Abstract
Objective
To identify prenatal risk factors for chronic lung disease (CLD) at 36 weeks postmenstrual age in very preterm infants.
Population
Data were collected prospectively as part of the ongoing audit of the Australian and New Zealand Neonatal Network (ANZNN) of all infants born at less than 32 weeks gestation admitted to all tertiary neonatal intensive care units in Australia and New Zealand.
Methods
Prenatal factors up to 1 minute of age were examined in the subset of infants born at gestational ages 22–31 weeks during 1998–2001, and who survived to 36 weeks postmenstrual age (n = 11 453). Factors that were significantly associated with CLD at 36 weeks were entered into a multivariate logistic regression model.
Results
After adjustment, low gestational age was the dominant risk factor, with an approximate doubling of the odds with each week of decreasing gestational age from 31 to less than 25 weeks (trend p<0.0001). Birth weight for gestational age also had a dose‐response effect: the lower the birth weight for gestational age, the greater the risk, with infants below the third centile having 5.67 times greater odds of CLD than those between the 25th and 75th centile (trend p<0.0001). There was also a significantly increased risk for male infants (odds ratio 1.51 (95% confidence interval 1.36 to 1.68), p<0.0001).
Conclusions
These population based data show that the prenatal factors low gestational age, low birth weight for gestational age, and male sex significantly predict the development of chronic respiratory insufficiency in very preterm infants and may assist clinical decision about delivery.
Keywords: preterm, chronic lung disease, brochopulmonary dysplasia, small for gestational age, population based
Very preterm infants commonly have respiratory failure in the first week after birth. With respiratory support, most infants survive without subsequent respiratory failure. In some, respiratory failure continues beyond one month and even up to the term equivalent age and beyond. This chronic lung disease (CLD), also known as bronchopulmonary dysplasia, is common in infants of very low gestational age (GA) or birth weight (BW)1 and is associated with other morbidities including growth failure, abnormal neurodevelopment,2,3,4 delayed discharge from hospital, need for home oxygen support,5 and more frequent readmission to hospital in the first year of life.6 Most studies on causality have emphasised lung injury from the physical effects of intermittent positive pressure ventilation and toxicity from high concentrations of oxygen.5,7 Prenatal factors and very preterm birth at a critical period of structural lung development8 may also be important.
As a large proportion of very immature infants are still receiving assistance at 28 days of age, respiratory support later, at near term age, has been used as a better measure of lung morbidity.9 This paper examines the prenatal factors associated with development of CLD in a population based regional cohort of infants born at very low gestation (less than 32 weeks). The focus is on variables that affect the infants' condition before they reach the neonatal intensive care unit (NICU), as this is the first phase of a project to examine variations in outcomes between NICUs and the model is to be used to adjust for case mix differences in risk of CLD between units.
Methods
The cohort consists of consecutive liveborn infants of 22–31 weeks gestation admitted in the first 28 days of life to the 25 tertiary NICUs in Australia and New Zealand's perinatal hospitals. These units are part of the Australian and New Zealand Neonatal Network (ANZNN), the members of which have prospectively collected a data set using agreed definitions since 1995.10 Based on data for the whole of New Zealand which are collected by ANZNN,11 and state maternity databases of all births in Australia, the cohort used here represents about 92% of all live births at less than 32 weeks and 99% of infants of that gestational age who develop CLD in the two countries. The predictive model was developed on babies born during the calendar years 1998 and 1999, then validated using a similar cohort from 2000 and 2001. Infants with lethal congenital malformations or hydrops were excluded. Analysis involved the 21 ANZNN variables covering the prenatal period and up to one minute after birth.
GA is expressed in completed weeks assessed on the basis of an obstetric ultrasound before 20 weeks, the first day of the last menstrual period, or clinical assessment of the infant.10 GA was analysed in weekly increments from 25 to the reference group at 31 weeks, with infants born at less than 25 weeks grouped together. Birth weight for gestational age (BW for GA) centile groups were derived from the Australian national birth weight centile charts for infants of each sex.12 BW for GA centile was divided into categories as follows ⩾75th, 25th–74th (reference group), 10th–24th, 3rd–9th, <3rd to test for trend using the Mantel‐Haenszel test. Symmetry around the reference category with divisions at the 90th and 97th centiles was explored, but these were collapsed into one large for GA (⩾75th) centile grouping because they had similar parameter estimates.
