Abstract
Background
Vasomotor nephropathy is a common renal dysfunction in very preterm neonates.
Objective
To determine whether theophylline could prevent vasomotor nephropathy in very preterm infants with respiratory distress syndrome.
Methods
A randomised, double blind, placebo controlled trial of 50 preterm infants of gestational age ⩽32 weeks needing assisted ventilation. Infants received an intravenous dose of theophylline (1 mg/kg) or placebo for three days. The 24 hour urine volume was measured daily. On days 2, 5, and 11, blood samples and 12 hour urine collections were analysed for electrolytes, creatinine, and urea.
Results
On day 1, urine output was significantly higher in the theophylline (2.4 (0.9) ml/kg/h) than the placebo (1.6 (1.0) ml/kg/h; p = 0.023) group (values are mean (SD)). The incidence of oligoanuria was significantly lower in the theophylline treated (5%) than the placebo (33%) group. Twenty four hours after the first administration of theophylline/placebo, serum creatinine concentration was significantly lower in the theophylline (0.76 (0.23) mg/dl) than the placebo (1.0 (0.41) mg/dl; p = 0.025) group. On day 5 an increase in serum creatinine was observed in both groups. On day 11 a significant reduction in serum creatinine was observed, compared with day 5, with no difference between the two groups.
Conclusion
The results suggest that, in very preterm infants with respiratory distress syndrome, early theophylline administration improves renal function during the first two days of life.
Keywords: theophylline, vasomotor nephropathy, renal dysfunction, very preterm neonates, respiratory distress syndrome
The premature infant is born with a very low glomerular filtration rate, under the control of a delicate balance of intrarenal vasoconstrictor and vasodilator factors, mainly angiotensin II and prostaglandins respectively. Disorders of the vasoactive mediators can further reduce glomerular filtration rate and cause acute renal failure or vasomotor nephropathy, a renal dysfunction resulting from reduced renal perfusion.1,2
Acute renal failure has been estimated to affect 8–26% of neonates admitted to neonatal intensive care units, and may result in persistent glomerular and/or tubular dysfunction in 35–40% of cases.3,4,5,6 Perinatal hypoxaemia or asphyxia, in the course of severe respiratory distress syndrome (RDS), is one of the most common conditions of neonatal acute renal failure caused by adenosine activation.1,5,7,8 Experimental data have shown that theophylline, a xanthine derivative with adenosine antagonistic properties, is able to reverse the intrarenal vasoconstriction observed during hypoxaemia.9,10,11,12,13
The aim of our study was to determine whether theophylline could prevent vasomotor nephropathy in very preterm infants with RDS, in a randomised, double blind, placebo controlled trial.
Methods
Inborn preterm neonates of ⩽32 weeks gestational age, who developed RDS within six hours of birth and needed mechanical ventilation or nasal continuous positive airway pressure, were included in the study.
Exclusion criteria were kidney and/or urinary tract congenital abnormalities, congenital heart defects, prenatal exposure to inhibitors of angiotensin converting enzyme or non‐steroidal anti‐inflammatory drugs (NSAIDs), and chromosomal disorders or multiple malformations.
The study protocol was approved by the hospital ethics committee. Babies were enrolled after written informed consent had been obtained from the parents.
Neonates were randomised by computer generated numbers to receive a daily intravenous dose of theophylline (1 mg/kg; Aminomal, Malesci) or an equal volume of placebo (5% dextrose in water) for three consecutive days. The first dose of drug/placebo was given soon after it had been confirmed that the inclusion criteria had been met. The medical and nursing staff of the neonatal intensive care unit were blinded to the patient assignment. Theophylline or placebo were prepared by a doctor from the neonatology section, not involved in the patients' care, using syringes with identical external appearance, following the randomisation allocation table.
