and elimination. The percent formation and elimination for each dendritic segment were compared to the upper quartile for non-transgenic % formation and elimination using contingency table analysis. With this analysis, we observe that significantly more spines are formed distant from plaques in PDAPP animals one hour after 3D6 treatment compared to control animals (Chi squared p=0.0177, c). Thus structural plasticity increases rapidly following anti Aβ antibody treatment but not control antibody treatment. Scale bar 2 μm.
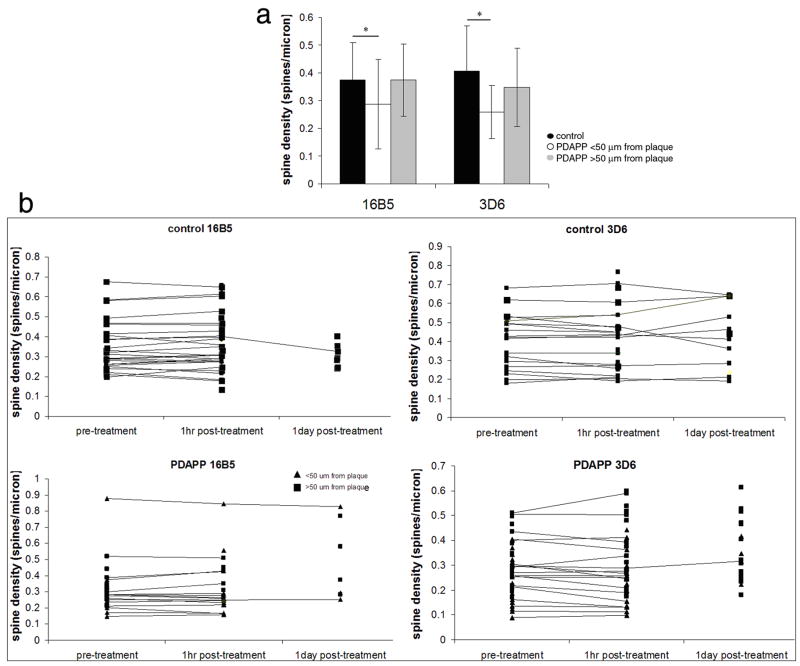
One hour after treatment with either 16B5 or 3D6, PDAPP animals still have reduced mean dendritic spine density compared to control animals (Fig 4, ANOVA genotype F[1,137]=10.77, p=0.0013). Despite the rapid effects of 3D6 treatment on spine plasticity, there is not an overall effect of treatment on mean spine density since the number of new spines formed in one hour is so small (ANOVA treatment F[1,137]=4.9×10−4, p=0.98).
Figure 4.
The average number of dendritic spines per micron of spiny dendrite length for each group (control dendrite segments, PDAPP dendrite segments within 50 μm of a plaque, and PDAPP dendrites far from plaques) was unchanged one hour after antibody treatment (a). Following individual dendritic segments over one hour and in some cases 1 day (b) shows no great changes in spine density over these time periods as would be expected since spine formation and elimination are very rare events. * p < 0.05 post-hoc one way ANOVA.
Plasticity effects precede gross morphological recovery
To determine the effects of passive immunotherapy on plaque burden, axon curvature, and axonal dystrophies associated with plaques, brains were collected post-mortem and immunostaining performed to label plaques or axons. Plaque burden in the PDAPP mice was highly variable. At one day post-treatment, there is no clearance of dense, ThioS positive plaques as assessed cross-sectionally. Mice treated with 3D6 had an average of 0.068 ± 0.082% ThioS positive plaque burden in the cortical area under the window, while animals treated with the control antibody 16B5 had 0.120% ± 0.11% plaque burden. Similarly, the diffuse plaque burden was variable but showed no recovery 1 day after treatment (3.40 ± 6.02% in 3D6 treated PDAPP animals, 0.93 ± 1.52% in 16B5 treated PDAPP animals). Non-transgenic animals had 0% dense and diffuse plaque burdens as expected.