CLD is defined as the use of any respiratory support including supplemental oxygen or any form of assisted ventilation, given for a chronic pulmonary disorder at 36 weeks postmenstrual age. Maternal ethnicity is defined by self report. Maternal age group compared teenagers and mothers over 34 with the other mothers. Maternal hypertension in pregnancy included all types, without information on drug treatment. Suspected intrauterine growth restriction (IUGR) is based on serial obstetric ultrasounds at any time. Babies were defined as being outborn if they were not born in the perinatal centre of registration. No information on chorioamnionitis was available.
Significant variables at p<0.05 on univariate analysis were entered into a multivariate logistic regression model, and the least significant variable was removed sequentially. Variables were retained in the model if they were significant at p<0.01 and were not collinear with other variables. The fit of the model was checked using Hosmer and Lemeshow's goodness of fit statistic,13 with the additional verification that models were not over‐fitted (indicated by very high p values). The discriminatory ability of the model was assessed using the area under the receiver operating characteristics (ROC) curve. Logistic regression diagnostics were also used to identify potential covariate patterns that were poorly fitted or highly influential. Analyses were performed using SAS statistical software, version 8.2 (SAS Institute, Cary, North Carolina, USA). Results are expressed as odds ratios (OR) with 95% confidence interval (CI) in parentheses. Ethical approval for this study was given by the Royal Prince Alfred Hospital Human Ethics Review Committee and by the administering institution, the University of Sydney.
To compare our results with those published by others, we undertook a systematic search of Medline (1966–November 2004) to identify similar population based studies of very preterm infants that examined prenatal risk factors for CLD at 36 weeks postmenstrual age using GA to define the cohort. The population base criterion is chosen to minimise selection or referral bias in hospital based studies, and the use of GA rather than birth weight was chosen to avoid GA bias.14,15
Results
There were 6249 infants born at 22–31 weeks GA in 1998 and 1999 and admitted to the NICUs of the ANZNN. Of these, 647 died before 36 weeks postmenstrual age, and for three the outcome CLD status was missing. The remaining 5599 infants had a median GA of 29 weeks with an interquartile range (IQR) of 27–30 weeks. The median birth weight was 1235 g (IQR 960–1505). The rate of CLD in this group was 22% (n = 1235). The validation cohort of 5854 surviving infants born in 2000–2001 had a CLD rate of 25%, median GA of 29 weeks (IQR 27–30), and birth weight of 1235 g (IQR 960–1510). The male to female ratio was similar in the two periods: 1.2:1 in 1998–1999 and 1.1:1 in 2000–2001.
Factors that were not significant on univariate analysis (table 1) were not included in multivariate analyses. Table 2 shows factors that were significantly associated with CLD. Previous perinatal death, hypertension in pregnancy (any form), antepartum haemorrhage, breech position at birth, and caesarean section before labour started were associated with an increased risk, but did not retain significance in multivariate analysis. Likewise, there was an unadjusted protective association for preterm prelabour rupture of membranes that did not persist in the adjusted model.
Table 1 Univariate analysis: perinatal variables not associated with chronic lung disease 1998–1999.
| Perinatal variable | Unadjusted OR ( 95% CI) | p Value | Missing data |
|---|---|---|---|
| Maternal age | 0.41 | 0 | |
| ⩾35 years | 1.07 ( 0.92 to 1.26) | ||
| 20–34 years | 1.00 reference group | ||
| <20 years | 0.91 ( 0.73 to 1.13) | ||
| Previous preterm birth | 1.02 ( 0.85 to 1.22) | 0.85 | 672 |
| Prolonged rupture of membranes (>24 h) | 0.99 ( 0.85 to 1.16) | 0.93 | 428 |
| Preterm labour | 0.93 ( 0.81 to 1.06) | 0.25 | 6 |
| Maternal corticosteroids (any dose) | 1.19 ( 0.99 to 1.43) | 0.06 | 218 |
| Fetal distress requiring intervention | 1.12 ( 0.95 to 1.32) | 0.17 | 518 |
| Plurality | 1.00 | 0 | |
| Singleton | 1.00 reference group | ||
| Multiple pregnancy | 1.00 ( 0.87 to 1.15) | ||
| Birth order | 0.95 | 2 | |
| Singleton or 1st of multiple pregnancy | 1.00 reference group | ||
| 2nd or higher of multiple pregnancy | 1.00 ( 0.86 to 1.15) | ||
| Outborn, transferred after birth | 1.11 ( 0.91 to 1.37) | 0.31 | 0 |
OR, Odds ratio; CI, confidence interval.
