Abstract
Aim
To assess the reproducibility of, and determine normative data for, flow volume measurements from the right common carotid artery (CCA) and its relation to left ventricular output (LVO) in stable term and preterm babies using Doppler ultrasound.
Methods
Right CCA flow volume was measured using a near focus, high frequency transducer by obtaining intensity weighted mean velocity and right CCA diameter. LVO was determined using standard Doppler techniques. Reproducibility studies were performed on 30 newborn infants by two observers. Normative data were obtained from 40 spontaneously breathing preterm babies and 21 term babies.
Results
The intraobserver coefficient of variation for CCA flow measurements was 10.5% for observer 1 and 15.4% for observer 2, whereas the interobserver coefficient of variation was 16.4%. In term and preterm infants, right CCA flow was about 20 ml/kg/min, accounting for 11% of cardiac output. Among the preterm infants, there was a positive correlation of right CCA flow with gestation (r = 0.61, p<0.001), weight (r = 0.64, p<0.001), and LVO (r = 0.59, p<0.001). Right CCA diameter also increased with weight (r = 0.63, p<0.001) and gestation (r = 0.58, p<0.001). The proportion of LVO distributed to the right CCA did not increase with gestation, nor did the right CCA flow per kg body weight.
Conclusions
It is possible to perform reproducible measurements of flow volume in the CCA of newborn infants. In stable, spontaneously breathing babies, both cardiac output and carotid flow increased with gestation and body weight. The proportion of cardiac output distributed to the right CCA remained relatively constant across gestation.
Keywords: common carotid artery blood flow, cardiac output, Doppler ultrasound, reproducibility
Abnormalities in cerebral blood flow (CBF) are thought to be an important contributor to the pathogenesis of periventricular white matter lesions in preterm infants.1 These lesions are often associated with cerebral palsy and an adverse neurodevelopmental outcome. Although several methods for quantitative assessment of CBF have been used in newborn infants,2 they are not widely available for clinical practice. Doppler ultrasound has traditionally been used to measure blood flow velocity in the cerebral artery in the newborn.3 Technological advances in ultrasound now allow more accurate assessment of vessel diameter4 and computation of volumetric flow using Doppler ultrasound.
More recently it has been used to measure CBF by evaluating extracranial cerebral arteries in adults and children.5,6 In the newborn, superior vena cava (SVC) flow has been assessed by Doppler ultrasound.7 We have assessed the validity of volumetric measurements using a near focus transducer on a Doppler ultrasound phantom and determined the intraobserver and interobserver reproducibility of right common carotid artery (CCA) flow in newborn infants.
Although CBF, SVC flow, and cardiac output has been measured in newborn babies, it is not known what proportion of cardiac output is cephalic flow. In this study, we wanted to establish normal values of flow volume measurement from the right CCA in stable preterm and term infants. We also aimed to assess if there was a relation between right CCA flow, left ventricular output (LVO), and traditionally assessed Doppler measurements of anterior cerebral artery blood flow velocity (ACABFV).
Methods
Patients
Preterm babies were recruited for this study once they were not receiving ventilatory support, and term babies were recruited from the postnatal ward. All were inpatients at the Royal London Hospital. Babies with large intraventricular haemorrhage, periventricular leucomalacia, known abnormality of the central nervous system, and cardiac anomaly were excluded from the study. Basic data and blood pressure at the time of measurement were recorded for each infant. The study was approved by the local ethics committee, and written parental consent was obtained. A total of 21 term babies and 40 preterm babies, all of whom were breathing spontaneously, were studied to obtain normative data. Two preterm babies were studied on two different days of life.
