Abstract
Objectives
Several studies have shown the efficacy of dilutional exchange transfusion (DET) in reducing haematocrit (Ht) and relieving clinical symptoms in neonatal polycythaemia. We conducted a systematic review to determine the efficacy of crystalloid versus colloid solutions used in DET in an effort to identify the best solution to replace red blood cells.
Methods
The Cochrane Library, MEDLINE, and EMBASE were searched for relevant randomised controlled trials. Quality assessment and data analysis were performed using the methods and software of the Cochrane Collaboration. Relative risk (RR) and weighted mean difference (WMD) were calculated as measures of effect for categorical and continuous outcome data, respectively. Ninety five percent confidence intervals (95% CI) were calculated and a fixed effect model was used for meta‐analysis.
Results
Six studies with a total of 235 newborns matched our inclusion criteria. When comparing crystalloid and colloid replacement solutions for DET, there was a clinically unimportant difference in Ht at 2–6 h and at 24 h in favour of colloidal solutions (WMD 2.29% (95% CI 1.28 to 3.31) and 1.74% (95% CI 0.80 to 2.68), respectively). This difference in post DET Ht was more evident when normal saline was compared to plasma but absent when normal saline was compared to 5% albumin.
Conclusion
There is little difference in effectiveness between plasma, 5% albumin, and crystalloid solutions. Since normal saline is cheap, readily available, and does not carry the potential risk of transfusion associated infection, normal saline is the optimal dilutional fluid for exchange transfusion in polycythaemic neonates.
Keywords: blood viscosity, infant, newborn, plasma exchange, polycythaemia, systematic review
Polycythaemia (PC) is a condition in which an increased red cell mass leads to increased viscosity of the blood. The most commonly used clinical definition of PC is a venous haematocrit greater than 65%.1 Hyperviscosity refers to an increase in internal friction of blood and is defined as a blood viscosity value greater than 2 standard deviations above the mean for the population of interest.2
Symptoms of PC can be mild to severe and may include plethora, lethargy, poor feeding, jitteriness, hypoglycaemia, tachypnea, and cyanosis. Untreated PC has been associated with severe complications such as cerebrovascular events, necrotising enterocolitis, and developmental delay.3,4,5
A partial or dilutional exchange transfusion (DET) is recommended for treatment of symptomatic neonatal PC.6,7 In addition, neonatal textbooks recommend a DET if a haematocrit value greater than 70% is present without clinical symptoms.6,7 Current practice reflects these recommendations, but few high quality trials supporting this have been reported.8
The efficacy of DET in reducing haematocrit and relieving clinical symptoms has been shown in several studies.10,11 However, there is uncertainty about what solution should be used to replace the excess red blood cells. We carried out a systematic review using the methods and software of the Cochrane Collaboration to determine the efficacy of crystalloid solutions versus colloid solutions used in DET for reducing the haematocrit and relieving clinical symptoms.
Methods
Search strategy
A computerised literature search was conducted independently by two reviewers (KW, WB) of the National Library of Medicine's MEDLINE from 1966 to May 2003, the Cochrane Controlled Trial Register, and EMBASE using the following search terms: (polycythaemia OR blood viscosity OR hyperviscosity OR exchange transfusion) AND (infant, newborn) combined with a sensitive filter for retrieval of reports of controlled trials.9 No language restrictions were applied. Bibliographies of all selected articles and review articles which included information on the topic were reviewed for other relevant articles. Abstracts from the Society for Pediatric Research and the European Society for Pediatric Research from 1989 to 2002 were also searched.
Inclusion criteria and outcome measures
To be included in the review, articles had to fulfil the following selection criteria: the study had to be a randomised controlled trial comparing the use of crystalloid with colloid solutions, in which the subjects were assigned prospectively to one or two (or more) interventions by random allocation or some quasi‐random method of allocation (for example, alternating); the participants had to be term or preterm newborn infants up to 28 days of age; outcome measures had to include a pre‐ and post‐DET haematocrit, our primary outcome; and there had to be a report on the relief of PC related clinical symptoms and/or complications of the procedure. Disagreement about inclusion was resolved by discussion.
Quality assessment and data abstraction
The quality of each article included in the review was assessed independently by two reviewers (KW, WB) in terms of the presence of treatment allocation concealment, blinding of carers and assessors to intervention, completeness of assessment of all randomised individuals, and blinded outcome measurement. Two reviewers extracted the data from each trial (KW, WB).