Table 2 Univariate analysis: perinatal variables significantly associated with chronic lung disease (CLD) 1998–1999.
| Perinatal variable | CLD/total | Unadjusted OR (95% CI) | p Value | Missing data |
|---|---|---|---|---|
| Maternal ethnicity | 0.002 | 505 | ||
| White | 955/4163 (23%) | 1.00 reference group | ||
| Asian | 71/331 (21%) | 0.92 ( 0.70 to 1.20) | ||
| Indigenous Australian | 51/243 (21%) | 0.89 ( 0.65 to 1.23) | ||
| Maori | 15/116 (13%) | 0.50 ( 0.29 to 0.86) | ||
| Pacific Islander | 35/241 (15%) | 0.57 ( 0.40 to 0.82) | ||
| Previous perinatal death | 89/316 (28%) | 1.41 ( 1.09 to 1.82) | 0.01 | 708 |
| Hypertension in pregnancy (any) | 298/1224 (24%) | 1.18 ( 1.02 to 1.37) | 0.03 | 63 |
| Antepartum haemorrhage | 315/1276 (25%) | 1.21 ( 1.04 to 1.40) | 0.01 | 483 |
| Preterm prelabour ROM | 249/1283 (19%) | 0.81 ( 0.70 to 0.95) | 0.008 | 0 |
| Suspected IUGR | 193/663 (29%) | 1.56 ( 1.30 to 1.87) | <0.0001 | 79 |
| Gestational age | <0.0001 | 0 | ||
| 31 weeks | 59/1343 (4%) | 1.00 reference group | ||
| 30 weeks | 83/1056 (8%) | 1.86 ( 1.32 to 2.62) | ||
| 29 weeks | 141/916 (15 %) | 3.96 ( 2.88 to 5.43) | ||
| 28 weeks | 159/737 (22%) | 5.99 ( 4.37 to 8.20) | ||
| 27 weeks | 202/554 (36%) | 12.49 ( 9.13 to 17.08) | ||
| 26 weeks | 228/463 (49%) | 21.11 ( 15.36 to 29.02) | ||
| 25 weeks | 193/300 (64%) | 39.25 ( 27.60 to 55.81) | ||
| ⩽24 weeks | 170/230 (74%) | 61.65 ( 41.60 to 91.36) | ||
| Weight for gestational age | <0.0001 | 0 | ||
| ⩾75th* | 173/1256 (14%) | 0.65 ( 0.54 to 0.79) | ||
| 25th–74th | 575/2930 (20%) | 1.00 reference group | ||
| 10th–24th | 257/864 (30%) | 1.73 ( 1.46 to 2.06) | ||
| 3rd–9th | 146/376 (39%) | 2.60 ( 2.07 to 3.26) | ||
| <3rd | 84/173 (49%) | 3.87 ( 2.83 to 5.28) | ||
| Sex | <0.0001 | 0 | ||
| Female | 497/2526 (20%) | 1.00 reference group | ||
| Male | 738/3073 (24%) | 1.28 ( 1.14 to 1.47) | ||
| Presentation | <0.0001 | 632 | ||
| Cephalic | 667/3314 (20%) | 1.00 reference group | ||
| Breech | 354/1390 (25%) | 1.36 ( 1.17 to 1.57) | ||
| Other | 75/263 (29%) | 1.58 ( 1.20 to 2.10) | ||
| Delivery | 0.01 | 156 | ||
| Vaginal birth | 476/2219 (21%) | 1.00 reference group | ||
| Caesarean in labour | 273/1358 (20%) | 0.92 ( 0.78 to 1.09) | ||
| Caesarean no labour | 454/1866 (24%) | 1.18 ( 1.02 to 1.36) | ||
| Apgar at 1 min <4 | 357/978 (37%) | 2.45 ( 2.11 to 2.85) | <0.0001 | 0 |
*Birth weight for gestational age groups 75th–89th, 90th–96th, ⩾97th were combined because ORs were similar.
OR, Odds ratio; CI, confidence interval. ROM, rupture of membranes; IUGR, intrauterine growth restriction.
