Abstract
Objective
To evaluate whether early neurofunctional assessment may be useful in predicting neurodevelopmental outcome in children of very low birth weight (VLBW).
Design
Observational longitudinal study.
Settings
Northern Italy.
Patients
A total of 250 VLBW children (129 boys, 121 girls) born consecutively 1996–1999.
Main outcome measures
Neurodevelopment at 36 months of chronological age, classified in accordance with the classification of Tardieu and the International classification of functioning.
Results
Of the infants exhibiting normal neurodevelopment (n = 183) or major dysfunction (n = 17) at 3 months of corrected age, 72% and 94% respectively did not change their score during the study. Minor dysfunctions at 3 months of corrected age were transient in 17 (34%) children. After adjustment for neonatal variables, neurodevelopment at 3 months of corrected age remained predictive of dysfunction at 36 months (odds ratio = 4.33, 95% confidence interval 2.05 to 9.12). If the results for the normal and minor dysfunction groups were pooled, the predictive qualities of the 3 month neurofunctional assessment were: sensitivity 0.5, specificity 0.99, positive predictive value 0.94, negative predictive value 0.93.
Conclusion
Early neurofunctional evaluation may be useful in predicting later neurodevelopmental outcome in VLBW children.
Keywords: neurofunctional assessment, neurodevelopmental outcome, very low birthweight infants
Advances in obstetric and neonatal care have dramatically improved the survival rate of very low birthweight (VLBW; birth weight <1500 g) infants over the last decade.1 As a result, questions arise about their long term neurodevelopmental outcome. Cerebral palsy occurs in 10–15% of the VLBW population, with higher rates in infants of lower birth weight and gestational age.2 An increased incidence of minor neurological dysfunctions, such as learning disabilities, cognitive defects, attention deficit/hyperactivity disorders, and behavioural problems, have been reported, especially in school children of extremely low birth weight (<1000 g) or gestational age <28 weeks.3 Identification of infants at higher risk of later negative developmental outcome remains a challenge for clinicians. This problem has been investigated mainly by using standard neurological assessments,4,5 which evaluate the relation between the nature and localisation of brain lesions and related dysfunctions.6 However, new approaches to disability are desirable considering the multidimensional nature of the problem.7
The International classification of functioning8 describes “functioning” as the dynamic interaction among three dimensions: body function/structure, activity and participation, and environmental factors. Several authors9,10,11 have proposed “neuromotor” or “neurobehavioral” assessments that evaluate the dynamic and developing systems in order to identify the “emerging functions” and the skill of adaptability to different stimuli. The hypothesis is that this functional approach to neurodevelopmental evaluation may better relate to later outcomes and focus on the consequences rather than on the disease itself. Few data are available on the relation between neurodevelopmental outcome of VLBW infants and early neurofunctional assessment.12
The aim of this study was to investigate whether neurofunctional evaluation at 3 months of age is predictive of neurodevelopmental outcome at 36 months of age.
Methods
Of all the consecutive newborns admitted to the same institution during 1996–1999, 299 entered the study. The inclusion criterion was birth weight <1500 g. Exclusion criteria were presence of congenital diseases or chromosomal abnormalities and death during postpartum hospital stay.
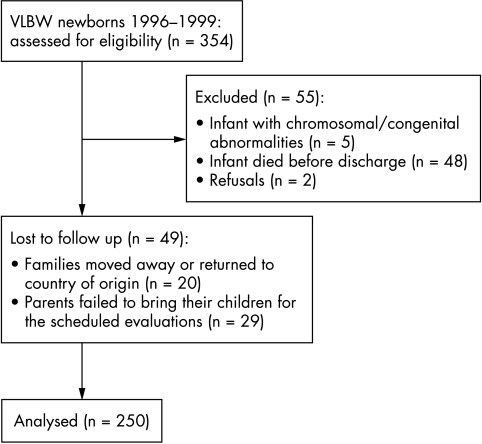
Infants were scheduled to be prospectively followed up to 36 months of age. Figure 1 shows the CONSORT flow chart of the study.
Figure 1 CONSORT flow chart of the study. VLBW, Very low birth weight.
Written informed consent was obtained from the parents, and the departmental ethics committee approved the study design.
