Abstract
Objective
To study the effects of continuous morphine infusion on arterial blood pressure in ventilated neonates.
Design
Blinded randomised placebo controlled trial.
Setting
Level III neonatal intensive care unit in two centres.
Patients
A total of 144 ventilated neonates. Inclusion criteria were postnatal age <3 days, ventilation <8 hours, and indwelling arterial line. Exclusion criteria were severe asphyxia, severe intraventricular haemorrhage, major congenital anomalies, neuromuscular blockers.
Intervention
Arterial blood pressure was measured before the start and during the first 48 hours of masked infusion of drug (morphine/placebo; 100 μg/kg + 10 μg/kg/h).
Outcome measures
Arterial blood pressure and blood pressure variability.
Results
There were no significant differences in overall mean arterial blood pressure between the morphine group (median (interquartile range) 36 mm Hg (6) and the placebo group (38 mm Hg (6)) (p = 0.11). Although significantly more morphine treated patients (70%) showed hypotension than the placebo group (47%) (p = 0.004), the use of volume expanders and vasopressor drugs was not significantly different (morphine group, 44%; placebo group, 48%; p = 0.87), indicating the limited clinical significance of this side effect. Blood pressure variability was not influenced by routine morphine analgesia (p = 0.81) or additional morphine (p = 0.80). Patients with and without intraventricular haemorrhage showed no differences in blood pressure (Mann‐Whitney U test 1953; p = 0.14) or incidence of hypotension (χ2 test 1.16; df 1; p = 0.28).
Conclusions
Overall arterial blood pressure, use of inotropes, and blood pressure variability were not influenced by morphine infusion. Therefore the clinical impact of hypotension as a side effect of low dose morphine treatment in neonates is negligible.
Keywords: randomised controlled trial, opioids, preterm/term infants, hypotension, blood pressure variability
Pain management of neonates admitted to neonatal intensive care units has gained increasing interest over the last few decades. This is reflected by the development of pain assessment tools,1,2,3 consensus statements,4,5 and developmental care strategies.6,7 However, potential serious adverse effects of opioids, such as morphine, have not been properly studied. This may explain the current variation in pain management policies, with limited use of analgesics still in most neonatal intensive care units.8,9,10,11
Hypotensive effects of morphine in the critically ill premature neonate are clearly undesired, However, there is still uncertainty about the amount of morphine needed to exert an effect on blood pressure in preterm neonates. Previous studies on haemodynamic effects of morphine in neonates report conflicting results,12,13,14,15,16,17 and therefore further evaluation is warranted. Suggested benefits from neonatal morphine use are relief of pain and stress13,18,19 and a decreased incidence of poor neurological outcome and intraventricular haemorrhage (IVH).19,20 The latter has been suggested to result from decreased blood pressure variability21,22 caused by morphine, but this has never been properly evaluated.
As part of a blinded, randomised, placebo controlled trial investigating the effects of morphine in ventilated newborns on their pain experience and stress response,20,23 we conducted a separate in depth analysis of the effects of continuous morphine infusion on arterial blood pressure. In this study we tested the hypothesis that continuous morphine infusion would (a) cause hypotension and (b) decrease blood pressure variability.
Methods
Participants
Patients enrolled in this study also participated in a randomised, placebo controlled trial evaluating the analgesic effects of continuous morphine in ventilated neonates.20 In short, all neonates admitted to two level III neonatal intensive care units (Erasmus MC‐Sophia, Rotterdam and Isala Clinics, Zwolle) between December 2000 and October 2002 who required mechanical ventilation were eligible for inclusion if postnatal age was <3 days, intubation and initiation of mechanical ventilation <8 hours, and an indwelling arterial catheter was already in place for clinical purposes. Neonates with severe asphyxia (Apgar score after five minutes <4 or cord blood pH<7),24 severe IVH (grade 3 or IVH + apparent periventricular haemorrhage infarction), major congenital or facial malformations (cleft lip and palate), neurological disorders, or those receiving continuous or intermittent neuromuscular blockers were excluded. The local ethics committees of the participating centres approved the study protocol.