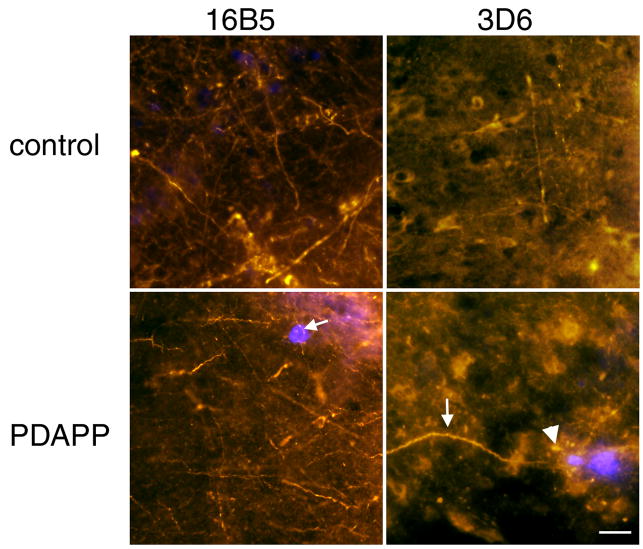
There is also no rescue of axonal curvature near plaques one day after antibody treatment (Fig 5). In non-transgenic animals, the curvature ratio was 1.029 in 16B5 treated animals and 1.035 in 3D6 treated animals (no effect of treatment, Mann-Whitney test p>0.05). PDAPP animals from both treatment groups had significantly curvier axons than control animals (Mann-Whitney test p<0.01). In PDAPP animals treated with 16B5, the curvature ratio was 1.043 within 50 μm of a plaque edge and 1.021 farther from plaques (significantly curvier near plaques Mann-Whitney p<0.01). In those PDAPP animals treated with 3D6, the curvature ratio was 1.050 within 50 μm of a plaque edge and 1.014 further from plaques (significantly curvier near plaques Mann-Whitney p<0.01). There was similarly no reduction in the mean number of dystrophic axons associated with each plaque. We would expect normalization of axonal trajectory to occur within several days of immunotherapy based on previous work [23, 25]. This experiment indicates that it takes more than 1 day for significant plaque clearance and recovery of axonal perturbations, although effects on spine plasticity can be detected after only 1 hour.
Figure 5.
Axonal curvature in PDAPP mice near plaques does not recover within 1 day of antibody treatment. Axonal neurofilament was stained with smi312 (red) to measure axon curvature near and far from ThioS labeled plaques (blue). Arrows show curvy dendrites and the arrowhead shows smi312 positive plaque-associated dystrophies persist for 1 day after 3D6 treatment. Scale bar 20 μm.
Discussion
Structural plasticity of dendritic spines is implicated in learning and memory. In particular, spines have been reported to be formed or enlarged with long-term potentiation and to shrink or disappear with long term depression over a time course of minutes to hours [24, 27, 28, 40]. Exposure to enriched environments, which enhances performance on behavioral tests even in the context of neurodegeneration, is also associated with new spine formation (reviewed by [35]). In Alzheimer’s disease, cognitive decline is associated with synapse loss and synapse loss precedes neuron death (reviewed by [36]). Is Aβ the toxic moiety that causes spine loss, or do Aβ deposits have a local toxic environment due to free radicals, activated glia, or other phenomena? We hypothesized that soluble Aβ directly impairs spine stability and tested this by acutely neutralizing soluble Aβ with an antibody. Since synapses and spines are plastic structures, even in the adult and aging brain, it is important to investigate their potential for recovery in animal models to know whether targeting prevention of spine loss or enhancing spine plasticity are worthwhile therapeutic targets.
In mouse models, both active and passive immunization strategies have been used successfully to remove amyloid pathology from the brain. Immunization of plaque-bearing mice with amyloid beta peptide decreases plaque load [29] and rescues behavioral deficits [15]. Treating mice systemically with anti amyloid beta antibodies also decreases plaque deposition and restores behavioral deficits [2, 12]. Even one dose of antibody topically applied to the brain clears diffuse plaques and causes recovery of neurite trajectory within days[1, 23]. Whether immunotherapy can ameliorate synaptic changes with removal of soluble Aβ, before dense plaques are cleared, was previously unknown.
We used the PDAPP mouse model of plaque formation to study the effects of removing soluble Aβ on dendritic spine plasticity. In this model, overexpression of the human amyloid precursor protein gene with the V717F mutation associated with familial Alzheimer’s disease causes plaque formation, synapse and dendrite loss, and memory and learning deficits [4, 8–10]. In several mouse models that develop plaque pathology, including PDAPP mice, dendritic spines are lost, particularly near dense plaques [26, 37, 39]. We previously showed that plaque-associated spine loss is due to an imbalance in the structural plasticity of dendritic spines with more spines being eliminated near plaques than formed [38]. Knowing that plaque formation causes neurite curvature and dystrophy formation within several days [25] and that these abnormalities can also clear over days after antibody treatment [23] led us to speculate that removing soluble Aβ with topical antibody treatment could act even more quickly on plasticity of dendritic spines which occurs more rapidly than changes in neurites (on the order of minutes to hours).
We tested the hypothesis that soluble Aβ causes disrupted dendritic spine plasticity using in vivo multiphoton imaging by observing spiny dendrites before treatment and one hour after antibody treatment. We found that antibody treatment causes an increase in structural plasticity in PDAPP animals within 1 hour, before there is any evidence of plaque clearance or recovery of neurite trajectory. We propose that this is due to removing soluble, probably oligomeric forms of Aβ that have been previously shown to be synaptotoxic in organotypic slice culture experiments [31, 32]. Experiments using peripheral administration of antibodies to Aβ have shown behavioural improvements within one to 14 days, without concomitant changes in soluble Aβ levels as detected by ELISA [7, 19, 22]. For example, three i.p. injections of an antibody specific to oligomeric Aβ ameliorated behavioral deficits in 7–10 days without clearing plaques, which strongly indicates that despite no change in soluble Aβ levels as measured by ELISA, removal of oligomeric Aβ from the brain has beneficial effects on cognition [5, 22]. It should be noted that these rapid effects were observed using an experimental protocol much more intensive than clinical trials of passive immunization. We topically applied a concentrated solution of the anti-Aβ antibody 3D6 directly to the brain between acquiring images of dendritic spines. The dye-labeled antibody was observed bound to plaques and amyloid angiopathy, but the concentrated solution contained an excess of antibody which was available to bind soluble Aβ, which we suggest prevents its synaptotoxic effects.