Mother's level of education and the presence of gestational hypertension and/or pre‐eclampsia were investigated as maternal variables. Maternal education (years) was categorised as follows: low (⩽8), medium (9–13), high (>13). Gestational hypertension and pre‐eclampsia were respectively defined as de novo hypertension (systolic blood pressure of ⩾140 mm Hg or diastolic blood pressure of ⩾90 mm Hg) arising after mid‐pregnancy and gestational hypertension accompanied by new onset proteinuria (⩾300 mg per 24 hours).
Neonatal characteristics (gestational age, being appropriate size (AGA) or small for gestational age (SGA), sex, birth weight, length, head circumference, need of mechanical ventilation) were recorded. Gestational age was based on the last date of menstruation and confirmed by ultrasound examination performed in the 20th week of pregnancy. Infants with birth weight ⩾10th centile or <10th centile for gestational age, according to the North Italian growth charts, were classified respectively as AGA or SGA.13 Corrected age was calculated, up to 24 months of life, from the chronological age adjusted for gestational age—that is, for the number of weeks different from the expected 40 weeks.
Cranial ultrasound scans were performed on infants within the first 14 days of life according to clinical protocol. Brain magnetic resonance imaging was performed at 40±2 weeks corrected age in infants who exhibited hypoxic‐ischaemic/haemorrhagic lesions on the cranial ultrasound examination and/or had a birth weight <1000 g (n = 125). Weight, length, and head circumference at birth were measured by standard procedures.14
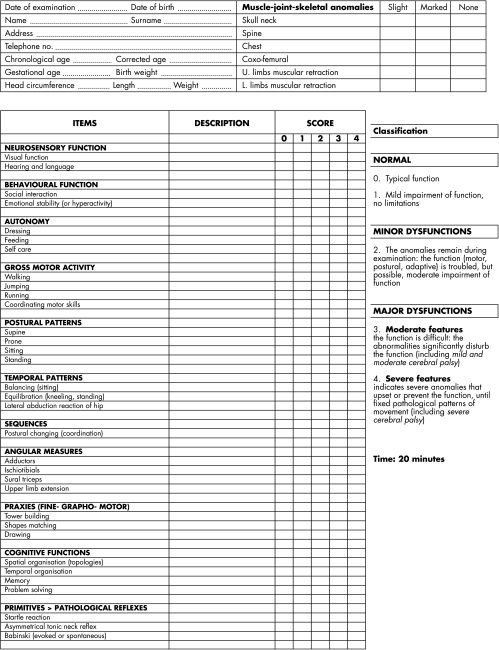
Infants entered a follow up programme that included measurement of anthropometric variables (weight, length, head circumference) and evaluation of the neurodevelopmental measures at 3, 6, 12, and 24 months of corrected age and at 36 months of chronological age. Neurodevelopmental assessment at 3 months of corrected age and at 36 months of chronological age was based on evaluation of items proposed by different authors,6,7,8,10,15,16,17,18,19,20,21,22,23and included evoked and spontaneous motility, postural adaptability, variability of motor patterns, and neuromotor and behavioural skills. The items were evaluated according to the emerging functions, characteristic of each age considered. Figures 2 and 3 give a detailed description of the neurofunctional assessment. A neurofunctional score, according to the classification of Tardieu21 and the International Classification of Functioning,8 was assigned to every item evaluated and categorised as follows: 0, normal function; 1, mild impairment of function (no limitations); 2, moderate impairment of function (possible but limited); 3, severe impairment of function (possible only with the use of facilitators or assisted devices); 4, function not possible. The maximum score was defined as the maximum value of the assessed items, reflecting the most severe functional impairment. The same trained examiner, blind to the infant's neuroimaging findings and unaware of previous scores when performing assessments, performed the neurofunctional evaluations. For the analysis, infants were further categorised into three groups, according to neurofunctional status, as: normal (score = 0–1); exhibiting minor dysfunctions (score = 2); exhibiting major dysfunctions (score = 3–4).
Figure 2 Neurofunctional assessment of premature baby at 3 months of age.
Figure 3 Neurofunctional assessment of premature baby at 3 years of age.