Ultrasound methodology
Right CCA flow volume was measured using a linear array 6–15 MHz near focus transducer designed for intraoperative vascular applications with a Hewlett‐Packard Sonos 4500 ultrasound unit. This transducer weighs 35 g, and its footprint is 1.2 cm×3.9 cm. The transducer was placed on the baby's neck to visualise the CCA longitudinally. Doppler shift waveforms were obtained, with visualisation of the artery on colour flow being used to correct for angle of insonation. The pulsed wave Doppler trace was obtained from the right CCA with sample volume placed to encompass the whole width of the artery and away from its branching to avoid turbulent flow. The intensity weighted mean velocity (IWM) was determined from at least five arterial waveforms (fig 1). From a two dimensional, cross sectional image (fig 2), the right CCA diameter was determined from an average of five maximum systolic diameters. CCA flow was calculated as IWM × π(d2/4) × 60. LVO was determined by assessing the ascending aortic Doppler waveform from a suprasternal view, and the LVO diameter from a two dimensional, cross sectional image from the long axis parasternal view. ACABFV was determined by standard Doppler techniques.
Figure 1 Pulsed wave Doppler waveform obtained from the common carotid artery of a newborn infant.
Figure 2 Measurement of maximum systolic carotid artery diameter from a cross sectional image. CCA, Common carotid artery; IJV, internal jugular vein.
Flow phantom studies
These studies were performed on a Doppler ultrasound phantom (Dophan; Department of Medical Physics & Bio‐Engineering, St Georges Hospital, London, UK). This phantom has a vessel of 4 mm internal diameter surrounded by tissue mimicking material. The vessel is angled at 60° from vertical (angle of insonation similar to carotid arteries), and both constant and pulsatile flow can be generated with blood mimicking fluid. Pulsed wave Doppler and diameter measurements were obtained by a single blinded observer (AKS) at 10 different peak flow rates varying between 60 and 600 ml/min.
Reproducibility of CCA flow measurement
Studies were performed on 30 spontaneously breathing newborn infants. On the day of the study, these infants had a mean weight of 1.62 kg (range 0.68–4.05) and mean corrected gestation of 34 weeks (range 28–43). Default factory settings for the transducer allowed a 60° minimum angle of insonation. For the last 10 infants, the setting of the transducer was changed to allow lower angles.
To ascertain the interobserver and intraobserver reproducibility, two observers (AKS and STK) blinded to each other's results alternately performed two measurements on each patient. Intraobserver reproducibility was assessed between two observations by the same observer. Interobserver reproducibility was determined on the basis of the mean of two measurements performed by each observer. For each measurement pair, a percentage error was calculated. The standard deviation of the percentage error was calculated as a coefficient of variation. The variation between measurements was assessed by using the method of Bland and Altman.
Calculations to allow comparison with other measurement methods
To enable comparison with other measurement methods, an estimate of brain weight was calculated from the head circumference of each baby, from which the blood flow per gram of brain tissue was then estimated. The estimated brain weight of the infants was calculated based on the interconversion procedure described by Dobbing and Sands (appendix 1).8 The standardised blood flow per 100 g brain weight was calculated, assuming a ratio of CCA flow to CBF similar to that found in adults (0.67:1.00; appendix 1).
Statistical analysis
Data are reported as mean (SD). Multiple regression analysis was performed using a statistical package (SSPS 12.0.1 for Windows).
Results
Phantom flow reproducibility
There was close agreement between the peak phantom flow and Doppler volumetric measurements (r = 0.998, error SD 7%). There was no systematic bias between the phantom flow and Doppler flow measurements.
Intraobserver reproducibility
Comparison of replications within observers shows similar mean CCA diameter, velocity, and flow measurement for the two replications. Intraobserver coefficient of variation was about 3% for CCA diameter for both observers, whereas for carotid flow measurements the coefficient of variation was 10.5% for observer 1 and 15.4% for observer 2. A Bland‐Altman plot showed no systematic bias.
Interobserver reproducibility
The interobserver coefficient of variation for two observers was 3.9% for CCA diameter and 16.4% for carotid flow measurements. For the last 10 patients, when lower angles of insonation were used, the interobserver coefficient of variation for the right CCA was 13.5%. The Bland‐Altman plot did not show any evidence of systematic difference between the values of the two observers.