Data analysis
Review Manager software developed by the Cochrane Collaboration (version 4.2) was used for data management and analysis. The relative risk (RR) and weighted mean difference (WMD) were calculated for categorical and continuous data, respectively. Ninety five percent confidence intervals (95% CI) were calculated and a fixed effect model was used for meta‐analysis.
Results
Description of selected studies and quality assessment
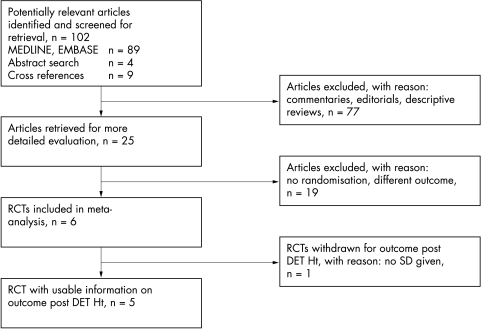
Figure 1 summarises the trial flow. Eighty nine studies were identified using the computerised search. Commentaries, descriptive reviews, and clinical trials that did not comply with our review questions were excluded, leaving 12 studies to be retrieved for more detailed evaluation. A further nine studies were identified from the cross references of selected articles and another four potentially relevant abstracts were found through a search of the abstracts from the Society for Pediatric Research and the European Society for Pediatric Research. Nineteen of these 25 papers were excluded according to the pre‐defined criteria, leaving six randomised trials with a total of 235 newborns that fulfilled the inclusion criteria.10,11,12,13,14,15 Jan et al did not provide standard deviations for the mean on the post‐DET Ht outcome, and as a consequence the results on this outcome cannot be weighted in the meta‐analyses. However, these authors did provide information on relief of clinical symptoms and complications of the procedure.
Figure 1 Trial flow characteristics. DET, dilutional exchange transfusion; Ht, haematocrit; SD, standard deviation; RTCs, randomised clinical trials.
The DET procedure was adequately described in five of the six studies and all studies used the same calculation to determine the exchange volume needed. Table 1 shows clinical details of included studies.
Table 1 Characteristics of included studies.
| Study | n | Participants | Interventions | Procedures |
|---|---|---|---|---|
| Tapia12* | 29 | Polycythaemic newborns | Plasma v 5% albumin v normal saline | Unknown route and desired Ht |
| Deorari13 | 30 | Venous Ht >65% with symptoms after | Plasma v normal saline | 22 umbilical, 8 peripheral, |
| screening high risk newborns | desired Ht 55% | |||
| Roithmaier10 | 20 | Venous Ht >65% with symptoms after | Plasma v Ringer solution | 20 umbilical, desired Ht 55% |
| screening cord blood | ||||
| Wong11 | 103 | Venous Ht ⩾65% with symptoms or | 5% albumin v normal saline | 16 umbilical, 87 peripheral, |
| venous Ht ⩾70% | desired Ht 55% | |||
| Krishnan14 | 27 | Venous Ht >65% with symptoms or | Plasma v normal saline | 27 peripheral, desired Ht 55%, |
| venous Ht >70% after screening all newborns | capillary post DET Ht | |||
| Jan15 | 26 | Venous Ht ⩾65% with symptoms after | Plasma v normal saline | Umbilical and peripheral, |
| screening all newborns | desired Ht 55% |
*Abstract only.
DET, dilutional exchange transfusion; Ht, haematocrit; n, number of patients participating.
The exact method of randomisation was described in only two of the six studies10,11 and no study reported on blinding of the intervention. In none of the six studies was it possible to determine whether the caregivers and those assessing the outcomes of interest were blinded to the treatment allocation. There was complete short term follow up of all randomised infants in the included studies. No long term follow up data were presented in any of the studies.
Quantitative data synthesis
The pooled results of the different trials are presented in tables 2 and 3.
Table 2 Quantitative data synthesis, post DET Ht.
| Outcome | Studies* | Crystalloid† | Colloid† | WMD (95% CI) |
|---|---|---|---|---|
| Crystalloid v colloid (all) | ||||
| 2–6 h post DET Ht | 5 | 115 | 111 | 2.29 (1.28 to 3.31) |
| 24 h post DET Ht | 5 | 115 | 120 | 1.74 (0.80 to 2.68) |
| Normal saline v plasma | ||||
| 2–6 h post DET Ht | 4 | 62 | 61 | 2.34 (0.94 to 3.73) |
| 24 h post DET Ht | 4 | 62 | 60 | 2.81 (1.74 to 3.89) |
| Normal saline v 5% albumin | ||||
| 2–6 h post DET Ht | 1 | 53 | 50 | 1.00 (−1.14 to 3.14) |
| 24 h post DET Ht | 2 | 63 | 60 | −0.35 (−2.06 to 1.35) |
*Number of studies reporting on outcome; †number of patients in group.