The rate of intravenous fluids was determined by the attending staff according to the protocol adopted in our neonatal intensive care unit (total fluid input: day 1, 70 ml/kg; day 2, 90 ml/kg; day 3, 110 ml/kg; day 4, 120 ml/kg; day 5, 140 ml/kg; day 6, 150 ml/kg; day 7, 150 ml/kg). On day 1, babies received an intravenous infusion of 10% dextrose in water; parenteral nutrition was begun on the second day with the same amounts of lipid and amino acid solutions in both groups. Enteral feeding of the neonates was started as soon as their clinical condition permitted; the volume of enteral feed was included in the fluid volume received. No sodium was added to maintenance intravenous fluids for the first 24 hours after birth. Subsequently, sodium intake was adjusted to maintain serum sodium concentration at 135–145 mEq/l. Antibiotics, inotropics (dopamine, dobutamine), diuretics (furosemide), analeptics (caffeine), NSAIDs (ibuprofen, indomethacin), and surfactant were prescribed as indicated by the clinical status of each patient. All fluid volumes of administered infusions and drugs were carefully recorded by the nursing staff. Body weight was determined at birth and every 24 hours; blood pressure was measured hourly by the oscillometric method; cardiorespiratory activity, oxygen saturation, and transcutaneous Pao2 and Paco2 were recorded continuously.
Urine was collected daily on open nappies after spontaneous voiding or application of suprapubic pressure.14 The 24 hour urine volume was measured starting from birth by weighing the nappies every two hours.
After the first 24 hours of life (day 2), blood samples were drawn and analysed for electrolytes, creatinine, and urea. A 12 hour urine collection was performed using an external device attached to the genitalia. Neonates were observed for spontaneous voiding, and the collection interval was initiated and ended immediately after the voiding. Bladder emptying was confirmed at this time by applying suprapubic pressure. The volume of all urine collected during this timed interval was measured, and an aliquot was analysed for electrolytes. Oliguria was defined as a urine output <1 ml/kg/h for at least 24 hours. The same measurements were performed in blood and urine samples on days 5 and 11. On day 5, serum theophylline concentration was measured. On days 5 and 11, the concentration of β2 microglobulin in the 12 h urine samples was measured as an index of tubular function; the normal upper limit was 4.0 mg/l (mean + two standard deviations in healthy infants).15 Blood urea was determined by the urease/glutamate dehydrogenase method, and creatinine by the Jaffè method.16 Serum theophylline concentrations were determined by the fluorescent polarisation immunoassay,17 and β2 microglobulin by the nephelometric immunoassay.18
Creatinine clearance was estimated using Schwartz's formula for preterm neonates19,20,21:
Creatinine clearance (ml/min/1.73 m2) = 0.33 × length (cm)/plasma creatinine (mg/dl).
The incidence of the following complications was considered: patent ductus arteriosus, intraventricular haemorrhage, periventricular leucomalacia, retinopathy of prematurity, bronchopulmonary dysplasia, and necrotising enterocolitis.
Statistical analysis was performed by the two way repeated measures analysis of variance followed by the Newman‐Keuls post hoc test, to evaluate differences in renal function between the two groups at different times. The baseline clinical data of the two groups were analysed by unpaired two tailed Student's t test or by the χ2 test with Yates correction, as required. The projected number of subjects needed was calculated by selecting a power of 0.9 and a two tailed α of 0.05. Sample size was estimated to be 20 infants for each study group to demonstrate a 20% difference in urine output, serum creatinine, glomerular filtration rate, or blood urea between the two groups. To compensate for non‐assessable patients, we planned to enrol 50 infants. p>0.05 was considered not significant. All computations were performed using a commercial statistical package (Statistica for Windows; StatSoft, Inc, Tulsa, Oklahoma, USA).
