Abstract
Background
Human milk has considerable short and long term benefits for preterm infants, but mothers may experience difficulties in expressing breast milk for infants too immature or sick to breast feed. Oxytocin has been used to assist breast feeding and milk expression, but few data are available to support this intervention in the neonatal unit setting.
Aim
To test the hypothesis that oxytocin nasal spray increases early milk output in mothers expressing milk for preterm infants.
Methods
A randomised, double blind trial of oxytocin nasal spray (100 µl per dose) versus placebo was conducted in mothers delivering infants <35 weeks gestation. Sprays were used before expression of milk using an electric pump up to day 5.
Main outcome
Total weight of milk expressed while using spray (study powered to detect >1SD difference between groups).
Secondary outcomes
Pattern of milk production; number of pumping sessions; weight/fat content of milk expressed during a fixed 20 minute period on day 5 (“physiological study”); mother's opinion of expressing and spray assessed by questionnaire.
Results
Fifty one mothers were randomised (27 oxytocin, 24 placebo). Total milk production did not differ between groups. Repeated measures analysis of variance suggested significantly (p = 0.001) different patterns of milk production, with initial faster production in the oxytocin group then convergence between groups. Parity did not influence the response to the intervention. No significant differences were seen in milk weight or fat content in the physiological study nor in mothers' opinions of milk expression and treatment.
Conclusions
Despite marginal differences in the pattern of early milk production, the use of oxytocin nasal spray did not significantly improve outcome. Most mothers believed they were receiving the active spray, suggesting a significant placebo effect (supported by limited data from historical controls) and benefits from the extra breast feeding support available during the study.
Keywords: breast milk, oxytocin, nasal spray, randomised trial, preterm infant
The importance of human milk for preterm infants is well established, with benefits both in the short term1,2,3 and also for longer term health.4,5,6 Consequently, mothers delivering prematurely are strongly encouraged to provide breast milk for their infant, even if they do not intend to breast feed beyond discharge. Infants born before 34 weeks gestation are usually too immature to suck, so the mother must express breast milk, which is then fed to the infant through a nasogastric tube. The difficulties experienced by mothers in this situation are well documented, and it is perhaps not surprising that the proportion that eventually establish full breast feeding is fairly low.7 Techniques designed to support mothers and maximise their milk production are therefore important. Most successful methods are based on the underlying physiology of lactation—for example, increasing the frequency of pumping,8 hand massage before expressing milk,9 breast pumps designed to operate in a more physiological manner,10,11,12 and the use of dopamine antagonists to increase plasma prolactin concentrations.13,14,15
The effect of exogenous oxytocin on milk production was investigated during the 1950s and 1960s. Results obtained in mothers of healthy term infants suggested that exogenous oxytocin administered by a variety of routes (sublingual, buccal, nasal) is effective at producing milk ejection.16,17,18,19,20,21,22 A single study comparing nasal oxytocin with placebo in mothers expressing milk for preterm infants was performed 25 years ago.23 The effect of oxytocin was so dramatic that the trial was stopped after eight primigravid women had been studied. However, despite this finding, and the fact that no safety concerns have ever been raised, oxytocin has not become established as a means of improving milk output in mothers of preterm infants in the United Kingdom; indeed, nasal oxytocin has not been licensed in the United Kingdom since the 1960s. To establish whether this intervention should be available to contemporary mothers, we therefore performed a randomised, double blind, placebo controlled trial to evaluate the safety and efficacy of nasal oxytocin in the neonatal unit setting.
Methods
Mothers who had delivered an infant at <35 weeks completed gestation and who were planning to express breast milk were recruited from the Elizabeth Garrett Anderson Hospital, UCLH, London, between March 2003 and April 2004. In most cases, women were provided with information about the trial before delivery. Written informed consent was obtained post partum. Ethical approval was obtained from the University College Hospitals Research Ethics Committee. A certificate of exemption (DDX certificate) was obtained from the Medicines Control Agency for use of oxytocin nasal spray in the context of this clinical trial.
