Abstract
Objective
To test the hypothesis that male compared with female prematurely born infants would have worse lung function at follow up.
Design
Prospective follow up study.
Setting
Tertiary neonatal intensive care units
Patients
Seventy six infants, mean (SD) gestational age 26.4 (1.5) weeks, from the United Kingdom oscillation study.
Interventions
Lung function measurements at a corrected age of 1 year.
Main outcome measures
Airways resistance (Raw) and functional residual capacity (FRCpleth) measured by whole body plethysmography, specific conductance (sGaw) calculated from Raw and FRCpleth, and FRC measured by a helium gas dilution technique (FRCHe).
Results
The 42 male infants differed significantly from the 34 female infants in having a lower birth weight for gestation, requiring more days of ventilation, and a greater proportion being oxygen dependent at 36 weeks postmenstrual age and discharge. Furthermore, mean Raw and FRCpleth were significantly higher and mean sGaw significantly lower. After adjustment for birth and current size differences, the sex differences in FRCpleth and sGaw were 15% and 26% respectively and remained significant.
Conclusion
Lung function at follow up of prematurely born infants is influenced by sex.
Keywords: prematurity, lung function, sex, plethysmography
Male compared with female children may be disadvantaged by having poorer respiratory function and more respiratory illnesses.1,2,3 Most studies,1,2,3 however, have focused on infants or older children born at full term. The only study that has examined prematurely born infants included those born only moderately preterm, and the infants were examined at discharge from the neonatal unit.4 We have recently reported that male sex is a significant risk factor for respiratory morbidity (troublesome symptoms and requirement for treatment) during infancy in children born very prematurely—that is, before 29 weeks of gestational age.5 We therefore hypothesised that male compared with female very prematurely born infants would have diminished lung function at 1 year of corrected age. It was important to test this hypothesis, as lung function results at 1 year corrected age have been shown to predict ongoing respiratory morbidity.6 We have previously shown that premature infants who were symptomatic at follow up had higher airways resistance and greater evidence of gas trapping than those who were asymptomatic, as highlighted by the difference in functional residual capacity measured by plethysmography and a helium gas dilution technique.7 We were therefore interested to determine if male compared with female very prematurely born infants also had higher airway resistance and greater gas trapping, which would be compatible with more male infants being symptomatic at follow up.
Methods
Infants were eligible to be entered into the United Kingdom oscillation study (UKOS) trial8 if their gestational age was between 23 weeks and 28 weeks plus six days. Infants whose parents gave informed written consent were randomised to either high frequency oscillation ventilation or conventional mechanical ventilation within one hour of birth. Pulmonary function was assessed at 1 year of age, corrected for prematurity, in a subgroup of trial infants who lived within reasonable travelling distance of King's College Hospital, London, where the lung function measurements were made. No significant differences were found in lung function related to the randomised respiratory support mode.9 The results of the whole subgroup9 were therefore examined to determine if there was an effect of sex on lung function at 1 year corrected age.
Infants were tested between the corrected ages of 11 and 14 months. All were seen in the paediatric lung function laboratory at King's College Hospital. On arrival, a history was taken and each infant was weighed, measured, and examined. No testing was undertaken within at least two weeks of a respiratory tract infection.
Respiratory function was assessed by plethysmographic measurement of airways resistance (Raw) and functional residual capacity (FRCpleth), from which specific conductance (sGaw) was calculated. In addition, functional residual capacity was assessed using a helium gas dilution technique (FRCHe). The infants were sedated with 80–120 mg/kg chloral hydrate, and monitored by pulse oximetry (3800; Datex‐Ohmeda, Hatfield, UK) throughout the pulmonary function testing and afterwards until they were awake.