Results
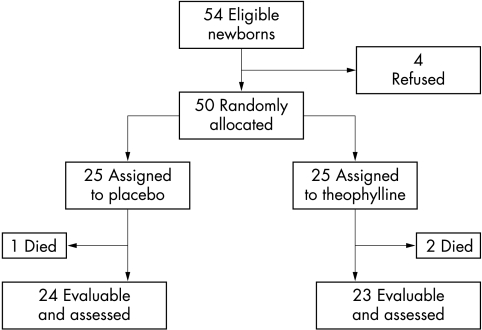
During a 12 month period, 54 consecutive preterm neonates met the entry criteria. Four sets of parents did not give informed written consent, therefore 50 babies were randomised. Three neonates died during the first 48 hours, leaving 47 infants who completed the study (fig 1).
Figure 1 Trial profile.
Patients in the two groups had similar characteristics. Twenty five neonates (16 boys, nine girls) were randomised to receive placebo (group P). Twenty five babies (13 boys, 12 girls) were assigned to the theophylline group (group T).
There were no significant differences in mean gestational age, birth weight, maximum fractional inspired oxygen requirement, hourly mean arterial blood pressure, or number of babies submitted to mechanical ventilation, synchronised intermittent mandatory ventilation, high frequency oscillation, or nasal continuous positive airway pressure between the two groups during the first five days (table 1).
Table 1 Clinical characteristics of infants on the first day of life and drug treatments during the study.
| Placebo (n = 25) | Theophylline (n = 25) | p Value | |
|---|---|---|---|
| Gestational age (weeks) | 28.7 (1.6) | 28.7 (2.0) | NS† |
| Birth weight (g) | 1157 (354) | 1192 (378) | NS† |
| SIMV | 15 | 14 | NS* |
| HFO | 3 | 4 | NS* |
| nCPAP | 7 | 7 | NS* |
| Fio2max | 0.39 (0.12) | 0.44 (0.18) | NS† |
| Mean arterial pressure (mm Hg) | 33.0 (7.0) | 35.4 (6.0) | NS† |
| Furosemide | 12 | 8 | NS* |
| Dopamine | 21 | 21 | NS* |
| Dobutamine | 12 | 9 | NS* |
| Ibuprofen | 4 | 6 | NS* |
| Indomethacin | 1 | 0 | NS* |
| Caffeine | 9 | 8 | NS* |
Values are either number or mean (SD). No differences in the use of diuretics, inotropic drugs, NSAIDs for patent ductus arteriosus or caffeine were found between the two groups.
*χ2 analysis.
†Student's t test.
nCPAP, Nasal continuous positive airway pressure; SIMV, synchronised intermittent mandatory ventilation; HFO, high frequency oscillation; Fio2, fractional inspired oxygen.
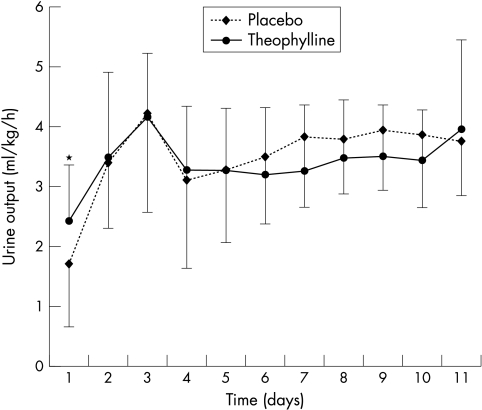
During the first 24 hours of life (day 1) urine output (ml/kg/h) was significantly higher in group T (2.4 (0.9) ml/kg/h) than group P (1.6 (1.0) ml/kg/h; p = 0.023; fig 2). The incidence of oligoanuria (urine output <1 ml/kg/h) was significantly lower in the theophylline group after the first study day (1/21 (5%) v 8/24 (33%); p = 0.017). No significant difference in urine output was observed over the next 10 study days between the two groups (fig 2).
Figure 2 Urine output (mean (SD)) during the study. On day 1 the neonates in the theophylline group had significantly higher urine output than those in the placebo group. *p = 0.023.