Statistical analysis
Descriptive data are shown as mean (SD) or number of observations (percentage). Comparison among groups was performed by the χ2 test for discrete variables, and by analysis of variance and the Kruskal‐Wallis test for continuous variables. Significance of multiple comparisons was adjusted by the Bonferroni correction. Logistic regression analysis was used to identify determinants of dysfunction at 36 months of age. Neurofunctional status at 3 month of corrected age, neonatal characteristics, and maternal hypertension during pregnancy entered the logistic model as confounders. Additional analysis assessed the ability of the 3 month neurofunctional evaluation to predict the 36 month neurofunctional evaluation. Results are presented as diagnostic validity analysis in terms of sensitivity, specificity, and positive and negative predictive values.
Table 2 shows the longitudinal variation of the neurofunctional status from 3 up to 36 months of age.
Table 2 Variation in neurofunctional status from 3 to 36 months of age (number of infants).
| 3 months | 36 months | |||
|---|---|---|---|---|
| Major dysfunction | Minor dysfunction | Normal | ||
| Major dysfunction | 17 | 16 | 1 | 0 |
| Minor dysfunction | 50 | 14 | 19 | 17 |
| Normal | 183 | 2 | 50 | 131 |
| Total | 250 | 32 | 70 | 148 |
Major dysfunction, maximum score 3–4; minor dysfunction, maximum score 2; normal, score 0–1.
Results
Follow up data at 36 months of chronological age were available for 250 (122 girls; 128 boys) infants. No infants died during the follow up. Table 1 shows the characteristics of the whole population at birth and according to neurofunctional status at 36 months of age.
Table 1 Characteristics of the whole population at birth and according to neurofunctional status at 36 months of age.
| Whole population (n = 250) | Neurofunctional status at 36 months | p Value† | |||
|---|---|---|---|---|---|
| Normal (n = 148) | Minor dysfunction (n = 70) | Major dysfunction (n = 32) | |||
| Birth weight (g) | 1125 (250.9) | 1184 (235)‡ | 1036 (251.6)§ | 1047 (247)§ | <0.0001* |
| Birth length (cm) | 37.1 (3.4) | 37.8 (3.1)‡ | 36 (3.7)§ | 36.3 (3.5) | <0.001* |
| Birth head circumference (cm) | 26.6 (2.1) | 27 (1.8)‡ | 25.1 (2.2)§ | 25.4 (2.5)§ | <0.0001* |
| Gestational age (weeks) | 30 (2.3) | 30.6 (2.2)‡ | 29.3 (2.1)§ | 28.7 (2.2)§ | <0.0001* |
| Males | 128 (51.2%) | 61 (41.2%)‡ | 45 (64%)§ | 22 (69%)§ | <0.001* |
| AGA | 141 (56.4%) | 78 (52.7%)‡ | 37 (53%)‡ | 26 (81%)§ | <0.001* |
| Infants ventilated | 129 (51.6%) | 58 (39.2%)‡ | 42 (60%)§ | 29 (91%)¶ | <0.0001* |
| Multiple birth | 66 (26.4%) | 37 (25%) | 18 (26%) | 11 (34%) | 0.556 |
Data are expressed as mean (SD) or number (%).
†One way analysis of variance, Kruskal‐Wallis test, or χ2 test. Different footnote symbols indicate significant difference between groups (Bonferroni correction).
*Statistically significant.
AGA, Appropriate size for gestational age.
At 3 months of corrected age, the neurofunctional score was 0 in 51 (20.4%) infants, 1 in 132 (52.8%), 2 in 50 (20%), 3 in 12 (4.8%), and 4 in 5 (2%). The corresponding values at 36 months of chronological age were 58 (23.2%), 90 (36%), 70 (28%), 21 (8.4%), and 11 (4.4%) (p<0.01). At the age of 36 months, the neurofunctional status had improved, remained unchanged, or deteriorated in 18 (7.2%), 166 (66.4%), and 66 (26.4%) infants respectively; 71.6% and 94% of infants with a score of ⩽1 or ⩾3 respectively at 3 months of corrected age had not changed their functional status at 36 months of age. Of the infants exhibiting minor dysfunction at 3 months of chronological age, 34%, 38%, and 28% showed normal neurodevelopment, minor, or major dysfunction respectively at 36 months.
The resulting sensitivity of the 3 month neurofunctional assessment for predicting the 36 month evaluation was 0.49, specificity was 0.88, positive predictive value 0.75, and negative predictive value 0.72. When the results for minor dysfunction and normal groups were pooled, sensitivity was 0.5, specificity 0.99, positive predictive value 0.94, and negative predictive value 0.93.