Once asleep, the infant was laid supine in the plethysmograph (Department of Medical Engineering, Hammersmith Hospital, London, UK), which had a total volume of 90 litres and included a heated, humidified rebreathing system. The infant breathed through an appropriately sized Rendell‐Baker face mask, sealed around the nose and mouth with silicone putty. The mask deadspace was calculated as 50% of the water displacement measurement with the silicone putty in situ.10 Pressure at the airway opening (Pao) was measured using a differential pressure transducer (range ± 5 kPa; MP45; Validyne Engineering Corporation, Northridge, California, USA) connected to a port in the mask support. The mask support also incorporated a thermistor measuring airway temperature and was connected to a heated pneumotachograph (Fleisch, Lausanne, Switzerland) to measure airflow. The pneumotachograph was attached to a differential pressure transducer (range ± 0.2 kPa). The total deadspace of the breathing apparatus, including the mask and all tubing connecting the transducers, measured by water displacement, was 31 ml.10 Pressure changes with the plethysmograph were measured using a differential pressure transducer (range ±0.2 kPa). All signals were amplified (CD18 carrier amplifiers; Validyne Engineering Corporation), and the flow signal integrated electrically to give tidal volume (FV 156 Integrator; Validyne Engineering Corporation). The resultant four channels of data were acquired, analysed, and displayed in real time on a personal computer (GP7‐500; Gateway, Dublin, Ireland) running a program custom designed using LabWindows software (National Instruments, Austin, Texas, USA) with analogue to digital sampling at 200 Hz (PC‐LPM‐16PnP; National Instruments). All channels were calibrated before each patient test, as previously described.11 FRCpleth was calculated from a minimum of three end inspiratory occlusions. A time based trace of all four data channels and an x/y plot of Vpleth/Pao during each occlusion were displayed by the computer. Occlusions were considered acceptable if Vpleth and Pao were in phase and no airflow was evident. The infant was then switched to the rebreathing bag. Individual breaths acquired during periods of rebreathing were displayed as x/y plots of Vpleth/flow by the computer. Only technically acceptable breaths—that is, the loop was closed or nearly closed at points of zero flow—were used in the analysis.12 Raw was calculated electronically using an established formula13 by applying a regression line to the selected portion of the loop. It was calculated during initial inspiration between 0% and 50% maximal inspiratory flow and during expiration between 0% and 50% maximal expiratory flow.11 During all Raw measurements, the computer calculated the apparatus resistance of the selected portion of the individual breath by relating ΔPao to Δflow, and then subtracted this value from the total measured resistance.14
On completion of the plethysmographic measurements, FRCHe was measured while the infant lay undisturbed on the base of the plethysmograph, using the same mask with silicone putty. During the initial stages of the study, FRCHe was determined using a water sealed spirometer (Pulmonet III; Gould, Bilthoven, the Netherlands) as described previously.15 The deadspace of the breathing apparatus for this system, including the mask and filter, was 66 ml. Subsequently infants were tested using the EBS 2615 system (Equilibrated Bio Systems, New York, USA), which consisted of a 500 ml rebreathing bag in a closed heliox circuit. The deadspace of this system, again including mask and filter, was 22 ml. The system was modified to produce a time based display of flow and tidal volume, allowing accurate switching into the circuit at end expiration.16 An online display of the helium dilution curve allowed precise determination of gas equilibration. For both FRCHe techniques, the mean of two recordings that were within 10% of each other was taken.17 The FRCHe of 12 infants was measured using both devices to assess comparability. Results from these 12 infants were included in the sex study, using the FRCHe measurement from the EBS 2615. The agreement between the two devices was assessed using the Bland‐Altman method.18 The mean (SD) difference in measured FRCHe was 3.4 (12.5) ml with 95% limits of agreement of −21.2 to 27.9 ml.
Patients
Lung function measurements were made on 76 infants with a mean (SD) gestational age of 26.4 (1.5) weeks. Their mean standard deviation score for birth weight was −0.52 (0.97), and 17% were below the tenth centile for birth weight. The standard deviation score for birth weight was calculated using published growth standards.19 Fifty five per cent had been randomised to high frequency oscillation ventilation. Mean (SD) days of ventilation was 21.1 (20.8), 59% were oxygen dependent at 36 weeks postmenstrual age (PMA), and 18% were oxygen dependent at time of discharge home. This study was approved by the King's College Hospital Research Ethics Committee, and the UKOS trial by the South Thames Multicentre Research Ethics Committee. Parents gave informed written consent for their infants to take part.
Analysis
Differences were assessed for statistical significance using Student's t test or χ2 test as appropriate. Multiple linear regression was used to adjust lung function using birth and anthropometric variables at measurement that were significantly related to sex in unifactorial analyses (see table 1 for the full list of variables tested). These multifactorial analyses were performed in two stages: modelling (a) significant birth variables and (b) significant birth variables and significant anthropometric variables at measurement. Results are therefore presented as the difference in mean lung function between male and female infants unadjusted and adjusted in the two stages as described above. Analyses were carried out using Stata 8 (Stata Corp 2003).