Procedure/intervention
After parents of eligible patients had given written informed consent, patients were randomly allocated to receive a loading dose (100 μg/kg) followed by a continuous infusion (10 μg/kg/h) of either morphine (morphine hydrochloride) or placebo (saline/NaCl), both dissolved in 5% glucose. The morphine doses used in this study are in line with the international recommended doses,5 and were the standard ones used in both centres before the start of this trial. No loading dose was given if a pre‐intubation morphine loading dose had been administered less than three hours before. Masked study drugs were continued for a maximum of seven days. If the attending doctor judged patients from either group to be in pain or distress, doses of 50 μg/kg followed by 5–10 μg/kg/h continuous additional “open label” morphine were administered.
After insertion of an indwelling arterial catheter, baseline mean arterial blood pressure (MAP) and systolic and diastolic blood pressure were determined every two hours before the start of the study medication. After the start, arterial blood pressures (again MAP and systolic and diastolic) were measured continuously and recorded every two hours. This was continued until 48 hours after the start of study medication. During this period, we collected data on all variables likely to influence blood pressure, such as volume expansion or inotropic support, and background characteristics. The clinical risk index for babies (CRIB) was used as a measure of severity of illness.25
Arterial blood pressure was measured by peripheral (Quick‐Cath 24 Ga, 16 mm; Baxter, Deerfield, Illinois, USA) or umbilical (3.5–5.0 French, 40 cm; Vygon, Brussels, Belgium) arterial catheters, using a disposable blood pressure system (Gabarith PMSET, Becton‐Dickinson, Franklin Lakes, New Jersey, USA) and 1DT‐XX blood pressure transducer.
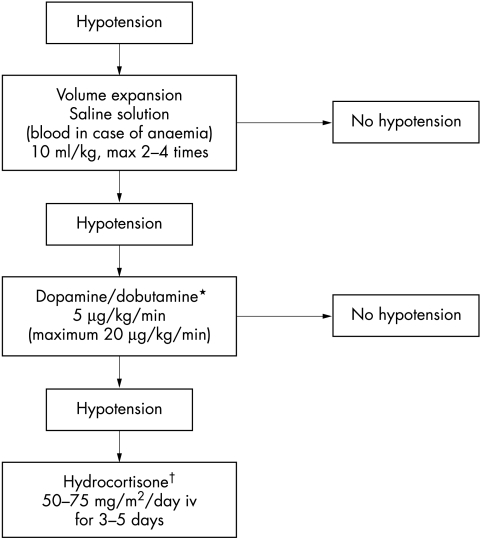
During the study, the attending physicians defined hypotension using a normative data model for different birth weights26 and, if necessary, applied inotropic therapy using a standardised algorithm (fig 1).
Figure 1 Schedule used in the case of hypotension, showing consecutive steps of treatment. *In term neonates with asphyxia, congenital heart disease, or other illnesses characterised by diminished myocardial contractility, dobutamine is the drug of choice to treat hypotension. Dopamine is used if dobutamine fails. In all other infants, dopamine is given before dobutamine. †In term neonates, noradrenaline (norepinephrine; 0.1–1.0 μg/kg/min) is used before hydrocortisone in the case of persistent hypotension.
Outcomes
Primary outcome was the hypotensive effect of morphine. Hypotension was determined by comparing: (a) overall arterial blood pressures (MAP, diastolic, and systolic); (b) administration of volume expanders and vasopressor drugs; (c) number of infants with hypotensive MAP measurements, as compared with “normal” values of blood pressure.
Normal values of MAP were calculated using an equation based on previous MAP modelling studies26,27:
MAP (mm Hg) = 29.80 + (5.16 × body weight (kg)) + (0.12 × postnatal age (hours))
Body weight was estimated from the birth weight. Hypotension was defined as a MAP below the lower value of the 95% confidence interval (MAP ± 9 mm Hg).26
Secondary outcome was variability in arterial blood pressure, defined as the interquartile range of ΔMAP (differences between each two consecutive MAP recordings) per patient. To determine the clinical significance of the haemodynamic effect of morphine, its relation to the development of IVH was analysed.
Sample size, randomisation, and blinding
A power analysis showed that 70 patients per group was needed to achieve a medium effect size (d = 0.55), with an α of 0.05, two sided, and a power of 90%.
Neonates had an equal probability of being assigned to either treatment group and were stratified into five gestational age groups (<27 weeks, 27–306 weeks, 31–336 weeks, 34–366 weeks, and ⩾37 weeks) to obtain a balanced number of morphine and placebo participants within each stratum. All research and clinical staff, as well as the parents of the participants, were blinded to the treatment.