Despite their statistical significance in the multivariate analysis, a low Apgar score at one minute, maternal ethnicity, and suspected IUGR were excluded from the final model. Reasons for this included evidence of collinearity between GA and Apgar score at one minute and, to some extent, Apgar score represents an infant's initial respiratory performance. Ethnicity is a difficult factor to assess, and its exclusion was justified by the amount of missing data (9%), the inherent problems of different indigenous populations in the two countries, and the difficulty of separating the effect of ethnicity from social factors, which were not measured. Suspected IUGR was rejected because it was collinear with BW for GA, is likely to be variably reported, and because BW for GA provides a range of categories rather than the dichotomous one of suspected IUGR.
Multivariate analysis retained the variables: GA group, BW for GA group, and male sex, which were significant at p<0.01 after simultaneous adjustment (table 3). Low GA was the dominant risk factor for CLD (table 3). The risk of CLD increases progressively with decreasing GA (trend χ21 = 997, p<0.0001) as indicated by the ORs, with the most preterm infants (⩽24 weeks) having 86 times the odds of those born at 31 weeks. A dose‐response effect was also apparent for BW for GA (trend χ21 = 272, p<0.0001), indicating that the lower the birth weight at each GA, the greater the risk of CLD. Male sex was associated with an increased risk of CLD (OR 1.42 ( 95% CI 1.22 to 1.65), χ21 = 20.1, p<0.0001). Similar results were also found after analysis of the validation cohort of babies born in 2000–2001 and the two cohorts combined—that is, born 1998–2001; see ORs in column 2 of table 3 and column 3 of table 4.
Table 3 Adjusted odds ratios for chronic lung disease (CLD) in the inception cohort (1998–1999) and the verification cohort (2000–2001).
| Adjusted OR 1998–1999 cohort (95% CI) | Adjusted OR 2000–2001 cohort (95% CI) | |
|---|---|---|
| Size of cohort | 5599 | 5854 |
| CLD (n (%)) | 1235 (22%) | 1475 (25%) |
| Predictor variable | ||
| Gestational age | ||
| 31 weeks | 1.00 reference group | 1.00 reference group |
| 30 weeks | 2.05 ( 1.44 to 2.91) | 1.66 ( 1.21 to 2.26) |
| 29 weeks | 4.25 ( 3.07 to 5.88) | 3.26 ( 2.43 to 4.39) |
| 28 weeks | 7.59 ( 5.49 to 10.51) | 6.56 ( 4.95 to 8.70) |
| 27 weeks | 15.58 ( 11.26 to 21.57) | 13.55 ( 10.16 to 18.06) |
| 26 weeks | 25.37 ( 18.22 to 35.33) | 22.96 ( 16.95 to 31.11) |
| 25 weeks | 56.14 ( 38.86 to 81.10) | 52.22 ( 37.03 to 73.64) |
| ⩽24 weeks | 85.70 ( 57.00 to 128.9) | 142.4 ( 93.14 to 217.7) |
| Weight for gestational age | ||
| ⩾75th* | 0.54 ( 0.44 to 0.67) | 0.64 ( 0.53 to 0.71) |
| 25th–74th | 1.00 reference group | 1.00 reference group |
| 10th–24th | 2.05 ( 1.67 to 2.51) | 1.82 ( 1.49 to 2.23) |
| 3rd–9th | 4.01 ( 3.06 to 5.24) | 2.87 ( 2.21 to 3.75) |
| <3rd | 6.25 ( 4.29 to 9.11) | 5.27 ( 3.43 to 8.09) |
| Male sex | 1.42 ( 1.22 to 1.65) | 1.63 ( 1.41 to 1.89) |
*Birth weight for gestational age groups 75th–89th, 90–96th and ⩾97th were combined because ORs were similar.
OR, Odds ratio; CI, confidence interval.