The mother was randomised to use either the active oxytocin spray or placebo spray. Randomisation assignments were prepared by DJP Clinical Trial Supplies (Abergevenny, Wales, UK) in permuted blocks of randomised length. Randomisation was stratified by parity (primigravida versus mulitigravida) and by infant gestation (<30 weeks versus 30+ weeks). Mothers and investigators were blind to the assignment until after data analyses were completed. Baseline characteristics of the mothers and obstetric data were collected, including obstetric history and details of previous breast feeding or experience of milk expression.
Sprays
Sprays were prepared by DJP Clinical Trials Supplies Ltd. Oxytocin sprays were obtained from the manufacturer (Syntocinon Nasal Spray; Novartis, Frimley, Surrey, UK) and contained 40 IU synthetic oxytocin (Syntocinon) per ml (total content 5 ml). Placebo sprays were prepared containing sterile normal saline plus benzalkonium chloride as preservative (to mimic the active spray). Empty oxytocin spray bottles were not available to us. To ensure blinding, oxytocin was therefore transferred (under sterile conditions) into the same type of bottle as the placebo, with the same dose per activation as the original oxytocin bottle. Once prepared, sprays were numbered and the randomisation schedule prepared as described above. The oxytocin content of the trial sprays was measured at the start of the study and again after six months, to check for potential loss of activity due to adsorption; no significant decrease was found in trial sprays relative to unaltered sprays at either time point.
Protocol
Each mother received the same standard advice about expressing milk and using the breast pump. The Egnell Ameda Elite pump was used by all subjects, generally in single pumping mode, which was the neonatal unit policy. Mothers were advised to express milk at least every three hours and were also instructed in the use of hand massage before pumping. Advice was provided by the staff on the postnatal ward and neonatal unit, but also by the study research nurse, who was available for contact by phone at all times and who saw each mother at least daily during the study.
The research nurse instructed the mothers in using the trial spray. Mothers were advised to administer one spray (100 µl) two to five minutes before expressing milk from each breast as per the manufacturer's instructions. To administer the spray, the mother sat upright, held the bottle upright, inserted the nozzle into one nostril, and inhaled gently through the nose while depressing the activator once. Each bottle contained the equivalent of 50 doses, and was expected to last at least five days, allowing for some wastage. In the unlikely event that a mother used up her spray before five days, a second spare spray with the same contents as the original was allocated by a member of the team not involved in other aspects of the project. Before allocation, sprays were kept at 4°C; once in use, they were kept at room temperature for the duration of the study.
Outcome measures
Primary outcome measure: milk volume
Each mother completed a daily record form until the spray was finished, or until day 7, recording the time started and finished and the weight of milk obtained each time she expressed milk. The mother was shown how to tare a digital scale with the empty bottle before expressing milk, and how to record the weight of milk afterwards.
Secondary outcome measures
Total number of pumping sessions during the study period
Milk volume and fat content over a fixed 20 minute period of milk expression (“physiological study”). On day 5, each mother was asked to express milk under observation for 10 minutes from each breast having first used her assigned spray. The milk expressed was collected using fine tubing in place of the normal bottle, and the volume for each one minute interval recorded. Small samples were taken at one minute intervals for determination of milk fat using the creamatocrit method.24 The remainder of the milk was retained for use by the infant.
Mothers' opinions. At the completion of the study, each mother was asked to complete a short questionnaire asking about her experience of expressing milk and use of the spray on an analogue scale, with 1 representing the best score, 7 the worst, and 4 a neutral position. The questionnaire asked four questions: (a) How easy did you find expressing milk? (b) How comfortable did you find expressing milk? (c) How pleasant did you find expressing milk? (d) Did you find the spray helpful? Mothers were also asked to report adverse effects of their spray if and when they occurred.
Historical control data
Comparable historical data on milk production were not available from the neonatal unit involved in this study. To examine whether the extra support available to both groups of mothers in this study resulted in beneficial effects on milk production, or whether there was a placebo effect of providing a spray regardless of its content, we therefore used historical data from a similar population of mothers studied previously on a different unit but using the same methodology. These mothers also used the Egnell Ameda electric pump, forming one limb of a randomised trial evaluating the use of two breast pumps in the neonatal unit setting.10 They received routine lactation support from the ward nursing staff without additional support from the research nurses. Data were available for median milk weights between days 2 and 5 and for the number of pumping sessions per day.