Statistical analysis
All infant data were analysed using the intention to treat principle. Outcomes were compared between the two treatment groups using χ2 tests (Yates corrected), Fisher's exact tests, and non‐parametric Mann‐Whitney U tests. Summary statistics (mean blood pressure values for each patient) were used to take repeated measures into account and to increase reliability. Multiple regression analysis was used with MAP variability as outcome variable, predicted by treatment group and the amount of additional open label morphine used, and with centre, CRIB score, sex, the use of volume expansion or inotropic therapy, gestational age, and deviation from mean birth weight as covariates. The model was checked on collinearity and overfitting. All data were analysed using SPSS version 10.1.
Results
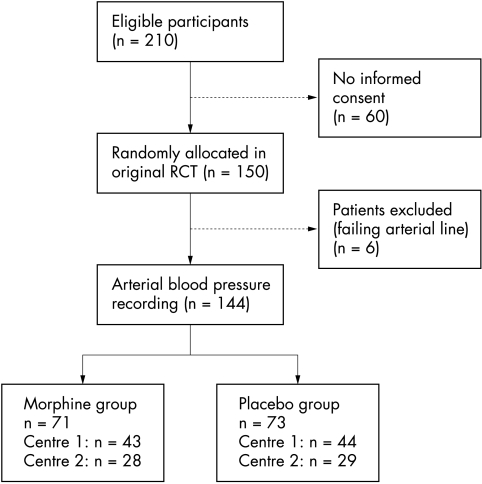
Data for 144 patients were included in the analyses (fig 2). Of these patients, 71 were allocated to receive continuous morphine infusion, and 73 to receive placebo. Median duration of infusion was 52 hours (25th–75th centile 21–97). It was discontinued for the following reasons: extubation (n = 102); seven days into the study (n = 24); death (n = 4); continuous neuromuscular blockers (n = 3); overdose (n = 1); surgery (n = 2); too much additional morphine was needed (n = 2). Table 1 lists the background characteristics for the morphine and placebo group. During blood pressure recordings, 15 morphine treated patients (21%) and 20 placebo treated patients (27%) received additional open label morphine (χ2 test 0.77; df 1; p = 0.38). The median (25th–75th centile) overall morphine dose (masked + open label) was 12.4 (11.0–16.1) and 3.3 (0–6.7) μg/kg/h in the morphine and placebo groups respectively.
Figure 2 Flow chart showing selection of patients for analysis. RCT, Randomised controlled trial.
Table 1 Background characteristics, blood pressures, and inotropic therapy for the morphine and placebo groups.
| Variable | Morphine (n = 71) | Placebo (n = 73) | p Value |
|---|---|---|---|
| Background characteristics | |||
| Sex (boys/girls) | 40/31 | 43/30 | |
| In/outborn | 55/16 | 52/21 | |
| Gestational age (weeks) | 29.0 (27.4–31.8) | 29.1 (27.3–31.3) | |
| Birth weight (g) | 1100 (835–1655) | 1215 (910–1511) | |
| Postnatal age (h) | 9 (5–13) | 8 (5–12) | |
| Apgar 1 min | 6 (4–8) | 6 (4–8) | |
| Apgar 5 min | 8 (7–9) | 8 (7–9) | |
| CRIB | 2 (1–6) | 3 (1–7) | |
| Duration of infusion (h) | 55 (23–97) | 46 (18–101) | |
| Patients receiving additional open label morphine | 21% | 27% | |
| Blood pressure | |||
| Baseline | |||
| Systolic | 43 (40–51) | 43 (39–48) | 0.43 |
| MAP | 33 (31–40) | 34 (30–37) | 0.43 |
| Diastolic | 27 (24–32) | 28 (23–32) | 0.67 |
| During study | |||
| Systolic | 44 (42–50) | 46 (43–51) | 0.22 |
| MAP | 36 (33–39) | 38 (35–41) | 0.11 |
| Diastolic | 28 (26–32) | 30 (27–34) | 0.06 |
| Inotropic therapy | |||
| Plasma expanders | 20% | 23% | |
| Dopamine | 3% | 5% | |
| Plasma expanders + dopamine | 17% | 14% | |
| Dopamine + dobutamine | 1% | 0% | |
| Plasma expanders + dopamine + dobutamine | 1% | 4% | |
| Hydrocortisone | 1% | 0% | |
| Noradrenaline | 0% | 1% | |
| Total | 44% | 48% | 0.87* |
Where applicable, values are median (25th–75th centile). Unless indicated otherwise, p values were calculated using the Mann‐Whitney U test (two sided). No significant differences were found between the background characteristics of the two groups.