Table 4 Final logistic regression model for chronic lung disease for 11 453 infants born in 1998–2001.
| Predictor variable | Parameter estimate | Standard error | Adjusted OR (95% CI) |
|---|---|---|---|
| Intercept | −3.4667 | 0.1008 | |
| Gestational age | |||
| 31 weeks | 0 | 1.00 reference group | |
| 30 weeks | 0.5972 | 0.1190 | 1.82 ( 1.44 to 2.29) |
| 29 weeks | 1.2996 | 0.1111 | 3.67 ( 2.95 to 4.56) |
| 28 weeks | 1.9475 | 0.1085 | 7.01 ( 5.67 to 8.67) |
| 27 weeks | 2.6616 | 0.1096 | 14.32 ( 11.55 to 17.75) |
| 26 weeks | 3.1625 | 0.1136 | 23.63 ( 18.91 to 29.53) |
| 25 weeks | 3.9639 | 0.1273 | 52.66 ( 41.04 to 67.58) |
| ⩽24 weeks | 4.6643 | 0.1467 | 106.1 ( 79.58 to 141.4) |
| BW for GA (centile grouping ) | |||
| ⩾75th | −0.5285 | 0.0731 | 0.59 ( 0.51 to 0.68) |
| 25th−74th | 0 | 1.00 reference group | |
| 10th−24th | 0.6503 | 0.0734 | 1.92 ( 1.66 to 2.21) |
| 3rd−9th | 1.2133 | 0.0961 | 3.37 ( 2.79 to 4.06) |
| <3rd | 1.7351 | 0.1438 | 5.67 ( 4.28 to 7.52) |
| Sex | |||
| Female | 0 | 1.00 reference group | |
| Male | 0.4144 | 0.0539 | 1.51 ( 1.36 to 1.68) |
OR, Odds ratio; CI, confidence interval; BW for GA, birth weight for gestational age
To investigate the possible effect of measuring GA in completed weeks rather than days, a subgroup of 1892 infants who had documented expected dates of delivery and thus gestation in weeks and days was examined. No trend in the proportion of infants with CLD across days of the week was found when data on days of the week were pooled across GAs in whole weeks (trend χ21 = 1.7, p = 0.20).
The predictive model (cohort 1998–1999, table 3) yields an area under the ROC curve of 0.84, indicating excellent discrimination, and the Hosmer and Lemeshow test statistic indicates a good fit (p = 0.33). The temporal stability also validates well for the 2000–2001 cohort, with excellent discrimination (area under ROC curve = 0.84) and goodness of fit (p = 0.27). The regression diagnostics supported the summary validation measures of goodness of fit and did not reveal any highly influential subjects. The temporal validation gives justification to the combined results (1998–2001), with 11 453 infants included, 24% rate of CLD, the same area under the ROC curve, and similar goodness of fit (p = 0.32). The larger cohort yields more precision, as evidenced by the narrower confidence intervals (column 2 of table 4).
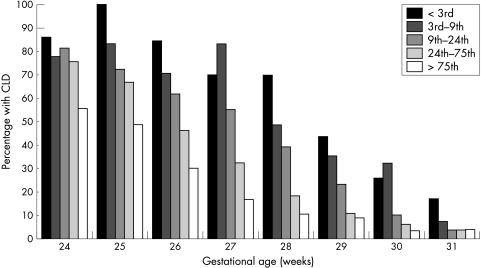
Figure 1 shows the effect of BW for GA centile on CLD at each GA. This indicates that the effect of BW for GA may vary somewhat by GA, although the interaction term is not significant (p = 0.36). For example, all infants below the 75th BW for GA centile have similarly high rates of CLD at ⩽24 weeks GA, whereas all those at or above the 10th centile have similarly low rates at 31 weeks, and a dose‐response effect is clearest in those at 25–29 weeks.
Figure 1 Percentage of infants with chronic lung disease (CLD) by gestation and birth weight for gestational age subgroups.
Discussion
Low GA, lower BW for GA, and male sex are independent prenatal risk factors for CLD. Low GA at birth is the strongest risk factor, with an approximate doubling of the odds with each week that GA decreases. This strong “dose‐response” relation has been a consistent finding in other studies.16,17,18 This is biologically plausible as the GA range covered is a critical period in structural lung development.8 An important stimulus to lung growth in utero is thought to come from distension of the lung with fluid to above its resting volume,19 and this would be lost after birth.
Independent of this GA effect, lower birth weight at each GA is associated with a progressive increase in the risk of developing CLD in survivors. Compared with the referent BW for GA in the 25th–75th centile range, there is almost a sixfold increase in odds in infants with a weight less than the 3rd centile, whereas in infants of higher weight, at or above the 75th centile, it is protective. In another population based cohort study of infants defined by GA less than 32 weeks and surviving to 36 weeks postmenstrual age, there is a similar dose‐response relation to BW for GA.20 Compared with referent infants born with BW for GA between the 25th and 75th centiles, those with a BW for GA less than the 10th centile had an increase in the OR of developing CLD (OR 2.84 (95% CI 2.01 to 4.00)), whereas infants with BW for GA above the 90th centile had a reduced risk (OR 0.41 (95% CI 0.26 to 0.65)). Being small for GA was also found to be a risk factor for CLD in two French regional studies17,21 of very preterm infants who were admitted to a NICU.