Sample size
The single previous study comparing oxytocin nasal spray with placebo in mothers with preterm infants reported a dramatic effect of oxytocin, with around 5SD difference in milk production between days 2 and 5 between groups in primiparous women.23 To detect a more modest 1SD difference in outcome at 5% difference with 80% power would require 16 women per group. For the physiological study, on the basis of our previous data on the pattern of milk flow during expression using the Egnell Ameda breast pump,10 the mean (SD) flow during the first four minutes was 5.7 (4.3) ml/min. Allowing for skewed distribution, a minimum of 19 mothers in each group would allow detection of a 1SD increase in milk flow in mothers using the oxytocin spray compared with the placebo at 5% significance with 80% power. To allow for mothers failing to complete all parts of the study, we aimed to recruit 25 subjects per group.
Statistical analysis
Analyses were performed on an intention to treat basis, then repeated for mothers with complete five day milk records. Categorical variables were compared using a χ2 test. Milk weight data were skewed and were therefore transformed to natural logarithms. To compare patterns of milk production over time between randomised groups and to examine the effect of parity, we used repeated measures analysis of variance. Subsequent comparisons were made between sprays for each day. Different models were investigated to obtain the best fit. Analyses were performed using SPSS and STATA.
Results
Fifty one mothers were randomised, 27 to the active oxytocin spray and 24 to placebo. Milk volume records were completed by 25 mothers using oxytocin and 23 using placebo. One mother from each group failed to fill in any records. An additional mother using oxytocin developed eclampsia post partum and had delayed onset of lactation with no milk production during the five day study period; she subsequently produced milk after day 7. Complete five day milk records were available for 42 mothers, 21 randomised to each spray. Reasons for not completing the full five day record were: spray ran out during day 5 (2 × oxytocin); mother did not express milk for one or more days (no particular reason identified; 2 × oxytocin, 1 × placebo); one mother using placebo developed a pulmonary embolus secondary to a central venous line used for parenteral nutrition and was unable to express milk after day 3. The physiological study was completed by 26 mothers using oxytocin and 22 using placebo. No adverse effects of either the active or placebo sprays were reported. Table 1 shows the baseline characteristics. There were no significant differences between the groups.
Table 1 Baseline characteristics of the groups randomised to oxytocin spray or placebo spray.
| Oxytocin (n = 27) | Placebo (n = 24) | |
|---|---|---|
| Maternal age (years)* | 31.5 (5.5) | 30.8 (6.7) |
| Mothers with degree or higher professional qualification | 18 (67) | 14 (58) |
| Mother married or with partner | 26 (96) | 21 (87) |
| Ethnic group of mother | ||
| White/Asian | 18 (67) | 16 (67) |
| Black | 9 (33) | 8 (33) |
| Multiple birth | 4 (15) | 5 (21) |
| Previous breast feeding experience | 5 (19) | 5 (21) |
| Previous pumping experience | 6 (22) | 3 (13) |
| Mode of delivery | ||
| Elective LSCS | 12 (44) | 5 (21) |
| Emergency LSCS | 4 (15) | 5 (21) |
| Vaginal delivery | 11 (41) | 14 (58) |
| Infant intubated at birth | 11 (41) | 16 (59) |
| Infant held by mother at birth | 3 (11) | 5 (21) |
| Male infants | 18 (56) | 14 (44) |
| Infant birth weight (g)* | 1380 (604) | 1315 (603) |
| Infant gestation (weeks)* | 29.9 (2.8) | 29.0 (3.7) |
Results are number (%) unless stated otherwise.
*Mean (SD).
LSCS, Lower segment caesarean section.