95% CI, 95% confidence interval; DET, dilutional exchange transfusion; Ht, haematocrit; WMD, weighted mean difference.
Table 3 Quantitative data synthesis, clinical symptoms, and complications.
| Outcome | n* | Crystalloid† | Colloid† | RR (95% CI) |
|---|---|---|---|---|
| Persistent jitteriness | 2 | 3/17 | 4/21 | 1.11 (0.27 to 4.60) |
| Persistent hypoglycaemia | 2 | 9/29 | 2/26 | 3.12 (0.91 to 10.65) |
| Persistent GE problems | 2 | 4/25 | 4/29 | 1.30 (0.41 to 4.16) |
| Second DET | 5 | 1/115 | 0/111 | Not estimable |
| Complications of procedure | 4 | 0/105 | 0/101 | Not estimable |
*Number of studies reporting on outcome; †number of patients with outcome/total number of patients.
95% CI, 95% confidence interval; DET, dilutional exchange transfusion; GE, gastro‐intestinal; RR, relative risk.
When comparing crystalloid and colloid replacement solutions, there was a clinically unimportant difference in Ht at 2–6 h and 24 h after the DET in favour of colloidal solutions (WMD 2.29% (95% CI 1.28 to 3.31) and 1.74% (95% CI 0.80 to 2.68), respectively). This difference in post DET Ht was slightly more evident when normal saline was compared to plasma but was not present if normal saline was compared to 5% albumin. There was evidence of statistical heterogeneity of treatment effect in these meta‐analyses.
Symptoms before and after the DET procedure were reported in three studies; however, “relief of symptoms” was not well defined in these studies. No differences in incidence of persistent jitteriness, persistent hypoglycaemia, or persistent gastro‐intestinal problems were indicated. In a total of 226 polycythaemic patients, only one repeat DET was necessary in a newborn in the saline group. No procedural complications were reported in any of the studies.
Two studies reported on viscosity, but pooling of these data was not feasible due to the different laboratory methods used. Both studies showed no significant differences in post‐DET viscosity.
Discussion
The results of this review indicate that a DET is effective in reducing the haematocrit in polycythaemic newborns. Colloid solutions are more effective in reducing the haematocrit than crystalloid solutions, with a mean difference of 2.29% at 2–6 h post DET. Plasma appears to be most effective in reducing haematocrit, followed by normal saline, then albumin. Higher loss to the extravascular space of crystalloid solutions as compared to plasma is the probable explanation for these findings.10
The small differences in post‐DET haematocrits between children treated with crystalloids and colloids seem to be of no clinical significance. We found no evidence that the proportion of patients relieved of clinical symptoms and the number of patients needing a repeat DET were different between treatment groups. It should be noted, however, that “relief of symptoms” was not well defined in the three studies reporting on this parameter and no clinical data were reported at all in the three remaining studies. One can only assume that relief of clinical symptoms was adequate in the latter, because no repeat DETs were necessary.
We found evidence of statistical heterogeneity of treatment effect in the meta‐analyses. This could be caused by methodological imperfections in several included studies, by differences in the selection of participants for the studies, or by differences in the DET solutions used. Allocation concealment was adequate in only two studies and blinding of carers and assessors of clinical outcome was not performed in any of the studies. The latter potential source of bias pertains to the scoring of relief of symptoms only, as a reading of Ht cannot be biased by knowledge of treatment allocation. There was no major clinical diversity between the studies. The proportion of small for gestational age patients in each treatment group was reported in four studies and did not differ significantly. Tapia et al did not provide a definition for PC, but mean pre‐DET haematocrit in this study was higher than 70%.12 We feel that pooling of the selected studies' results is justified despite these differences.