Cerebral palsy was found in 23 (9%) infants: diplegia occurred in 12, quadriplegia in eight, and hemiplegia in three. Table 3 presents the clinical features of cerebral palsy, according to the neurofunctional score at the 36 months evaluation of children with cerebral palsy.
Table 3 Clinical features of cerebral palsy, according to the neurofunctional maximum score at 36 months of age (number of infants).
| Cerebral palsy | Total no | Clinical features | Score 3 | Score 4 |
|---|---|---|---|---|
| Diplegia | 12 | Diplegia | 8 | 2 |
| Diplegia with hearing loss | 1 | 0 | ||
| Ataxic diplegia | 1 | 0 | ||
| Quadriplegia | 8 | Quadriplegia | 0 | 6 |
| Quadriplegia with blindness | 0 | 1 | ||
| Dystonic quadriplegia | 0 | 1 | ||
| Hemiplegia | 3 | Hemiplegia | 2 | 0 |
| Hemiplegia with epilepsy | 0 | 1 | ||
| Total | 23 |
Hypertension during pregnancy and/or pre‐eclampsia characterised 42.8% (n = 107) of mothers. Maternal hypertension occurred in 47.9%, 44.3%, and 18.8% of infants with normal neurofunction or minor or major dysfunction respectively at 36 months of chronological age (p = 0.011).
Educational level of mothers was: low, 30%; medium, 52%; high, 18%. Antenatal steroids were administered to 64% (n = 160) of infants; 90% (n = 224) were born by caesarean section. None of these variables was associated with neurodevelopment outcome at 36 months of chronological age (p>0.415).
Hypoxic‐ischaemic/haemorrhagic lesions were found in 41 (16.4%) cases. Of the infants who had brain magnetic resonance imaging, 27 (22%) showed clinically relevant central nervous system injury.
Lower weight, length, and head circumference at birth, shorter gestational age, being male, AGA, or ventilated were associated with the presence of dysfunction at 36 months of age. No significant difference was found between infants with major or minor dysfunction, except for AGA and being ventilated (table 1).
A logistic regression analysis was performed to identify determinants of dysfunction at 36 months of age (table 4).
Table 4 Odds ratio (95% confidence interval) of variables associated with dysfunction at 36 months of age.
| Variable | Comparison | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|---|
| Maternal hypertension during pregnancy | Yes v no | 0.59 (0.34 to 1.02) | 0.61 (0.32 to 1.18) |
| Birth weight | <1000 v ⩾1000 g | 2.99 (1.76 to 5.11)*** | 1.23 (0.50 to 2.99) |
| Birth length | <10th v ⩾10th centile | 2.97 (1.68 to 5.26)** | 1.54 (0.75 to 4.4) |
| Birth head circumference | <10th v ⩾10th centile | 1.27 (0.72 to 2.28) | 1.25 (0.68 to 2.30) |
| Gestational age | ⩽30 v >30 weeks | 3.01 (1.75 to 5.15)*** | 1.04 (0.435 to 2.47) |
| Sex | Male v female | 2.70 (1.60 to 4.50)*** | 2.30 (1.21 to 4.37)* |
| AGA | Yes v no | 1.44 (0.87 to 2.41) | 1.25 (0.586 to 2.71) |
| Ventilation | Yes v no | 3.51 (2.06 to 6.01)*** | 1.77 (0.78 to 4.01) |
| Neurofunctional status at 3 months chronological age | Impaired v normal | 7.35 (3.88 to 13.91)*** | 4.33 (2.05 to 9.12)*** |
*p<0.05; **p<0.001; ***p<0.0001 (logistic regression analysis).
AGA, Appropriate size for gestational age.
Neurofunctional status at 3 months (p<0.0001) and being male (p<0.05) were found to be independently associated with dysfunction at 36 months.
Discussion
The results of our study show that the 3 month neurofunctional evaluation is predictive of major dysfunction at 36 months of age, with a sensitivity and specificity of 0.5 and 0.99 respectively; in addition a sex effect (being male) was found to be associated with later dysfunction after adjustment for confounders.