CCA flow
Table 1 shows the patient characteristics and flow measurements. Median age (range) at the time of study was 9 (1–83) days for preterm infants and 1 (1–3) day for term infants. In all the preterm infants, the ductus arteriosus was closed at the time of their Doppler study. In the term babies, the ductus had already closed in 13/21 at the time of the study. In seven infants the ductus was ⩽1 mm in size with a restrictive flow pattern. In one infant, the ductus was 1.5 mm in diameter with bidirectional flow.
Table 1 Patient characteristics, right common carotid artery (CCA) flow index, left ventricular output (LVO) index, and proportion of LVO to CCA flow.
| Preterm (n = 42) | Term (n = 21) | |||||
|---|---|---|---|---|---|---|
| Mean (95% CI) | SD | Range | Mean (95% CI) | SD | Range | |
| Gestation (weeks) | 33.2 | 2.9 | 28–40 | 40 | 1.2 | 38–42 |
| Weight (kg) | 1.45 | 0.46 | 0.68–3.25 | 3.3 | 0.42 | 2.6–4.1 |
| Right CCA flow index (ml/kg/min) | 20.0 (18.5 to 21.5) | 4.9 | 12.6–30.3 | 17.7 (15.6 to 19.8) | 4.9 | 9.2–28.9 |
| CBFe (ml/min/100 g brain weight) | 24.6 (22.4 to 26.9) | 7.3 | 13.5–43.9 | 23.0 (20.4 to 25.7) | 6.3 | 12.9–35.9 |
| LVO index (ml/kg/min) | 196 (184 to 208) | 40.6 | 121–284 | 164 (150 to 177) | 31 | 112–230 |
| % of LVO to right CCA flow | 10.6 (9.6 to 11.5) | 3.1 | 5.1–18.7 | 10.9 (9.9 to 11.8) | 2.2 | 6.2–15.3 |
CBFe, Estimated cerebral blood flow.
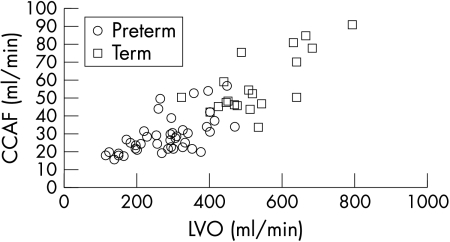
In term and preterm infants, right CCA flow was about 20 ml/kg/min, accounting for 11% of cardiac output. Among the preterm infants, there was a positive correlation of right CCA flow with gestation (r = 0.61, p<0.001), weight (r = 0.64, p<0.001), LVO (r = 0.59, p = 0.001; fig 3), and postnatal age (r = 0.41, p<0.01; table 2). In stepwise multiple regression analysis, the correlation with weight and postnatal age remained significant. Once right CCA flow was expressed as ml/kg, the only remaining significant correlation was with postnatal age, with no significant correlation with LVO or cardiac index. There was no correlation of right CCA flow in ml/kg with mean arterial blood pressure in either preterm or term infants.
Figure 3 A scatterplot of common carotid artery flow (CCAF) against left ventricular output (LVO) in preterm and term infants.
Table 2 Results of stepwise multiple regression analysis in preterm infants; dependent variable is carotid flow.
| Independent variable | Simple correlation coefficient | Partial correlation coefficient | Regression coefficient | Confidence interval (95%) |
|---|---|---|---|---|
| Day | 0.41** | 0.44** | 0.16 | 0.056 to 0.27 |
| Gestation (weeks) | 0.61** | −0.13 | Excluded from model | |
| Weight (kg) | 0.64** | 0.65** | 13.6 | 8.49 to 18.70 |
| Cardiac output (ml/min) | 0.59** | −0.20 | Excluded from model | |
| Intercept | – | – | 5.72 | −2.12 to 13.56 |
Although all the independent variables were associated with carotid flow in simple correlation, multiple regression analysis showed that only weight and age in days were independently associated with carotid flow.