As a last point, viscosity data were reported in only two studies. For practical purposes, indications for DET and evaluation of outcome of the procedure were based on haematocrit values in most studies. The question remains whether haematocrit or viscosity is most accurate in the assessment of the severity of polycythaemia and of the efficacy and safety of alternative dilutional fluids. This question can only be answered in prospective studies involving both haematocrit and viscosity measurements, in combination with standardised documentation of clinical symptoms
Conclusion
Dilutional exchange transfusion is effective in reducing haematocrit and in relieving clinical symptoms related to PC. There is no clinically important difference among plasma, 5% albumin, normal saline, or Ringers solution in reducing haematocrit. Since normal saline is cheap, readily available, and does not carry the potential risk of transfusion associated infection, normal saline is the optimal fluid for dilutional exchange transfusion in polycythaemic neonates.
What is already know about this topic
A partial or dilutional exchange transfusion is recommended for treatment of symptomatic neonatal polycythaemia
The efficacy of dilutional exchange transfusion in reducing haematocrit and relieving clinical symptoms has been shown
What this study adds
There is no clinically important difference among plasma, 5% albumin, normal saline, or Ringers solution when used in a dilutional exchange transfusion in reducing haematocrit
Normal saline is the optimal fluid for dilutional exchange transfusion in polycythaemic neonates
Abbreviations
95% CI - 95% confidence interval
DET - dilutional exchange transfusion
Ht - haematocrit
PC - polycythaemia
RR - relative risk
WMD - weighted mean difference
Footnotes
Competing interests: none declared
References
- 1.Werner E J. Neonatal polycythemia and hyperviscosity. Clin Perinatol 199522(3)693–710. [PubMed] [Google Scholar]
- 2.Wirth F H, Goldberg K E, Lubchenco L O. Neonatal hyperviscosity: I. Incidence. Pediatrics 197963(6)833–836. [PubMed] [Google Scholar]
- 3.Amit M, Camfield P R. Neonatal polycythemia causing multiple cerebral infarcts. Arch Neurol 198037(2)109–110. [DOI] [PubMed] [Google Scholar]
- 4.Kliegman R M, Fanaroff A A. Neonatal necrotizing enterocolitis: a nine‐year experience. Am J Dis Child 1981135(7)603–607. [DOI] [PubMed] [Google Scholar]
- 5.Black V D, Lubchenco L O, Luckey D W.et al Developmental and neurologic sequelae of neonatal hyperviscosity syndrome. Pediatrics 198269(4)426–431. [PubMed] [Google Scholar]
- 6.Luchtman‐Jones L, Swartz A, Wilson D. The blood and the hematopoietic system. In: Fanaroff AA, Martin RJ, eds. Neonatal‐perinatal medicine: diseases of the fetus and newborn. St Louis, MO: Mosby, 19971201–1297.
- 7.Letsky E. Polycythemia in the newborn. In: Rennie JM, ed. Textbook of neonatology. Edinburgh, UK: Churchill Livingstone, 1999834–838.
- 8.Schimmel M S, Bromiker R, Soll R F. Neonatal polycythemia: is partial exchange transfusion justified? Clin Perinatol 200431(3)545–553. [DOI] [PubMed] [Google Scholar]
- 9.Robinson K A, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol 200231(1)150–153. [DOI] [PubMed] [Google Scholar]
- 10.Roithmaier A, Arlettaz R, Bauer K.et al Randomized controlled trial of Ringer solution versus serum for partial exchange transfusion in neonatal polycythaemia. Eur J Pediatr 1995154(1)53–56. [DOI] [PubMed] [Google Scholar]
- 11.Wong W, Fok T F, Lee C H.et al Randomised controlled trial: comparison of colloid or crystalloid for partial exchange transfusion for treatment of neonatal polycythaemia. Arch Dis Child Fetal Neonatal Ed 199777(2)F115–F118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapia J, Solivelles X, Grebe G.et al Evaluation of different solutions for erythropheresis in the treatment of neonatal polycythemia. Pediatr Res 1992311614, 271A [Google Scholar]
- 13.Deorari A K, Paul V K, Shreshta L.et al Symptomatic neonatal polycythemia: comparison of partial exchange transfusion with saline versus plasma. Indian Pediatr 199532(11)1167–1171. [PubMed] [Google Scholar]
- 14.Krishnan L, Rahim A. Neonatal polycythemia. Indian J Pediatr 199764(4)541–546. [DOI] [PubMed] [Google Scholar]
- 15.Jan M, Ahmad S, Charoo B.et al Neonatal polycythemia: comparison of partial exchange transfusion with plasma versus normal saline. JK Practitioner 20007(3)195–196. [Google Scholar]