Although the sensitivity of the 3 month evaluation was relatively low, the positive predictive value was as high as 0.94. When the results for normal babies and infants with minor dysfunction were pooled, the specificity and negative predictive value of the method increased to 0.99 and 0.93 respectively, suggesting that early neurofunctional assessment may be extremely useful in reassuring parents on the integrity of central nervous system function. According to this neurofunctional approach, an infant who scores ⩽2 at the 3 month assessment has a 93% chance of being free from major dysfunction at 36 months. In the population studied, only two babies out of 183 who scored 0–1 at the 3 month evaluation developed major dysfunction at 36 months of age, with a score of 3—that is, partial function was maintained. Identification of long term neurodevelopmental adverse outcomes in VLBW infants is important for understanding the full extent of morbidity and making referrals for early intervention.2,3 However, early diagnosis of major dysfunction remains difficult, as the central nervous system is still too immature to exhibit pathological signs associated with corticospinal lesions.16 Moreover early neuromotor abnormalities may be transient,24 because of the immaturity of the motor paths and the intrinsic strength deficit of the premature infant, which do not allow exhibition of acquired skills.10 It is also tricky to separate transient effects of cardiorespiratory and metabolic problems from specific expression of brain damage, in the early stages of extrauterine adaptation.25 Therefore early identification of “normal” infants and infants at risk of poor later neurodevelopmental outcome continues to be a challenge. Which clinical assessment is the most useful for predicting risk in monitoring neurodevelopment in preterm infants is still under investigation.15,25 Indeed, only relatively recently have guidelines of neurological findings in preterm infants, examined at term, been proposed.26
The predictive qualities, estimated in this study in terms of specificity, positive and negative predictive values, of the 3 month neurofunctional evaluation are in agreement with those of previously reported methods. Specificity and negative predictive values compare favourably with the 3 month test of infant motor performance (TIMP; 0.76 and 0.98 respectively at 12 months),12 the 4 month Alberta infant motor scale (AIMS; 0.81 and 0.96 at 18 months),27 movement assessment of infants (MAI; 0.93 and 0.96 at 18 months),28 the Peabody developmental gross motor scale (PDGMS; 0.71 and 0.96),27 and the 18 week infant neuromotor assessment (INA; 0.98 and 0.99 at 12 months).29 Although the sensitivity (0.50) of the 3 month neurofunctional assessment is lower than that of TIMP (0.92), AIMS (0.77), MAI (0.72), PDGMS (0.81), and INA (0.90), the positive predictive value (0.94) is higher than that of these infant motor tests (TIMP, 0.39; AIMS, 0.39; MAI, 0.58; PDGMS, 0.31; INA, 0.56).
In addition, the scoring system used in this study provides a qualifier of the neurofunctional status that better describes both the severity of the impairment and the potential outcome of the infant; it could also be used to compare repeated assessments over time as well as neurofunctional evaluations performed in different centres.
Our findings indicate that being male and having a poor neurofunctional assessment at 3 months of corrected age were independently associated with later dysfunction. Other authors have reported that being male is a risk factor for disability.2
What is already known on this topic
Neurofunctional assessment and study of emerging functions in premature infants relates to prognosis better than standard neurological examination
The role of neurofunctional assessment has been emphasised by authors involved in rehabilitative fields: neurodevelopmental treatment and bobathian practice in the United Kingdom, éducation thérapeutique and évaluation factorielle by Tardieu in France, and pattern analysis and neurorehabilitative semeiotics by Milani in Italy
Maternal hypertension, ventilation, AGA, and lower birth weight, length, head circumference, and gestational age were associated with dysfunction at 36 months on the univariate analysis only.
Studies in the literature are inconclusive about the role of maternal hypertension,30,31 ventilation,32,33 and AGA.34,35 There is agreement that lower birth weight is associated with increased morbidity and abnormal neurological findings.2 Bhutta et al3 report that mean cognitive scores are directly proportional to birth weight and gestational age. Anderson et al36 showed that children of birth weight <750 g or gestational age <26 weeks have lower cognitive scores. However, results on the effect of gestational age on neurodevelopment in VLBW infants are contradictory.37,38 Hagberg et al39 report that the risk of cerebral palsy is inversely proportional to gestational age, and the relative risk is 60 times higher at <28 weeks of gestation than at term. Latal‐Hajnal et al40 and Cooke and Foulder‐Hughes41 found that poor postnatal growth in preterm infants, in particular of the head, rather than SGA (symmetrical and otherwise) status seems to be associated with increased levels of later motor and cognitive impairment. Amin et al34 report persistence of microcephaly in intrauterine growth retardation as a risk factor for adverse neurodevelopmental outcome.