Intention to treat analysis
Main outcome measure: milk production
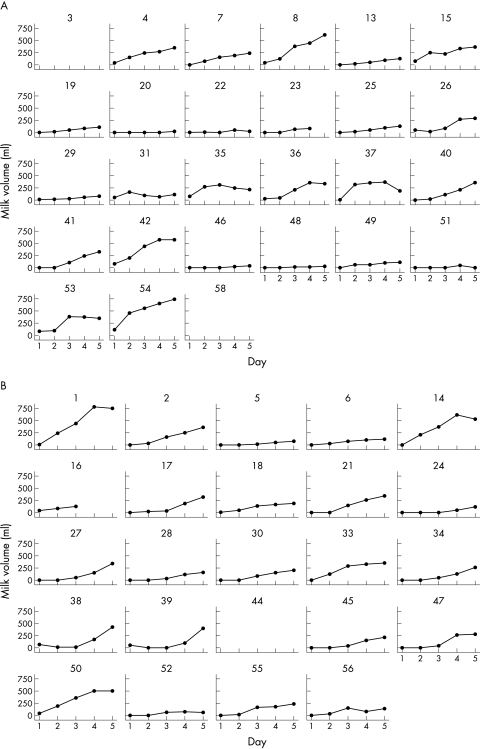
Table 2 (which can be found at http://adc.bmjjournals.com/supplemental/) shows results for reported milk production during the study. Milk production was slightly higher in mothers using oxytocin spray on days 1–3. After correction for multiple comparisons, the difference was significant only for day 2. There was no significant difference in the cumulative weight of milk produced over days 1–5 between oxytocin and placebo groups (oxytocin group median 667 (25th, 7th centiles 206, 1203) g, placebo group 530 (394, 778) g; p = 0.9). The variability in milk production between individual mothers in both randomised groups is well demonstrated by the individual plots shown in fig 1.
Figure 1 Plots of daily milk production data for individual women, according to randomised spray assignment. (A) Oxytocin spray; (B) placebo spray.
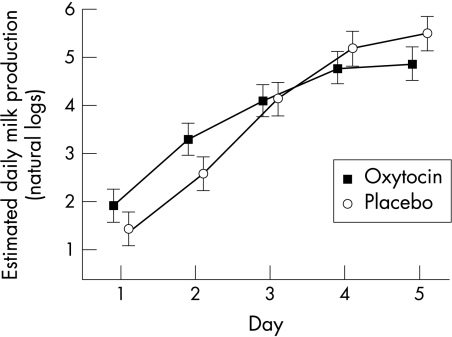
The pattern of milk production was significantly different between the randomised groups (p = 0.001 for linear trend, repeated measures analysis of variance), with the oxytocin group producing more milk over the first two days, but the placebo group then matching and exceeding them (fig 2). We investigated the use of other relations between milk weight and study day, such as quadratic and logistic models. All models produced broadly similar results, suggesting that there was some evidence of a difference in the pattern of milk production between the two sprays.
Figure 2 Mean daily milk production on days 1–5 of the study according to spray used (error bars represent 95% confidence intervals).
The mean (SD) number of pumping sessions per day over days 1–5 did not differ significantly between groups (oxytocin 3.4 (0.8) v placebo 3.6 (0.9); p = 0.4). The mean time spent expressing milk per day over the five day study period was 84 (24) minutes for the oxytocin group and 95 (29) minutes for the placebo group (p = 0.14). The mean volume of milk expressed per minute over the study period was 1.89 (1.6) for the oxytocin group and 1.51 (1.2) for the placebo group (p = 0.4). The values of these parameters for individual study days are provided in table 2 for information.
Physiological test
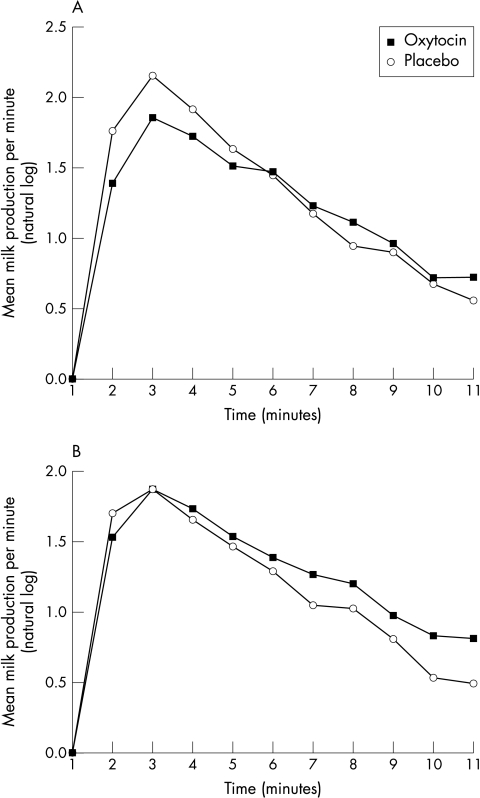
The cumulative milk volumes for both first and second breasts were not significantly different between oxytocin and placebo groups (side 1, oxytocin 34.8 (18.3) ml v placebo 40.5 (22.2) ml; p = 0.3; side 2, oxytocin 37.1 (23.9) ml v placebo 33.0 (20.7) ml; p = 0.5). Using repeated measures analysis of variance, the pattern of milk production was not significantly different between spray groups for either breast (fig 3).