**p<0.01.
ACABFV correlated with gestation and absolute right CCA flow, but there was no significant relation between flow expressed as ml/kg and the ACABFV.
Calculated CBF
Based on calculated brain weight and allowing for distribution of right CCA flow to non‐cerebral structures, the median (SD) cerebral blood flow per 100 g brain weight (CBFe) was estimated to be 22.1 (7.3) ml/min in preterm and 21.4 (6.3) ml/min for term infants. There was no significant correlation of CBFe with gestational age, weight, postnatal days, or cardiac output.
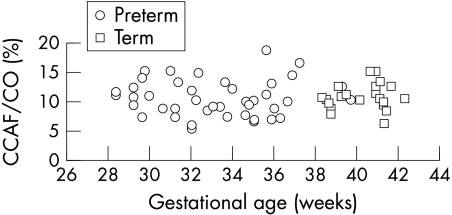
The mean (SD) right CCA diameter was 2.1 (0.3) mm for preterm infants and 3.0 (0.3) mm for term infants. Among the preterm infants, right CCA diameter increased with weight (r = 0.63, p<0.001) and gestation (r = 0.58, p<0.001). The proportion of LVO distributed to the right CCA did not increase with gestation (fig 4), nor did the right CCA flow per kg body weight.
Figure 4 Proportion of cardiac output (CO) distributed to right common carotid artery flow (CCAF) as a percentage plotted against gestational age in weeks at the time of study.
Discussion
This study has shown that in well term and preterm babies, 11% of cardiac output is distributed to the right CCA. Assuming equal flow in the carotid arteries, this would suggest that cephalic blood flow (defined as twice the right CCA flow) accounts for about 22% of cardiac output. This should be interpreted taking into consideration the fact that some spinal cord and cerebral blood flow is derived from the vertebral arteries, and some of the right CCA flow is directed to non‐cerebral structures via the external carotid artery. In adults, flow in the external carotid artery is about twice that of vertebral artery flow. As a result, in the adult, total CBF is about 25% less than the sum of flow in both CCAs.9
The reproducibility of any Doppler measurement is important, and we have shown that this measurement has good reproducibility compared with other volumetric Doppler measurements such as cardiac output and SVC flow.10 Its reproducibility also compares favourably with other methods of measuring CBF in the newborn, with test‐retest variation of 17–24% for near infrared spectroscopy using an oxyhaemoglobin tracer,11,12 15% for near infrared spectroscopy using indocyanine green tracer,12 and 10–15% for 133Xe clearance methods.13,14 Errors in ultrasound volumetric measurement relate to determination of the diameter and intensity weighted mean velocity. An incorrect assumption of vessel shape can be prevented by obtaining the diameter from a circular cross sectional image. The use of higher frequencies (15 MHz) ensures improved accuracy for diameter measurement. The diameter changes during the cardiac cycle; as most blood flow occurs during systole, it is reasonable to use the maximum systolic diameter for calculation of flow, although it results in a marginal overestimation. Doppler shift is related to the cosine of the insonation angle, with errors increasing above 60°. Measurements on the flow phantom show that, even with a 60° angle, reproducible and valid measurements can be obtained with careful attention to angle correction.
What is already known on this topic
Superior vena cava flow accounts for about 37% of right ventricular output
Cerebral or carotid blood flow as a proportion of cardiac output is not known
We have used a group of preterm and term infants to define normal CCA flow. None of the preterm infants were ventilated, and their ductus arteriosus was closed. How do the values we have obtained compare with other studies of CBF?