As the survival rate of VLBW infants has dramatically improved, especially that of extremely low birthweight infants (<1000 g),1 monitoring neurodevelopmental outcome of these children and early detection of “normality” and dysfunction is important for initiating early intervention programmes and optimising potential outcome. Follow up should be continued at least to school age3 in order to identify children in need of special support, to define more precisely minor dysfunctions, and to evaluate the interaction of biological and environmental factors in outcome. These results indicate that early neurofunctional assessment is a useful method for obtaining a better understanding of the full extent of morbidity of VLBW infants. Larger studies are required to clarify the role of the neurofunctional assessment in early diagnosis of later dysfunction in VLBW children.
What this study adds
Neurofunctional assessment is an additional method for evaluating neurodevelopmental disability in infants at high risk; this approach allows early intervention and targeted follow up, besides reassuring parents on integrity of function
The study applies the functional approach to paediatric and neonatological fields according to WHO recommendations
Abbreviations
AGA - appropriate size for gestational age
SGA - small for gestational age
VLBW - very low birth weight
Footnotes
Competing interests: none declared
References
- 1.Draper E S, Manktelow B, Field D J.et al Prediction of survival for preterm births by weight and gestational age: retrospective population based study. BMJ 19993191093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vohr B R, Wright L L, Dusick A M.et al Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 20001051216–1226. [DOI] [PubMed] [Google Scholar]
- 3.Bhutta A T, Cleves A M, Casey P H.et al Cognitive and behavioural outcomes of school‐aged children who were born preterm. JAMA 2002288728–737. [DOI] [PubMed] [Google Scholar]
- 4.Frisone M F, Mercuri E, Laroche S.et al Prognostic value of the neurologic optimality score at 9 and 18 months in preterm infants born before 31 weeks' gestation. J Pediatr 200214057–60. [DOI] [PubMed] [Google Scholar]
- 5.Haataja L, Mercuri E, Regev R.et al Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr 1999135153–161. [DOI] [PubMed] [Google Scholar]
- 6.Volpe J J. Neurological examination: normal and abnormal features. In: Neurology of the newborn. Philadelphia: WB Saunders, 199595–124.
- 7.Msall Me, Avery R C, Tremont M R.et al Functional disability and school activity limitations in 41300 school age children: relationship to medical impairments. Pediatrics 2003111548–553. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization International classification of functioning, disability and health. Geneva: WHO, 2001
- 9.Brazelton TB: Neonatal behavioural assessment scale Clin Dev Med. 1973;50:1–66. [Google Scholar]
- 10.Grenier A, Hernandorena X, Sainz M.et al Examen neuromoteur complementaire des nourrissons à risque de sequelles. Pourquoi? Comment? Arch Pediatr 199521007–1012. [DOI] [PubMed] [Google Scholar]
- 11.Milani A, Gidoni A. Pattern analysis of motor development and its disorders. Dev Med Child Neurol 19679625–630. [DOI] [PubMed] [Google Scholar]
- 12.Campbell S, Kolobe T H A, Wright B D.et al Validity of the test of infant motor performance for prediction of 6‐,9‐, and 12‐ month scores on the Alberta Infant Motor Scale. Dev Med Child Neurol 200244263–272. [DOI] [PubMed] [Google Scholar]
- 13.Robertson C. Catch‐up growth among very‐low‐birth‐weight preterm infants: a historical perspective. J Pediatr 2003143145–146. [DOI] [PubMed] [Google Scholar]
- 14.Agostoni C, Grandi F, Scaglioni S.et al Growth pattern of breastfed and non breastfed infants with atopic dermatitis in the first year of life. Pediatrics 2000106E73. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari F, Cioni G, Prechtl H F R. Qualitative changes of general movements in preterm infants with brain lesions. Early Hum Dev 199023193–231. [DOI] [PubMed] [Google Scholar]
- 16.Amiel‐Tison C, Grenier A.Neurological assessment during the first year of life. New York: Oxford University Press, 1986