Figure 3 Milk production per minute in the physiological test. (A) First breast; (B) second breast.
The mean creamatocrit of milk expressed was not significantly different between spray groups (side 1, oxytocin 7.95 (2.4) v placebo 7.96 (3.1); p = 0.99; side 2, oxytocin 8.00 (2.7) v placebo 8.85 (3.1); p = 0.4). The pattern of creamatocrit over each 10 minute period was not affected by the randomised spray.
Mothers' opinions of sprays
Figure 4 (which can be found at http://adc.bmjjournals.com/supplemental/) summarises the results of the questionnaire. There were no significant differences between the ratings awarded by mothers using the different sprays for any parameter. Twenty eight mothers expressed a strong opinion of whether they had the active spray or placebo. Twenty two were convinced that they had the active spray; many asked for a replacement spray and/or reported that their milk production dropped when the spray ran out. Of these 22 mothers, nine (41%) were actually receiving placebo. In contrast, only one of the six (16%) mothers convinced that she was using placebo was actually receiving the active spray.
Analysis of daily milk volume data for mothers with complete five day records (21 per group)
This produced similar results to those from the whole cohort (data not shown).
Effect of parity
Thirty three subjects were primiparous (17 randomised to oxytocin, 16 to placebo) and 18 subjects were multiparous (10 oxytocin, eight placebo). There was a trend towards greater milk production by multiparous mothers (cumulative weights (median (25th, 75th centiles): day 1, 6 (0, 61) v 1 (0, 13) g; day 2, 54 (14, 238) v 15 (6, 74) g; day 3, 177 (77, 604) v 69 (34, 232) g; day 4, 356 (199, 939) v 237 (143, 422) g; day 5, 691 (429, 1236) v 473 (254, 717) g. However, in a repeated measures analysis of variance, there was no significant main effect of parity on milk production, and no interaction between parity and oxytocin spray (p = 0.8), suggesting that the effect of the oxytocin spray was similar in the two groups. Parity did not significantly influence the weight or creamatocrit of milk expressed during the physiological study and there was no interaction between oxytocin and parity.
Comparison with historical reference data
Milk production in this study was slightly higher on days 2 and 3 than in 17 women from our previous study (table 2), but the difference was not significant. The mean (SD) number of times that mothers expressed per day was 3.6 (1.0) for the present study and 3.5 (0.9) for the previous study.
Discussion
The use of nasal oxytocin spray did not significantly improve milk production during the first five days post partum in mothers expressing milk for their preterm infant. Although there was some evidence that oxytocin resulted in a different pattern of milk production, with greater amounts expressed during the first two days, by five days post partum the placebo group were producing greater amounts, and the total milk production over the five day period was not significantly different between the groups.
Mothers of preterm infants are increasingly encouraged to provide breast milk for their infant, and it is important to investigate techniques for supporting them and maximising milk production. Several methods aimed at improving milk production in the neonatal unit setting have been evaluated; they include increasing the frequency of pumping,8 hand massage before pumping,9 the use of more physiologically designed breast pumps,10,11,12 and different pumping regimens.9,12 Pharmacological manipulation of the hormones involved in lactation has also been attempted. For example, dopaminergic antagonists such as metoclopramide are often used in an attempt to increase plasma prolactin concentrations and milk output. A few published data13,14 suggest that this intervention may be beneficial beyond the first month post partum when plasma prolactin concentrations decline in some women.15 However, in our experience, many mothers stop expressing milk earlier in the postnatal period, often because they are disheartened by their apparently poor milk production. Simply advising such mothers to express more frequently may not be very effective in this situation. Problems with milk output early in the postpartum period may relate more to inadequate production of oxytocin than to prolactin deficiency. Oxytocin secretion is highly sensitive to psychological cues and is easily inhibited by stress. During normal lactation, oxytocin production is stimulated by numerous visual, auditory, and olfactory stimuli from the infant that are missing in the situation of a mother expressing milk in the neonatal unit.