These data indicate that soluble Aβ contributes to impaired dendritic spine plasticity and synapse loss in this model of AD and further that passive immunotherapy has the potential to enhance structural plasticity very rapidly.
Acknowledgments
This work was supported by NIH grants AG08487, AG00277, a John D French Foundation Fellowship, and Alzheimer’s Association Pioneer Award and grant EB00768.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22(18):7873–8. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 3.Brendza RP, Bacskai BJ, Cirrito JR, Simmons KA, Skoch JM, Klunk WE, Mathis CA, Bales KR, Paul SM, Hyman BT, Holtzman DM. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest. 2005;115(2):428–33. doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408(6815):975–9. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 5.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 6.D’Amore JD, Kajdasz ST, McLellan ME, Bacskai BJ, Stern EA, Hyman BT. In vivo multiphoton imaging of a transgenic mouse model of Alzheimer disease reveals marked thioflavine-S-associated alterations in neurite trajectories. J Neuropathol Exp Neurol. 2003;62(2):137–45. doi: 10.1093/jnen/62.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci. 2002;5(5):452–7. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 8.Dodart JC, Mathis C, Saura J, Bales KR, Paul SM, Ungerer A. Neuroanatomical abnormalities in behaviorally characterized APP(V717F) transgenic mice. Neurobiol Dis. 2000;7(2):71–85. doi: 10.1006/nbdi.1999.0278. [DOI] [PubMed] [Google Scholar]
- 9.Dodart JC, Meziane H, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral disturbances in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Behav Neurosci. 1999;113(5):982–90. doi: 10.1037//0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- 10.Games D, Adams D, Alessandrini R, Barbour R, Borthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F [beta]-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Alloza M, Dodwell SA, Meyer-Luehmann M, Hyman BT, Bacskai BJ. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J Neuropathol Exp Neurol. 2006;65(11):1082–9. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- 12.Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25(26):6213–20. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–43. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62(6):925–31. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 15.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408(6815):979–82. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 16.Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT. Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002;61(9):797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- 17.Knowles RB, Gomez-Isla T, Hyman BT. Abeta associated neuropil changes: correlation with neuronal loss and dementia. J Neuropathol Exp Neurol. 1998;57(12):1122–30. doi: 10.1097/00005072-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer’s disease. Proc Natl Acad Sci U S A. 1999;96(9):5274–9. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22(15):6331–5. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanz TA, Carter DB, Merchant KM. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol Dis. 2003;13(3):246–53. doi: 10.1016/s0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 21.Le R, Cruz L, Urbanc B, Knowles RB, Hsiao-Ashe K, Duff K, Irizarry MC, Stanley HE, Hyman BT. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J Neuropathol Exp Neurol. 2001;60(8):753–8. doi: 10.1093/jnen/60.8.753. [DOI] [PubMed] [Google Scholar]
- 22.Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281(7):4292–9. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 23.Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT. Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci. 2003;23(34):10879–83. doi: 10.1523/JNEUROSCI.23-34-10879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451(7179):720–4. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML. Dendrite and dendritic spine alterations in alzheimer models. J Neurocytol. 2004;33(3):377–87. doi: 10.1023/B:NEUR.0000044197.83514.64. [DOI] [PubMed] [Google Scholar]
- 27.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44(5):759–67. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7(10):1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 29.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 30.Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 31.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27(11):2866–75. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skoch J, Hickey GA, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of amyloid-beta deposits in mouse brain with multiphoton microscopy. In: Sigurdsson EM, editor. Amyloid proteins: methods and protocols. Humana Press; Totowa: 2004. pp. 349–364. [DOI] [PubMed] [Google Scholar]
- 34.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–8. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 35.Spires TL, Hannan AJ. Nature, nurture and neurology: gene-environment interactions in neurodegenerative disease. FEBS Anniversary Prize Lecture delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw Febs J. 2005;272(10):2347–61. doi: 10.1111/j.1742-4658.2005.04677.x. [DOI] [PubMed] [Google Scholar]
- 36.Spires TL, Hyman BT. Neuronal structure is altered by amyloid plaques. Rev Neurosci. 2004;15(4):267–78. doi: 10.1515/revneuro.2004.15.4.267. [DOI] [PubMed] [Google Scholar]
- 37.Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25(31):7278–87. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. Impaired Spine Stability Underlies Plaque-Related Spine Loss in an Alzheimer’s Disease Mouse Model. Am J Pathol. 2007;171(4):1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7(11):1181–3. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44(5):749–57. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]