*Calculated using the χ2 test.
CRIB, Clinical risk index for babies.
Hypotensive effect of morphine
Blood pressure data were collected over a median of 46 hours (25–75th centile 32–54) per infant. Neither the baseline nor study values (mean values per patient) of MAP, systolic and diastolic blood pressure differed significantly between the two groups (table 1). In six patients, the attending physicians had the impression that the masked study medication was morphine and was probably causing hypotension and for this reason they stopped the infusion. Two of these patients turned out to be receiving placebo (afterwards hypotension calculated during 0% and 7% of the study), and four had been receiving morphine (hypotension measured during 25%, 52%, 64%, and 80% of the study).
During the infusion, 44% of the morphine treated patients and 48% of those receiving placebo were also given plasma expanders and/or pharmacological treatment of hypotension (table 1). There was no significant difference in antihypotensive treatments between the two groups (χ2 test 2.46; df 6; p = 0.87). Hypotension was measured in 50 morphine treated patients (70%) compared with 34 patients (47%) of the placebo group (χ2 test 8.42; df 1; p = 0.004). However, the overall median MAP did not differ between these morphine (34 mm Hg; interquartile range 4.5) and placebo (34 mm Hg; interquartile range 6.0) treated infants (Mann‐Whitney U test 830; p = 0.88).
Taking the use of additional morphine into consideration, 70% (14/20) of the placebo treated infants who received additional morphine compared with 38% (20/53) who did not showed hypotension (Fisher's exact test: p = 0.018). In comparison, 80% (12/15) of the infants treated with morphine plus additional morphine showed hypotension versus 68% (38/56) of those treated with morphine who did not require additional morphine (Fisher's exact test: p = 0.53).
Blood pressure variability
Variability (median (25th–75th centile)) in MAP during infusion was 5.5 (4.5–7.0) mm Hg in the morphine group and 5.0 (4.0–7.0) mm Hg in the placebo group. Multiple regression analyses (table 2) revealed that variability in blood pressure was not predicted by treatment group (B = 0.094; 95% confidence interval (CI) −0.67 to 0.85; p = 0.81), nor by the amount of additional open label morphine used (B = 0.019; 95% CI −0.12 to 0.16; p = 0.80) or by gestational age (B = 0.0090; 95% CI −0.008 to 0.026; p = 0.30). The use of inotropic therapy significantly predicted higher blood pressure variability (B = 0.90; 95% CI −0.095 to1.70; p = 0.03). No significant difference in the incidence of large MAP changes (⩾5 mm Hg within one hour) occurred between patients in the morphine and placebo group (45.1% and 34.2% respectively; χ2 test 1.76; df 1; p = 0.184) or between patients with or without additional administration of morphine (48.0% and 35.1% respectively; χ2 test 2.27; df 1; p = 0.132).
Table 2 Results of multiple regression analyses with variability in mean arterial blood pressure as outcome variable.
| IQR ΔMAP (mm Hg) | |||
|---|---|---|---|
| B | 95% CI of B | p Value | |
| Treatment group | 0.094 | −0.67 to 0.85 | 0.81 |
| Amount of open label morphine | 0.019 | −0.12 to 0.16 | 0.80 |
| Centre | 0.25 | 0.60 to 1.10 | 0.56 |
| CRIB score | 0.068 | −0.076 to 0.21 | 0.35 |
| Sex | −0.094 | −0.88 to 0.69 | 0.81 |
| Use of inotropics | 0.90 | 0.095 to 1.70 | 0.03 |
| Gestational age | 0.0090 | −0.008 to 0.026 | 0.30 |
| Developmental mean birth weight* | 0.033 | −0.32 to 0.25 | 0.82 |
IQR ΔMAP, Interquartile range of ΔMAP (difference between two consecutive mean arterial blood pressure recordings), used as a measure of blood pressure variability; B, unstandardised regression coefficients; CRIB, clinical risk index for babies.
*Birth weights were compared with normal values of birth weight for each infant's gestational age.