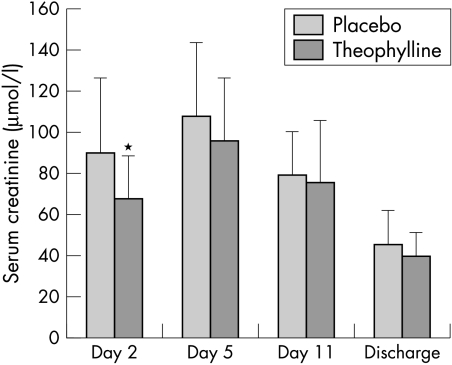
On day 2, 24 hours after the first administration of theophylline/placebo, neonates in group T had significantly lower serum creatinine concentration (67.3 (20.4) μmol/l) than those in group P (89.8 (36.3) μmol/l; p = 0.025; fig 3). Creatinine clearance was significantly higher in group T (19.0 (9.6) ml/min/1.73 m2) than in group P (13.5 (3.6) ml/min/1.73 m2).
Figure 3 Serum creatinine (mean (SD)) on days 2, 5, and 11 and at discharge. On day 2 the neonates in the group given theophylline had significantly lower serum creatinine concentration than those in the group given placebo. *p = 0.025.
Similar results were found in the two subgroups of neonates with severe RDS who required tracheal intubation and mechanical ventilation (17 infants in each group): group T creatinine 64.6 (21.2) μmol/l, group P creatinine 92.0 (40.7) μmol/l, p = 0.035; group T creatinine clearance 19.9 (10.7) ml/min/1.73 m2, group P creatinine clearance 13.7 (3.7) ml/min/1.73 m2, p = 0.037.
At 5 days of life, a reduction in renal function, evaluated by serum creatinine concentration and creatinine clearance, was observed in both groups compared with the second day; mean serum creatinine in group T was lower (95.1 (30.6) μmol/l) than in group P (107.4 (36.1) μmol/l), and creatinine clearance in group T was higher (13.1 (4.4) ml/min/1.73 m2) than in group P (11.1 (3.6) ml/min/1.73 m2), although these differences were not significant. At day 11, a significant improvement in renal function compared with day 5 was observed in both groups, with no differences in serum creatinine concentration or creatinine clearance.
Blood urea in group T was lower than in group P on day 2 (4.9 (1.9) mmol/l v 5.9 (2.6) mmol/l), although the difference was not significant (p = 0.052). On days 5 and 11, serum urea concentration, like creatinine, was lower in the neonates receiving theophylline although not significantly.
Mean serum sodium and potassium concentrations were within normal limits on days 2, 5, and 11, with no significant differences between the two groups. The incidence of hyponatraemia (Na+ <130 mEq/l) and hyperkalaemia (K+ >6.5 mEq/l) was very low in both groups (group T: two infants with hyponatraemia, three infants with hyperkalaemia; group P: two infants with hyponatraemia, two infants with hyperkalaemia). Sodium intake (mEq/kg), sodium excretion (mEq/24 h), and sodium balance were similar in the two study groups on days 2, 5, and 11.
Urinary β2 microglobulin concentrations on days 5 and 11 of the study were higher than normal in five infants in group T and four in group P. At these times no difference was found in the mean urinary β2 microglobulin concentrations between groups (group T: 4.0 (4.6) mg/l on day 5 and 3.7 (4.5) mg/l on day 11; group P: 4.0 (4.5) mg/l on day 5 and 2.5 (2.8) mg/l on day 11; p = NS).
Group T achieved a mean serum theophylline concentration of 27 (23) μg/l on day 5, whereas the concentration was not measurable in group P.
No significant differences in the incidence of complications (table 2) were found. One baby in group T died after the study.