Raju et al15 reported CCA blood flow in a small group of preterm and term infants. They found the mean right CCA flow to be 33.2 ml/kg for preterm babies and 34.6 ml/kg for term infants with a wider range of values. They used a 7.5 MHz pulsed wave system with a fixed sample gate length that was approximately half of the mean vessel diameter. This could result in overestimation of the blood flow.16 The inherent error in the measurement of the diameter of the carotid artery using a 7.5 MHz pulsed wave system would be larger than the higher resolution obtained by our system using 15 MHz, possibly explaining the larger variability in their flow data.17
Kehrer et al18 reported measurement of CBF from extracranial arteries using Doppler ultrasound in more mature preterm infants and term infants. Although they did not give CBF in relation to body weight, they calculated it to be 20 ml/min/100 g brain weight (assuming brain weight at term to be 350 g). Although their findings are very similar to our data, we have studied more premature babies, who would be at risk of periventricular white matter injury.19
Kluckow and Evans10 reported SVC flow to constitute 37% of right ventricular output in preterm infants, and 46–52% of right ventricular output in term infants. Assuming that the flow in the two carotid arteries is equal, we found cephalic flow to constitute 22% of LVO in both preterm and term infants. It is possible that the difference between these two methods represents the contribution of the non‐cephalic circulation and blood flow from vertebral arteries to SVC flow.
Measurements of CBF using near infrared spectroscopy and 133Xe from sick preterm infants in the first few days of life have been reported to be lower than the values calculated from our study.11,20 Both CBF and cardiac output increase in early postnatal life,21 and sick ventilated preterm infants may have lower CBF.22,23 In 133Xe studies, babies without respiratory distress and infants receiving continuous positive airway pressure have been shown to have higher CBF than preterm infants requiring mechanical ventilation,24 with our results being comparable to those found in well, non‐ventilated patients using 133Xe.
This technique could be more widely used than near infrared spectroscopy because of the availability of ultrasound machines in many neonatal units and the possibility of repeated measurements. Technological advances in ultrasound transducers and processing since a previous study reporting cephalic blood flow15 have led to more accurate measurements with greater validity.
What this study adds
Measurement of common carotid artery flow has good intraobserver and interobserver reproducibility, which compares favourably with other methods of measuring cerebral blood flow
In well term and preterm infants, about 11% of cardiac output is distributed to the right common carotid artery; common carotid artery flow correlates well with gestation, weight, and left ventricular output
This is the first study to describe cephalic flow and the proportion of cardiac output contributing to it in stable preterm and term infants. The reference data from this study may be useful for further research into factors affecting cephalic flow, or the fraction of cardiac output directed to the cephalic circulation in sick newborns.
Abbreviations
ACABFV - anterior cerebral artery blood flow velocity
CCA - common carotid artery
LVO - left ventricular output
SVC - superior vena cava
Appendix 1
(1) Dobbing and Sands interconversion procedure for estimating brain weight
The study compared the occipitofrontal circumference and brain weight during the last trimester of gestation and the first 2 years of postnatal life. The following expression was suggested to obtain the brain weight:
G = X3/a – b/2X
where G = brain weight (g), X = head circumference (cm), a = 100, and b = 3000.
(2) CBF in ml/100 g brain tissue
CBF was estimated assuming that the ratio of CCA flow to CBF is similar to that of the adult. Schoning et al9 have found that mean CCA flow was about 67% of the CBF in adults.
Estimated CBF/100 g brain weight (CBFe) = 100 × (right CCA flow/0.67)/brain weight (g).
Footnotes
Competing interests: none declared
References
- 1.Pape K E, Wigglesworth J S. Haemorrhage, ischaemia and the perinatal brain. Clinics in developmental medicine. London: Spastics International Medical Publications, 19791–196.