Clinical significance
In a previous report of the original trial in which the present study was embedded, we showed a significantly lower incidence of IVH in the morphine group compared with the placebo group.20 In the current analyses, blood pressure did not differ significantly between patients with IVH and those without (Mann‐Whitney U tests 1953; p = 0.14), and the incidence of IVH was not higher in patients with hypotension than in patients without hypotension (χ2 test 1.16; df 1; p = 0.28). As neonates with and without IVH showed no significant difference in blood pressure variability (Mann‐Whitney U test 2950; p = 0.51), a relation between blood pressure variability and the development of IVH could not be demonstrated.
Discussion
This study aimed to determine the effects of continuous morphine infusion on blood pressure in ventilated newborn infants. No significant differences in overall blood pressure or use of volume expanders and vasopressor drugs between the morphine and placebo treated infants were found.
Attending physicians discontinued the masked medication in six patients (4%) because they assumed, despite being blinded to the treatment that these patients were receiving, that “morphine” infusion was the cause of low blood pressure. Two of these patients had been receiving placebo infusions only, but the other four were receiving morphine. This illustrates the concerns and uncertainty of clinicians about the potential adverse effects of morphine on blood pressure in neonates.
One of the major limitations of our study, as well as of previous studies investigating haemodynamic effects of morphine in newborns, is that effects of prolonged morphine use on blood pressure were not studied. As the existing models for calculating “normal blood pressure” seem to be reliable during the first postnatal days only, we analysed blood pressures for the first 48 hours. Another limitation of our study is that blood pressure data were analysed in two hour time intervals. Blood pressure was measured using indwelling arterial lines, and monitors immediately sounded an alarm if hypotension occurred, giving the attending clinicians the opportunity to treat it. However, the blood pressures analysed seem to realistically reflect actual continuous blood pressures. This is confirmed by the fact that, like the blood pressure data, the use of vasopressors/inotropic agents did not differ between the two treatment groups.
Previous small studies, which are difficult to compare because of different settings and morphine dosage regimens, have produced conflicting results.12,13,15,16,17 In contrast with our data, the NEOPAIN trial of Anand et al28 showed a significant increase in intravenous vasopressors and fluid boluses after morphine boluses and after the first 24 hours of morphine infusion compared with placebo. This difference may be explained by the higher morphine doses (up to 30 μg/kg/h) used in some gestational age groups of the NEOPAIN trial. In analogy, the use of additional morphine was related to an increased incidence of hypotension in our study, although this was not significant in the morphine treated infants. Obviously, there appears to be a dose‐response relation between the amount of morphine used and the incidence of hypotension. Extrapolation of these data therefore suggests that, if higher morphine doses are used, more frequent periods of hypotension can be expected. The significant effect of morphine on blood pressure can be further explained by a difference in the populations studied in the two studies—for instance, a difference in gestational ages—and a different definition of hypotension. Furthermore, as the NEOPAIN trial included a larger study sample (898 patients), it may have been able to detect a smaller decrease in blood pressure than our study.
In the individual neonatal patient, however, even the low morphine doses used in our study may well exert hypotensive effects. Seventy percent of the morphine treated infants showed hypotension for at least some of the time compared with 47% of the placebo treated infants (p = 0.004), and the use of additional open label morphine in the placebo group was also related to hypotension. Although overall blood pressures were comparable, this indicates that hypotension probably occurs for limited periods of time in morphine treated neonates. As the use of volume expanders and vasopressor drugs was not increased in the morphine group, the hypotension induced by morphine did not appear to affect the haemodynamic stability of these infants. The clinicians who prescribed these volume expanders and vasopressor drugs were not able to detect the increased frequency of hypotension or decided that no treatment was necessary for this decrease in blood pressure. The latter is more likely because the hypotension was minimal or self limiting, resulting in higher blood pressures before the start of treatment. Why this difference between the incidence of hypotension found by the researchers and treatment by clinicians was not found in the placebo group remains unclear. In a previous report,20 we have already shown no negative consequences of morphine on clinical outcome measures, such as length of stay in intensive care, duration of ventilation, or incidence of secondary morbidity. We conclude therefore that major clinical consequences of the increased incidence of hypotension are not very likely, and that the morphine doses used in this study are safe for ventilated neonates.