A single study of nasal oxytocin versus placebo in mothers expressing milk for preterm infants reported such a dramatic effect of oxytocin on milk production that the trial was discontinued after eight primigravid mothers had been studied.23 Interestingly there was a significant interaction between parity and treatment on milk volume, with a greater effect of oxytocin in primiparous women than in multiparous women. The study was criticised on the basis that mothers only expressed milk four times a day; however, our own data suggest that this is true of many mothers today in the United Kingdom. These findings seem difficult to reconcile with those from our own trial, particularly as the protocols (including the oxytocin dose) were similar, and we measured milk production (by design) over the same period. Potential explanations for the differences include the possibility that the small number of women studied in the earlier trial may not have been representative of the wider population, and differences in data presentation and analysis. The previous study presented means and standard deviations. We log‐transformed our data or used non‐parametric statistics because of the highly skewed nature of the data; in fact, differences in milk volumes between groups in our own study are greater when parametric statistics on untransformed data are (inappropriately) used.
We considered the possibility that the activity of our oxytocin sprays may have decreased during the course of the study, as, in order to blind the trial, it was necessary to decant the active oxytocin into different bottles. However, we found no effect of recruitment date (representing the “age” of the spray) on outcome. Moreover, chemical analyses showed no significant decline in oxytocin in the repackaged sprays compared with the manufacturer's unaltered sprays over a six month period.
What is already known on this topic
Human milk has considerable short and long term benefits for preterm infants, but mothers often experience difficulties in expressing breast milk for their infant
Methods shown to increase milk production include increasing the frequency of pumping, hand massage before expressing milk, breast pumps designed to operate in a more physiological manner, and the use of dopamine antagonists to increase plasma prolactin concentrations
What this study adds
Despite marginal differences in the pattern of early milk production, the use of oxytocin nasal spray did not significantly improve milk production in the early postpartum period in mothers expressing milk for their preterm infant
There was some evidence that mothers benefited from the extra lactation support available during the study period plus the provision of a spray that they believed might improve their milk production, illustrating the importance of psychological factors and support in lactation
Although our study showed no significant difference in total milk production between randomised groups, or in the parameters assessed by the questionnaire, it was apparent that most of the mothers believed that they were receiving the active spray. Indeed, many mothers complained that their milk production declined when the spray was discontinued, although, unfortunately, data were not collected for milk production after the spray was stopped. The nursing staff on the neonatal unit also felt that more milk was available for the infants.
In summary, our randomised trial provided no evidence for a significant beneficial effect of oxytocin nasal spray on milk production during the first five days post partum. The study population included a mix of social class and ethnic groups, and our findings should therefore be generalisable to other neonatal units. There was some evidence that mothers benefited from the extra lactation support available during the study period plus the provision of a spray that they believed might improve their milk production, illustrating the importance of support and psychological factors in lactation, particularly in this vulnerable group of mothers.
Acknowledgements
We thank the mothers who participated in the study, the staff on the delivery suite, postnatal wards, and neonatal intensive care unit; Mr Pat O'Brien; George Mukwaya, Celia Dos Santos and John Muir from EMU, Steve Williams at DJP Clinical Trials Supplies for his help in preparing the sprays, and Professor Alan McNeilly for his contributions to the design of the study.