Other studies have shown that, in infants at all gestations, those that are small for GA have more lung dysfunction in childhood.22,23 Size at birth is also predictive of lung disease in adults.24 Experimental studies in a range of animal species have shown a causal relation between reduced growth at a critical time of structural lung development and reduced lung size and function. This has been observed when fetal growth was reduced through undernutrition in rats,25 guinea pigs,26 and lambs,27 as well as when fetal growth was reduced through reduced placental function in sheep.28,29
A potential problem for our study was using GA measured in whole weeks. As GA is such a strong predictor of CLD, the risk may change from day zero to day six of any given week. In our study no such trend was found.
Male sex increases the odds of CLD by 51%, and this finding is consistent with other reports.18 In some population based studies in which postnatal acute respiratory function has been included in models predicting CLD, male sex does not remain significant.17,21 It is possible that sex acts by determining the risk of acute respiratory disease. The risk of mortality and morbidity has been consistently higher in male neonates, and the underlying mechanisms, including differential rates of maturation, have been discussed.30
The prenatal risk factors for CLD reported here also predict the severity of acute neonatal lung disease.31 The consequent treatments, such as mechanical ventilation and oxygen therapy, are associated with lung injury5,7 and may explain some of the increase in the risk of CLD. In studies reporting multivariate models for predicting CLD, which have included prenatal and neonatal factors such as acute respiratory failure, GA remains a significant predictor.17,18,21
CLD is defined as receiving respiratory support and this was determined by individual clinical practice. Differences in clinical policies such as targeting higher or lower oxygen saturation have been shown to significantly affect the length of respiratory support with oxygen.32 However, this bias is between NICUs and is unlikely to affect infants in any systematic manner.
In very low birthweight infants admitted to a NICU in the ANZNN, GA, sex, and BW for GA were also found to be risk factors for death33 and retinopathy of prematurity,34 whereas GA and sex are risk factors for intraventricular haemorrhage.35 Regardless of whether the risk factors found in the model here act partly through increased neonatal complications, they are the factors that are available before the birth of the infant. Obstetricians could use them to assess the risks of delivery and to inform parents about likely outcomes.
What is already known on this topic
Chronic lung disease in infants born very preterm is common, and the incidence increases with decreasing gestational age at birth and if the infant is small for gestational age. It is associated with the amount of intermittent positive pressure ventilation and level of oxygen therapy given to the infant
Most of these results are based on a combination of prenatal and neonatal data from individual hospitals and on single cohorts
What this study adds
This study used prenatal data available for use in prognostication by the time of birth from large population based cohorts in two countries. The model was developed on a two year cohort and evaluated for stability on the cohort from the subsequent two years, thus increasing its validity
The results confirm the large effect of low gestational age at birth and indicate the increased risk in male infants. Of particular note is the dose‐response relation between lower birth weight for gestational age based on five measures across the whole range of birth weight
Acknowledgements
This research was supported by the National Health and Medical Research Council of Australia (grant 211088). Collaboration of members of the ANZNN (appendix) is gratefully acknowledged.