What is already known on this topic
Hypotension is a very undesirable side effect of morphine
Previous studies, often using high morphine doses, have produced conflicting results on the haemodynamic effects of morphine in newborn infants
As we previously showed a significant reduction in IVH in morphine treated newborns,20 in this study we analysed if this reduction could be explained by changes in blood pressure. Hypotension and alterations in blood pressure have been associated with variability in cerebral blood flow (CBF).29,30 However, we found no relation between blood pressure or blood pressure variability and the incidence of IVH. This is confirmed by the results of a study from Hunt et al,31 who showed that abnormal developmental outcome was not related to arterial blood pressure. Arterial blood pressure is not a good surrogate measure for superior vena cava flow or CBF, which seem to be more sensitive markers of cerebral haemodynamic pathology.32 Hypoperfusion‐reperfusion and associated postnatal alterations in CBF have been implicated in the pathogenesis of IVH.21,33,34 This study, to our knowledge, provides the first data on the effect of continuous morphine infusion on blood pressure variability in neonates. As no relation was found, future research should focus on the association between morphine and CBF, which may explain the protective effect of morphine against IVH.
In summary, with this randomised, placebo controlled trial, we were not able to link the protective effect of morphine on the development of IVH with changes in blood pressure. Although hypotension was suggested to be a side effect of morphine, the importance of this hypotension in clinical practice is probably minimal, as overall blood pressures as well as the use of volume expanders and vasopressor drugs were not significantly different between the morphine and placebo treated infants. Furthermore, morphine treatment was not related to negative clinical outcome. Follow up of our patients is, however, necessary to study the effects of morphine treatment during the neonatal period on the long term perspective.
What this study adds
Recommended morphine doses for pain management have no major clinical consequences on neonatal arterial blood pressure; whereas significantly more morphine treated neonates showed hypotension during the study, the use of volume expanders and vasopressor drugs was comparable with that in the placebo group
No relation between morphine use, arterial blood pressure, and intraventricular haemorrhage could be determined
Acknowledgements
This work was supported by a grant (MW‐NWO 940‐31‐048) from the Netherlands Organization for Scientific Research (NWO, The Hague). We thank N Jongeneel, RN, for helping with the SPSS data files, and J Hagoort for his help in preparing the manuscript.
Abbreviations
CBF - cerebral blood flow
IVH - intraventricular haemorrhage
MAP - mean arterial blood pressure
Footnotes
Competing interests: none declared
References
- 1.Lawrence J, Alcock D, McGrath P.et al The development of a tool to assess neonatal pain. Neonatal Netw 19931259–66. [PubMed] [Google Scholar]
- 2.Stevens B, Johnston C, Petryshen P.et al Premature Infant Pain Profile: development and initial validation. Clin J Pain 19961213–22. [DOI] [PubMed] [Google Scholar]
- 3.van Dijk M, de Boer J B, Koot H M.et al The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3‐year‐old infants. Pain 200084367–377. [DOI] [PubMed] [Google Scholar]
- 4.Prevention and management of pain and stress in the neonate American Academy of Pediatrics. Committee on Fetus and Newborn. Committee on Drugs. Section on Anesthesiology. Section on Surgery. Canadian Paediatric Society. Fetus and Newborn Committee. Pediatrics 2000105454–461. [PubMed] [Google Scholar]
- 5.Anand K J S. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med 2001155173–180. [DOI] [PubMed] [Google Scholar]
- 6.Als H. A synactive model of neonatal behavioral organization. Phys Occup Ther Pediatr 198663–55. [Google Scholar]
- 7.Als H. Developmental care in the newborn intensive care unit. Curr Opin Pediatr 199810138–142. [DOI] [PubMed] [Google Scholar]
- 8.Johnston C C, Collinge J M, Henderson S J.et al A cross‐sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. Clin J Pain 199713308–312. [DOI] [PubMed] [Google Scholar]
- 9.Kahn D J, Richardson D K, Gray J E.et al Variation among neonatal intensive care units in narcotic administration. Arch Pediatr Adolesc Med 1998152844–851. [DOI] [PubMed] [Google Scholar]