- 2.Pryds O, Edwards A D. Cerebral blood flow in the newborn infant. Arch Dis Child Fetal Neonatal Ed 199674F63–F69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempley S T, Gamsu H R. Arterial blood pressure and blood flow velocity in major cerebral and visceral arteries. I. Interindividual differences. Early Hum Dev 199334227–232. [DOI] [PubMed] [Google Scholar]
- 4.Kiserud T, Saito T, Ozaki T.et al Validation of diameter measurements by ultrasound: intraobserver and interobserver variations assessed in vitro and in fetal sheep. Ultrasound Obstet Gynecol 19991352–57. [DOI] [PubMed] [Google Scholar]
- 5.Scheel P, Ruge C, Petruch U R.et al Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke 200031147–150. [DOI] [PubMed] [Google Scholar]
- 6.Schoning M, Hartig B. Age dependence of total cerebral blood flow volume from childhood to adulthood. J Cereb Blood Flow Metab 199616827–833. [DOI] [PubMed] [Google Scholar]
- 7.Evans N, Kluckow M, Simmons M.et al Which to measure, systemic or organ blood flow? Middle cerebral artery and superior vena cava flow in very preterm infants. Arch Dis Child Fetal Neonatal Ed 200287F181–F184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobbing J, Sands J. Head circumference, biparietal diameter and brain growth in fetal and postnatal life. Early Hum Dev 1978281–87. [DOI] [PubMed] [Google Scholar]
- 9.Schoning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke 19942517–22. [DOI] [PubMed] [Google Scholar]
- 10.Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed 200082F182–F187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skov L, Pryds O, Greisen G. Estimating cerebral blood flow in newborn infants: comparison of near infrared spectroscopy and 133Xe clearance. Pediatr Res 199130570–573. [DOI] [PubMed] [Google Scholar]
- 12.Patel J, Marks K, Roberts I.et al Measurement of cerebral blood flow in newborn infants using near infrared spectroscopy with indocyanine green. Pediatr Res 19984334–39. [DOI] [PubMed] [Google Scholar]
- 13.Greisen G. Cerebral blood flow in mechanically ventilated, preterm neonates. Dan Med Bull 199037124–131. [PubMed] [Google Scholar]
- 14.Pryds O, Schneider S. Aminophylline reduces cerebral blood flow in stable, preterm infants without affecting the visual evoked potential. Eur J Pediatr 1991150366–369. [DOI] [PubMed] [Google Scholar]
- 15.Raju T N, Go M, Ryva J C.et al Common carotid artery flow velocity measurements in the newborn period with pulsed Doppler technique. Biol Neonate 198752241–249. [DOI] [PubMed] [Google Scholar]
- 16.Evans D H. On the measurement of the mean velocity of blood flow over the cardiac cycle using the Doppler ultrasound. Ultrasound Med Biol 198511735–741. [DOI] [PubMed] [Google Scholar]
- 17.Volumetric blood flow measurement In: Evans DH, McDicken WN, eds. Doppler ultrasound: physics, instrumentation and signal processing. Chichester: J Wiley, 2000288–310.
- 18.Kehrer M, Goelz R, Krageloh‐Mann I.et al Measurement of volume of cerebral blood flow in healthy preterm and term neonates with ultrasound. Lancet 20023601749–1750. [DOI] [PubMed] [Google Scholar]
- 19.Larroque B, Marret S, Ancel P Y.et al White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. J Pediatr 2003143477–483. [DOI] [PubMed] [Google Scholar]
- 20.Tyszczuk L, Meek J, Elwell C.et al Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics 1998102337–341. [DOI] [PubMed] [Google Scholar]
- 21.Meek J H, Tyszczuk L, Elwell C E.et al Cerebral blood flow increases over the first three days of life in extremely preterm neonates. Arch Dis Child Fetal Neonatal Ed 199878F33–F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baenziger O, Mueller A M, Morales C G.et al Cerebral blood flow and neurological outcome in the preterm infant. Eur J Pediatr 1999158138–143. [DOI] [PubMed] [Google Scholar]
- 23.Baenziger O, Jaggi J L, Mueller A C.et al Cerebral blood flow in preterm infants affected by sex, mechanical ventilation, and intrauterine growth. Pediatr Neurol 199411319–324. [DOI] [PubMed] [Google Scholar]
- 24.Greisen G. Cerebral blood flow in preterm infants during the first week of life. Acta Paediatr Scand 19867543–51. [DOI] [PubMed] [Google Scholar]