Table 2 Main complications in the two infant groups.
| Placebo (n = 24) | Theophylline (n = 23) | p Value | |
|---|---|---|---|
| Length of stay (days) | 62.3 (25.9) | 62.7 (29.9) | NS† |
| PDA | 5 | 6 | NS* |
| IVH | |||
| Grade 1 | 4 | 2 | NS* |
| Grade 2 | 1 | 0 | NS* |
| Grade 3 | 1 | 1 | NS* |
| PVL | 2 | 2 | NS* |
| ROP | 2 | 2 | NS* |
| BPD | 4 | 1 | NS* |
| NEC | 1 | 0 | NS* |
Values are mean (SD) or number of patients.
*χ2 analysis.
†Student's t test.
PDA, Patent ductus arteriosus; IVH, intraventricular haemorrhage; PVL, periventricular leucomalacia; ROP, retinopathy of prematurity; BPD, bronchopulmonary dysplasia; NEC, necrotising enterocolitis.
Discussion
Our results show that, in very preterm infants, low dose theophylline administration at the beginning of RDS improves renal function in the first 2 days of life. Indeed, treated infants showed a better urine output, with a significant reduction in oliguria, and serum creatinine concentration significantly lower and endogenous creatinine clearance significantly higher than the placebo group. Creatinine clearance was estimated by the Schwartz equation. Although this adds limited additional information to serum creatinine concentration alone, because of the difficulty of obtaining an accurate timed urine collection, the Schwartz equation remains the most widely used marker of creatinine clearance in the neonatal period.19,20,21
The approach to acute renal failure today is mainly prevention.1 It is therefore possible to attempt early modulation of the mediators responsible for kidney hypoperfusion, reduced glomerular filtration rate, and subsequent cellular injury.
What is already known on this topic
Vasomotor nephropathy is a common renal dysfunction in very preterm neonates
Theophylline can reverse the intrarenal vasoconstriction observed during hypoxaemia
Several pieces of experimental evidence seem to confirm the key role of adenosine in the pathogenesis of renal failure secondary to hypoxaemia.8,22,23,24,25 Theophylline may antagonise renal endogenous adenosine, which is increased after renal hypoxia or ischaemia.26,27 Some authors have described an acute effect of theophylline on urine output when given to preterm babies for apnoea of prematurity.28,29,30 A significant increase in urine output and creatinine clearance was reported in five of six neonates with RDS 12 hours after the administration of a single dose of 1 mg/kg theophylline.13 In a prospective randomised study on term asphyxiated neonates, prophylactic early theophylline given at birth as a single 8 mg/kg dose had a protective effect on renal function, with a significant increase in urine output and creatinine clearance from the second to fifth day of life, and reduction in urinary β2 microglobulin.15
In our study the theophylline preventive effect on renal function did not persist after the first two days. It is well known that preterm infants show a brief initial increase in plasma creatinine concentration because of tubular reabsorption.31,32,33,34 Therefore renal failure can be hypothesised when a further increase, or lack of reduction, in serum creatinine concentration is observed during the first week of life.
The preterm kidney is very vulnerable to multiple factors that can modify its haemodynamics and determine renal failure.35,36 In addition to hypoxaemia, several pathological and iatrogenic conditions during the neonatal period can modify renal haemodynamics by activation of vasoactive factors other than adenosine, such as the renin‐angiotensin system, endothelin, atrial natriuretic peptide, prostaglandins, and thromboxane.1,2
The theophylline protective effect on renal function noticed at the beginning of respiratory distress in our preterm neonates may be induced by inhibition of renal endogenous adenosine, which increases during hypoxaemia. Other pathogenetic factors that activate mediators not affected by theophylline, such as sepsis, mechanical ventilation, and nephrotoxic drugs, may explain the worsening of renal function after the first few days.
As most of the patients included in the study, in both the control and treatment group, received dopamine treatment, we may hypothesise that the observed effects of theophylline are applicable principally to patients receiving dopamine.
Further studies are necessary to verify whether the short term improvement in renal function induced by low dose theophylline will prove useful for improving the prognosis of very preterm infants with RDS. Indeed, even a moderate improvement in renal function in critical situations is appreciable and may ease the whole management of many patients.