Abbreviations
ANZNN - Australian and New Zealand Neonatal Network
CLD - chronic lung disease
IUGR - intrauterine growth restriction
NICU - neonatal intensive care unit
ROC - receiver operating characteristics
Appendix
Members of the ANZNN Advisory Committee and Executive*
Australia: Centre for Perinatal Health Services Research, NSW: David Henderson‐Smart*. Flinders Medical Centre, SA: Peter Marshall. John Hunter Hospital, NSW: Chris Wake. King Edward Memorial and Princess Margaret Hospitals, WA: Noel French, Ron Hagan and Karen Simmer. Launceston General Hospital, Tas: Chris Bailey. Liverpool Health Service, NSW: Robert Guaran. Mater Mother's Hospital, Qld: David Tudehope. Mercy Hospital for Women, Vic: Andrew Watkins. Monash Medical Centre, Vic: Kaye Bawden*, Andrew Ramsden, Victor Yu. National Perinatal Statistics Unit, NSW: Paul Lancaster*. Nepean Hospital, NSW: Lyn Downe. Newborn Emergency Transport Service (Vic): Michael Stewart. NSW Newborn & Paediatric Emergency Transport Service: Andrew Berry. Perinatal Research Centre, Qld: Paul Colditz. Royal Children's Hospital, Vic: Linda Johnstone, Peter McDougall. Royal Darwin Hospital, NT: Charles Kilburn. Royal Hobart Hospital, Tas: Peter Dargaville. Royal Hospital for Women, NSW: Kei Lui. Royal North Shore Hospital, NSW: Jennifer Bowen. Royal Prince Alfred Hospital, NSW: Nick Evans Royal Women's Hospital, Qld: David Cartwright*. Royal Women's Hospital, Vic: Colin Morley, Neil Roy. Sydney Children's Hospital, NSW: Barry Duffy. The Canberra Hospital, ACT: Graham Reynolds. The Children's Hospital at Westmead, NSW: Robert Halliday. The Townsville Hospital, Qld: John Whitehall. Western Australia Neonatal Transport Service: Jenni Sokol. Westmead Hospital, NSW: William Tarnow‐Mordi. Women's & Children's Hospital, SA: Ross Haslam. Deborah Donoghue is the ANZNN coordinator.
New Zealand: Christchurch Women's Hospital: Nicola Austin. Christchurch School of Medicine: Brian Darlow*. Dunedin Hospital: Roland Broadbent. Gisborne Hospital: Graeme Lear. Hastings Hospital: Jenny Corban. Hutt Hospital: Robyn Shaw. Middlemore Hospital: Lindsay Mildenhall. National Women's Hospital: Carl Kuschel. Nelson Hospital: Peter McIlroy. Palmerston North Hospital: Jeff Brown. Rotorua Hospital: Stephen Bradley. Southland Hospital: Paul Tomlinson. Taranaki Hospital: John Doran*. Tauranga Hospital: Hugh Lees. Timaru Hospital: Philip Morrison. University of Auckland: Jane Harding. Waikato Hospital: David Bourchier. Wairau Hospital: Ken Dawson. Wanganui Hospital: Neil MacKenzie. Wellington Women's Hospital: Vaughan Richardson. Whakatane Hospital: Chris Moyes. Whangarei Hospital: Peter Jankowitz.
Footnotes
Competing interests: none declared
References
- 1.Stevenson D K, Wright L L, Lemons J A.et al Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol 19981791632–1639. [DOI] [PubMed] [Google Scholar]
- 2.Sauve R S, Singhal N. Long‐term morbidity of infants with bronchopulmonary dysplasia. Pediatrics 198576725–733. [PubMed] [Google Scholar]
- 3.Skidmore M D, Rivers A, Hack M. Increased risk of cerebral palsy among very low‐birthweight infants with chronic lung disease. Dev Med Child Neurol 199032325–332. [DOI] [PubMed] [Google Scholar]
- 4.Vohr B R, Coll C G, Lobato D.et al Neurodevelopmental and medical status of low‐birthweight survivors of bronchopulmonary dysplasia at 10 to 12 years of age. Dev Med Child Neurol 199133690–697. [DOI] [PubMed] [Google Scholar]
- 5.Jobe A H, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 20011631723–1729. [DOI] [PubMed] [Google Scholar]
- 6.Chye J K, Gray P H. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. J Paediatr Child Health 199531105–111. [DOI] [PubMed] [Google Scholar]
- 7.Bancalari E, Abdenour G E, Feller R.et al Bronchopulmonary dysplasia: clinical presentation. J Pediatr 197995819–823. [DOI] [PubMed] [Google Scholar]
- 8.Burri P H. Fetal and postnatal development of the lung. Annu Rev Physiol 198446617–628. [DOI] [PubMed] [Google Scholar]
- 9.Shennan A T, Dunn M S, Ohlsson A.et al Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 198882527–532. [PubMed] [Google Scholar]
- 10.Donoghue D A. Report of the Australian and New Zealand Neonatal Network 2002. Sydney: ANZNN, 2004