- 10.Debillon T, Bureau V, Savagner C.et al Pain management in French neonatal intensive care units. Acta Paediatr 200291822–826. [DOI] [PubMed] [Google Scholar]
- 11.Simons S H P, van Dijk M, Anand K J S.et al Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med 20031571058–1064. [DOI] [PubMed] [Google Scholar]
- 12.Wood C M, Rushforth J A, Hartley R.et al Randomised double blind trial of morphine versus diamorphine for sedation of preterm neonates. Arch Dis Child Fetal Neonatal Ed 199879F34–F39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn M W, Wild J, Dean H G.et al Randomised double‐blind controlled trial of effect of morphine on catecholamine concentrations in ventilated pre‐term babies. Lancet 1993342324–327. [DOI] [PubMed] [Google Scholar]
- 14.Hartley R, Green M, Quinn M.et al Pharmacokinetics of morphine infusion in premature neonates. Arch Dis Child 199369(1 spec no)55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabatino G, Quartulli L, Di Fabio S.et al Hemodynamic effects of intravenous morphine infusion in ventilated preterm babies. Early Hum Dev 199747263–270. [DOI] [PubMed] [Google Scholar]
- 16.Dyke M P, Kohan R, Evans S. Morphine increases synchronous ventilation in preterm infants. J Paediatr Child Health 199531176–179. [DOI] [PubMed] [Google Scholar]
- 17.Rutter N, Evans N. Cardiovascular effects of an intravenous bolus of morphine in the ventilated preterm infant. Arch Dis Child Fetal Neonatal Ed 200083F101–F103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott C S, Riggs K W, Ling E W.et al Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr 1999135423–429. [DOI] [PubMed] [Google Scholar]
- 19.Anand K J S, Barton B A, McIntosh N.et al Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med 1999153331–338. [DOI] [PubMed] [Google Scholar]
- 20.Simons S H P, van Dijk M, van Lingen R A.et al Routine morphine infusion in preterm neonates who received ventilatory support: a randomized controlled trial. JAMA 20032902419–2427. [DOI] [PubMed] [Google Scholar]
- 21.Perlman J M, McMenamin J B, Volpe J J. Fluctuating cerebral blood‐flow velocity in respiratory‐distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med 1983309204–209. [DOI] [PubMed] [Google Scholar]
- 22.Ghazi‐Birry H S, Brown W R, Moody D M.et al Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am J Neuroradiol 199718219–229. [PMC free article] [PubMed] [Google Scholar]
- 23.Simons S H P, van Dijk M, van Lingen R A.et al Randomised‐controlled‐trial evaluating effects of morphine on (nor)adrenaline plasma‐concentrations in newborns. Arch Dis Child Fetal Neonatal Ed 200590F36–F40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Committee on Fetus and Newborn, American Academy of Pediatrics, and Committee on Obstetric Practice, American College of Obstetricians and Gynecologists Use and abuse of the Apgar score. Pediatrics 199698141–142. [PubMed] [Google Scholar]
- 25.The International Neonatal Network The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet 1993342193–198. [PubMed] [Google Scholar]
- 26.Versmold H T, Kitterman J A, Phibbs R H.et al Aortic blood pressure during the first 12 hours of life in infants with birth weight 610 to 4,220 grams. Pediatrics 198167607–613. [PubMed] [Google Scholar]
- 27.LeFlore J L, Engle W D, Rosenfeld C R. Determinants of blood pressure in very low birth weight neonates: lack of effect of antenatal steroids. Early Hum Dev 20005937–50. [DOI] [PubMed] [Google Scholar]
- 28.Anand K J, Hall R W, Desai N.et al Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 20043631673–1682. [DOI] [PubMed] [Google Scholar]
- 29.Bada H S, Korones S B, Perry E H.et al Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. J Pediatr 1990117607–614. [DOI] [PubMed] [Google Scholar]
- 30.Coughtrey H, Rennie J M, Evans D H. Variability in cerebral blood flow velocity: observations over one minute in preterm babies. Early Hum Dev 19974763–70. [DOI] [PubMed] [Google Scholar]
- 31.Hunt R W, Evans N, Rieger I.et al Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr 2004145588–592. [DOI] [PubMed] [Google Scholar]
- 32.Seri I. Low superior vena cava flow during the first postnatal day and neurodevelopment in preterm neonates. J Pediatr 2004145573–575. [DOI] [PubMed] [Google Scholar]
- 33.Volpe J J. Neurologic outcome of prematurity. Arch Neurol 199855297–300. [DOI] [PubMed] [Google Scholar]
- 34.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed 200082F188–F194. [DOI] [PMC free article] [PubMed] [Google Scholar]