What this study adds
In very preterm infants with RDS, early theophylline administration improves renal function during the first 2 days of life
Treated infants showed a better urine output, significantly lower serum creatinine concentration, and significantly higher endogenous creatinine clearance than the placebo group
Acknowledgements
We thank Ms Anna Dorza and the nursing staff of the neonatal intensive care unit and neonatology sections for their invaluable help in this research.
Abbreviations
NSAID - non‐steroidal anti‐inflammatory drug
RDS - respiratory distress syndrome
VLBW - very low birth weight
ELBW - extremely low birth weight
Footnotes
Competing interests: none declared
References
- 1.Toth‐Heyn P, Drukker A, Guignard J P. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol 200014227–239. [DOI] [PubMed] [Google Scholar]
- 2.Awazu M, Hunley T E, Kon V. Pathophysiology of acute renal failure in the neonatal period. In: Polin RA, Fox WW, eds. Fetal and neonatal physiology. 2nd ed. Philadelphia: Saunders, 19981691–1696.
- 3.Hentschel R, Lodige B, Bulla M. Renal insufficiency in the neonatal period. Clin Nephrol 19964654–58. [PubMed] [Google Scholar]
- 4.Stapleton F, Jones D, Green R. Acute renal failure in neonates: incidence, etiology and outcome. Pediatr Nephrol 19871314–320. [DOI] [PubMed] [Google Scholar]
- 5.Karlowicz M G, Adelman R D. Acute renal failure in the neonate. Clin Perinatol 199219139–158. [PubMed] [Google Scholar]
- 6.Abibtol C L, Bauer C R, Montane B.et al Long‐term follow‐up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol 200318887–893. [DOI] [PubMed] [Google Scholar]
- 7.Karlowicz M G, Adelman R D. Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol 19959718–722. [DOI] [PubMed] [Google Scholar]
- 8.Gouyon J B, Guignard J P. Functional renal insufficiency: role of adenosine. Biol Neonate 198853237–242. [DOI] [PubMed] [Google Scholar]
- 9.Gouyon J B, Arnaud M, Guignard J P. Renal effects of low‐dose aminophylline and enprofylline in newborn rabbits. Life Sci 1988421271–1278. [DOI] [PubMed] [Google Scholar]
- 10.Gouyon J B, Guignard J P. Theophylline prevents the hypoxemia‐induced renal hemodynamic changes in rabbits. Kidney Int 1988331078–1083. [DOI] [PubMed] [Google Scholar]
- 11.Osswald H, Gleiter C, Muhlbauer B. Therapeutic use of theophylline to antagonize renal effects of adenosine. Clin Nephrol 199543S33–S37. [PubMed] [Google Scholar]
- 12.Huet F, Semana D, Grimaldi M.et al Effects of theophylline on renal insufficiency in neonates with respiratory distress syndrome. Intens Care Med 199521511–514. [DOI] [PubMed] [Google Scholar]
- 13.Jenik A G, Ceriani Cernadas J M, Gorenstein A.et al A randomised double‐blind, placebo‐controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics 20001051–6. [DOI] [PubMed] [Google Scholar]
- 14.Hermansen M C, Buches M. Urine output determination from superabsorbent and regular diapers under radiant heat. Pediatrics 198881428–431. [PubMed] [Google Scholar]
- 15.Tack E D, Perlman J M, Robson A M. Renal injury in sick newborn infants: a prospective evaluation using urinary β2‐microglobulin concentrations. Pediatrics 198881432–440. [PubMed] [Google Scholar]
- 16.Newman D J, Price C P. Renal function and nitrogen metabolites. In: Burtis CA, Ashwood ER, eds. Tietz textbook of clinical chemistry. 3rd ed. Philadelphia: Saunders, 19991204–1270.