- 17.Bobath B. The very early treatment of cerebral palsy. Dev Med Child Neurol 19679373–390. [DOI] [PubMed] [Google Scholar]
- 18.Brazelton T B. Neonatal behavioural assessment scale. Clin Dev Med 1973501–66. [Google Scholar]
- 19.Le Métayer M.Rééducation cérébro‐motrice du jeune enfant. Education thérapeutique. Paris: Masson, 1993
- 20.Piaget J.The origins of intelligence in the child. London: Penguin Educational, 1977
- 21.Tardieu G.Le dossier clinique de l'infirmité motrice cérébrale. Paris: Cahiers du CDI, 1984
- 22.Touwen B C L.Examination of the child with minor neurological dysfunction. London: Spastic International Medical Publisher, 1979
- 23.Vojta V V.Die cerebralen Bewegungsstorungen im Sauglingsalter. Fruhdiagnose und Fruhtherapie. Stuttgart: Verlag, 1974
- 24.Bennet C F, Scott D T. Long‐term perspective on premature infant outcome and contemporary intervention issues. Semin Perinatol 199721190–201. [DOI] [PubMed] [Google Scholar]
- 25.Amiel‐Tison C. Update of the Amiel‐Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr Neurol 200227196–212. [DOI] [PubMed] [Google Scholar]
- 26.Mercuri E, Guzzetta A, Laroche S.et al Neurologic examination of preterm infants at term age: comparison with term infants. J Pediatr 2003142647–655. [DOI] [PubMed] [Google Scholar]
- 27.Darrah J, Piper M, Watt M ‐ J. Assessment of gross motor skills of at‐risk infants: predictive validity of the Alberta Infant Motor Scale. Dev Med Child Neurol 199840485–491. [DOI] [PubMed] [Google Scholar]
- 28.Swanson M, Bennett F, Shy K.et al Identification of neurodevelopmental abnormality at four and eight months by the Movement Assessment of Infants. Dev Med Child Neurol 199234321–327. [DOI] [PubMed] [Google Scholar]
- 29.Magasiner V, Molteno C, Lachman P.et al A neuromotor screening test for high risk infants in a hospital or community settino. Pediatric Physical Therapy 19979166–172. [Google Scholar]
- 30.Szymonowwicz W, Yu V Y H. Severe preeclampsia and infants of very low birth weight. Arch Dis Child 198762712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng S W, Chou H C, Tsou K I.et al Delivery before 32 weeks of gestation for maternal pre‐eclampsia: neonatal outcome and 2‐year developmental outcome. Early Hum Dev 20047639–46. [DOI] [PubMed] [Google Scholar]
- 32.Overstreet D W, Jackson J C, van Belle G.et al Estimation of mortality risk in chronically ventilated infants with bronchopulmonary dysplasia. Pediatrics 1991881153–1160. [PubMed] [Google Scholar]
- 33.Gaillard E A, Cooke R W I, Shaw N J. Improved survival and neurodevelopmental outcome after prolonged ventilation in preterm neonates who have received antenatal steroids and surfactant. Arch Dis Child Fetal Neonatal Ed 200184F194–F196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin H, Singhal N, Sauve R S. Impact of intrauterine growth restriction on neurodevelopmental and growth outcomes in very low birthweight infants. Acta Paediatr 199786306–314. [DOI] [PubMed] [Google Scholar]
- 35.Kok J H, den Houden AL; Verloove‐Vanhorick S P.et al Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol 1998105162–168. [DOI] [PubMed] [Google Scholar]
- 36.Anderson P, Doyle L W, and the Victorian Infant Collaborative Study Group Neurobehavioral outcomes of school‐age children born extremely low birth weight or very preterm in the 1990s. JAMA 20032893264–3272. [DOI] [PubMed] [Google Scholar]
- 37.Piecuch R E, Leonard C H, Cooper B A.et al Outcome of extremely low birth weight infants (500 to 999 grams) over a 12‐year period. Pediatrics 1997100633–639. [DOI] [PubMed] [Google Scholar]
- 38.Dezoete A, Mac Arthur B A, Aftimos S. Developmental outcome at 18 months of children less than 1000 grams. NZ Med J 1997110205–207. [PubMed] [Google Scholar]
- 39.Hagberg B, Hagberg G, Olow I. The changing panorama of cerebral palsy in Sweden.VII. Prevalence and origin in the birth year period 1987–1990. Acta Paediatr 199685954–960. [DOI] [PubMed] [Google Scholar]
- 40.Latal‐Hajnal L H, von Siebenthal K, Kovari H.et al Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr 2003143163–170. [DOI] [PubMed] [Google Scholar]
- 41.Cooke R W I, Foulder‐Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch Dis Child 200388482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]