Footnotes
Funding source: MRC Programme grant
Competing interests: none declared
References
- 1.Lucas A, Gore S M, Cole T J.et al Multicentre trial on feeding low birth weight infants: effects of diet on early growth. Arch Dis Child 198459722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas A, Cole T J. Breast milk and neonatal necrotizing enterocolitis. Lancet 19903361519–1523. [DOI] [PubMed] [Google Scholar]
- 3.Narayanan I, Prakash K, Gujral V V. The value of human milk in the prevention of infection in the high‐risk low birth weight infant. J Pediatr 198299496–498. [DOI] [PubMed] [Google Scholar]
- 4.Lucas A, Morley R, Cole T J.et al Breast milk and subsequent intelligence quotient in children born preterm. Lancet 1992339261–264. [DOI] [PubMed] [Google Scholar]
- 5.Singhal A, Cole T J, Lucas A. Early nutrition and later blood pressure: two cohorts after a randomised trial. Lancet 2001357413–419. [DOI] [PubMed] [Google Scholar]
- 6.Singhal A, Cole T J, Fewtrell M S.et al Breast‐milk feeding and the lipoprotein profile in adolescents born preterm. Lancet 20043631571–1578. [DOI] [PubMed] [Google Scholar]
- 7.Furman L, Minich N M, Hack M. Breastfeeding of very low birth weight infants. J Hum Lact 19981429–34. [DOI] [PubMed] [Google Scholar]
- 8.DeCarvalhoet al Frequency of milk expression and milk production by mothers of non‐nursing premature neonates. Am J Dis Child 1985139483–485. [DOI] [PubMed] [Google Scholar]
- 9.Jones E.et al A randomised controlled trial to compare methods of milk expression after preterm delivery. Arch Dis Child Fetal Neonatal Ed 200185F91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fewtrell M S.et al Randomised trial comparing the efficacy of a novel manual breast pump with a standard electric breast pump in mothers who delivered preterm infants. Pediatrics 20011071291–1297. [DOI] [PubMed] [Google Scholar]
- 11.Alekseev N P.et al Compression stimuli increase the efficacy of breast pump function. Eur J Obstet Gynecol Reprod Biol 199877131–139. [DOI] [PubMed] [Google Scholar]
- 12.Kent J C, Ramsay D T, Doherty D.et al Response of breasts to different stimulation patterns of an electric breast pump. J Hum Lact 200319179–186. [DOI] [PubMed] [Google Scholar]
- 13.da Silva O P.et al Effect of domperidone on milk production in mothers of premature newborns: a randomised, double‐blind, placebo‐controlled trial. CMAJ 200116417–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenkrantz R A. Metoclopramide effect on faltering milk production by mothers of premature infants. Pediatrics 198678614–620. [PubMed] [Google Scholar]
- 15.Chatterton R T, Jr, Hill P D, Aldag J C.et al Relation of plasma oxytocin and prolactin concentrations to milk production in mothers of preterm infants: influence of stress. J Clin Endocrinol Metab 2000853661–3668. [DOI] [PubMed] [Google Scholar]
- 16.Newton M, Egli G E. Effect of intranasal oxytocin on let‐down of milk. Am J Obstet Gynecol 195876103–107. [DOI] [PubMed] [Google Scholar]
- 17.Stewart R H, Nelson R N. Synthetic oxytocin. Obstet Gynecol 195913204. [PubMed] [Google Scholar]
- 18.Huntingford P J. Intranasal use of synthetic oxytocin in management of breast feeding. BMJ 1961I709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman E A, Sachtleben M R. Oxytocin in lactation. Am J Obstet Gynecol 196182846–855. [DOI] [PubMed] [Google Scholar]
- 20.Douglas G R, Dillon T F. Use of oxytocin and vasopressin in obstetrics and gynecology. Obstet Gynecol 196220852–858. [PubMed] [Google Scholar]
- 21.Luhman L A. The effect of nasal oxytocin in lactation. Obstet Gynecol 196321713–717. [PubMed] [Google Scholar]
- 22.Ingerslev M, Pinholt K. Oxytocin treatment during the establishment of lactation. Acta Obstet Gynecol Scand 196241159–167. [Google Scholar]
- 23.Ruis H, Rolland R, Doesburg W.et al Oxytocin enhances onset of lactation among mothers delivering prematurely. BMJ 1981283340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas A, Gibbs J H, Lyster R L J.et al Creamatocrit: a simple technique for estimating fat concentration and energy value in human milk. BMJ 19781(6119)1018–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]