- 17.Mounie J, Richard L, Ribon B.et al Methods of theophylline assay and therapeutic monitoring of this drug. Ann Biol Clin 199048287–293. [PubMed] [Google Scholar]
- 18.Lievens M M, Woestyn S, De Nayer P.et al Measurement of beta 2‐microglobulin in serum by a particle‐enhanced nephelometric immunoassay. Eur J Clin Chem Clin Biochem 199129401–404. [DOI] [PubMed] [Google Scholar]
- 19.Brion L P, Fleischman A R, McCarton C.et al A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr 1986109698–707. [DOI] [PubMed] [Google Scholar]
- 20.Zacchello G, Bondio M, Saia O S. Simple estimate of creatinine clearance from plasma creatinine in neonates. Arch Dis Child 198257297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mussap M, Plebani M. Cystatin C in neonatal and pediatric nephrology. Pediatr Med Chir 200426364–367. [Google Scholar]
- 22.Miller W L, Thomas R A, Berne R M.et al Adenosine production in the ischemic kidney. Circ Res 197843390–397. [DOI] [PubMed] [Google Scholar]
- 23.Ramos‐Salazar A, Baines A D. Role of 5′‐nucleotidase in adenosine‐mediated renal vasoconstriction during hypoxia. J Pharmacol Exp Ther 1986236494–499. [PubMed] [Google Scholar]
- 24.Hall J E, Granger J P. Adenosine alters glomerular filtration control by angiotensin II. Am J Physiol 1986250F917–F923. [DOI] [PubMed] [Google Scholar]
- 25.Prevot A, Huet F, Semana D S.et al Complementary effects of adenosine and angiotensine II in hypoxemia‐induced renal dysfunction in the rabbit. Life Sci 200271779–787. [DOI] [PubMed] [Google Scholar]
- 26.Hedquist P, Fredholm B, Olund H. Antagonistic effects of theophylline and adenosine on adrenergic neuroeffector transmission in the rabbit kidney. Circ Res 197843592–598. [DOI] [PubMed] [Google Scholar]
- 27.Persson C G A, Andersson K E, Kjellin G. Effects of enprofylline and theophylline may show the role of adenosine. Life Sci 1986381057–1072. [DOI] [PubMed] [Google Scholar]
- 28.Nobel P A, Light G S. Theophylline‐induced diuresis in the neonate. J Pediatr 197790825–826. [DOI] [PubMed] [Google Scholar]
- 29.Mazkereth R, Laufer J, Jordan S.et al Effects of theophylline on renal function in premature infants. Am J Perinatol 19971445–49. [DOI] [PubMed] [Google Scholar]
- 30.Shannon D C, Gotay F. Effects of theophylline on serum and urine electrolytes in preterm infants with apnea. J Pediatr 197994963–965. [DOI] [PubMed] [Google Scholar]
- 31.Gouyon J B, Guignard J P. Management of acute renal failure in newborns. Pediatr Nephrol 2000141037–1044. [DOI] [PubMed] [Google Scholar]
- 32.Matos P, Duarte‐Silva M, Drukker A.et al Creatinine reabsorption by the newborn rabbit kidney. Pediatr Res 199844639–641. [DOI] [PubMed] [Google Scholar]
- 33.Miall L S, Henderson M J, Turner A J.et al Plasma creatinina rises dramatically in the first 48 hours in preterm infants. Pediatrics 1999104e76. [DOI] [PubMed] [Google Scholar]
- 34.Gallini F, Maggio L, Romagnoli C.et al Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol 200015113–124. [DOI] [PubMed] [Google Scholar]
- 35.Guignard J P, Gouyon J B, John E G. Vasoactive factors in the immature kidney. Pediatr Nephrol 19915443–446. [DOI] [PubMed] [Google Scholar]
- 36.Guignard J P. Postnatal development of glomerular filtration rate in neonates. In: Polin RA, Fox WW, Abman SH, eds. Fetal and neonatal physiology. 3rd ed. Philadelphia: Saunders, 20041256–1266.