- 11.Darlow B A, Cust A E, Donoghue D A. Improved outcomes for very low birthweight infants: evidence from New Zealand national population based data. Arch Dis Child Fetal Neonatal Ed 200388F23–F28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts C L, Lancaster P A. Australian national birth weight percentiles by gestational age. Med J Aust 1996170114–118. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer D W, Lemeshow S.Applied logistic regression. New York: John Wiley and sons, 2000
- 14.Arnold C C, Kramer M S, Hobbs C A.et al Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol 1991134604–613. [DOI] [PubMed] [Google Scholar]
- 15.Lapeyre D, Klosowski S, Liska A.et al Very preterm infant (<32 weeks) vs very low birth weight newborns (1500 grammes): comparison of two cohorts. Arch Pediatr 200411412–416. [DOI] [PubMed] [Google Scholar]
- 16.Henderson‐Smart D J. Postnatal consequences of chronic intrauterine compromise. Reprod Fertil Dev 19957559–565. [DOI] [PubMed] [Google Scholar]
- 17.Egreteau L, Pauchard J Y, Semama D S.et al Chronic oxygen dependency in infants born at less than 32 weeks' gestation: incidence and risk factors. Pediatrics 2001108E26. [DOI] [PubMed] [Google Scholar]
- 18.Costeloe K, Hennessy E, Gibson A T.et al The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 2000106659–671. [DOI] [PubMed] [Google Scholar]
- 19.Harding R. Hooper SB. Regulation of lung expansion and lung growth before birth. J Appl Physiol 199681209–224. [DOI] [PubMed] [Google Scholar]
- 20.Lal M K, Manktelow B N, Draper E S.et al Chronic lung disease of prematurity and intrauterine growth retardation: a population‐based study. Pediatrics 2003111483–487. [DOI] [PubMed] [Google Scholar]
- 21.Truffert P, Mailard F, Burguet A.et al BPD and CLD in very preterm infants: incidence in relation to SGA. Pediatr Res 200252797 [Google Scholar]
- 22.Rona R J, Gulliford M C, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ 1993306817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vik T, Vatten L, Markestad T.et al Morbidity during the first year of life in small for gestational age infants. Arch Dis Child Fetal Neonatal Ed 199675F33–F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker D J P, Godfrey K M, Fall C.et al Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991303671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaultier C. Malnutrition and lung growth. Pediatr Pulmonol 199110278–286. [DOI] [PubMed] [Google Scholar]
- 26.Lechner A J. Perinatal age determines the severity of retarded lung development induced by starvation. Am Rev Respir Dis 1985131638–643. [DOI] [PubMed] [Google Scholar]
- 27.Harding J E. Periconceptual nutrition determines the fetal growth response to acute maternal undernutrition in fetal sheep of late gestation. Perinat Neonat Med 19972310–319. [Google Scholar]
- 28.Maloney J E, Bowes G, Brodecky V.et al Function of the future respiratory system in the growth retarded fetal sheep. J Dev Physiol 19824279–297. [PubMed] [Google Scholar]
- 29.Joyce B J, Louey S, Davey M G.et al Compromised respiratory function in postnatal lambs after placental insufficiency and intrauterine growth restriction. Pediatr Res 200150641–649. [DOI] [PubMed] [Google Scholar]
- 30.Ingemarsson I. Gender aspects of preterm birth. Br J Obstet Gynaecol 2003110(suppl 20)34–38. [DOI] [PubMed] [Google Scholar]
- 31.Tyson J E, Kennedy K, Broyles S.et al The small for gestational age infant: accelerated or delayed pulmonary maturation? Increased or decreased survival? Pediatrics 199595534–538. [PubMed] [Google Scholar]
- 32.Askie L M, Henderson‐Smart D J, Irwig L.et al Oxygen‐saturation targets and outcomes in extremely preterm infants. N Engl J Med 2003349953–961. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson J, Donoghue D, Henderson‐Smart D.et al Risk factors for death in very preterm babies of the Australian and New Zealand Neonatal Network. Proceedings of the Perinatal Society of Australia and New Zealand, Sydney 2004
- 34.Darlow B A, Hutchinson J L, Henderson‐Smart D J.et al Prenatal risk factors for severe retinopathy of prematurity in very preterm infants of the Australian and New Zealand Neonatal Network (ANZNN). Pediatrics 2005115990–996. [DOI] [PubMed] [Google Scholar]
- 35.Heuchan A M, Evans N, Henderson Smart D J.et al Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995 to 1997. Arch Dis Child Fetal Neonatal Ed 200286F86–F90. [DOI] [PMC free article] [PubMed] [Google Scholar]