Table 1 Characteristics of study infants according to sex.
| Variable | Male (n = 42) | Female (n = 34) | Difference* (95% CI) | p Value |
|---|---|---|---|---|
| Gestational age (weeks) | 26.4 (1.5) | 26.4 (1.4) | 0.02 (−0.93 to 0.97) | 0.960 |
| Birth weight (g) | 833 (187) | 877 (161) | −44 (−155 to 67) | 0.282 |
| Birthweight SDS† | −0.81 (0.97) | −0.17 (0.85) | −0.64 (−1.22 to −0.06) | 0.003 |
| Below 10th birthweight centile‡ | 24% | 9% | 15% (−1% to 31%) | 0.126 |
| Randomised to HFOV | 57% | 53% | 4% (−18% to 27%) | 0.714 |
| Multiple birth | 14% | 29% | −15% (−34% to 3%) | 0.108 |
| Days ventilated¶ | 17.0 | 6.9 | 2.45 (1.37 to 4.38) | 0.003 |
| O2 dependent at 36 weeks PMA | 69% | 47% | 22% (0% to 44%) | 0.052 |
| O2 dependent at discharge | 29% | 6% | 23% (7% to 38%) | 0.016 |
| White ethnic group | 79% | 68% | 11% (−9% to 31%) | 0.282 |
| Smoking in pregnancy | 24% | 14% | 10% (−9% to 28%) | 0.343 |
| Maternal smoking after birth | 24% | 18% | 6% (−14% to 26%) | 0.585 |
| Corrected age at measurement (months) | 12.55 (0.86) | 12.59 (0.99) | −0.04 (−0.46 to 0.38) | 0.849 |
| Length at measurement (cm) | 73.34 (2.96) | 75.24 (2.74) | −1.90 (−3.22 to −0.58) | 0.005 |
| Weight at measurement (kg) | 8.54 (1.15) | 9.25 (1.34) | −0.71 (−1.28 to −0.14) | 0.016 |
Values are mean (SD) or percentages.
*Differences in means and proportions are expressed as male minus female.
†Standard deviation score, standardised for gestational age and sex.19
‡Below 10th centile for gestational age and sex.19
¶Geometric mean used as variable is skewed. Difference is expressed as a ratio of geometric means male/female.
HFOV, High frequency oxygen ventilation; PMA, postmenstrual age.
Results
The male infants were lighter at birth (p = 0.003 for birth weight standard deviation score), required more days of ventilation (p = 0.003), and were more likely to be oxygen dependent at 36 weeks PMA (p = 0.052) and at discharge home (p = 0.016) (table 1). They were also shorter (p = 0.005) and lighter (p = 0.016) when they underwent lung function measurements (table 1).
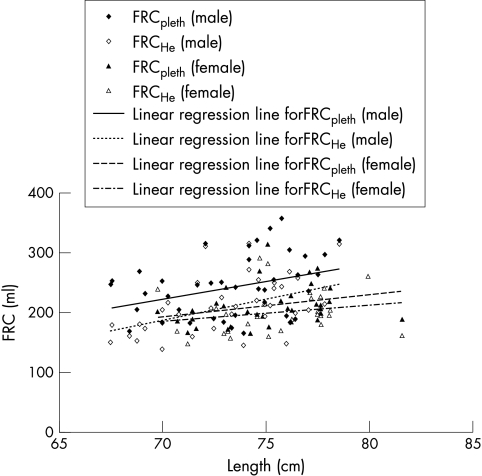
The mean Raw (p = 0.005) and FRCpleth (p = 0.0045) (fig 1) of the male infants were significantly higher and their mean sGaw lower (p<0.0001) than those of the female infants (table 2). They also had a higher mean FRCHe (fig 1), but this difference did not reach statistical significance. The male infants had a tendency to greater gas trapping as indicated by the difference in FRCpleth and FRCHe: mean (SD) FRCpleth – FRCHe 32.0 (37.5) v 17.1 (22.7) ml; difference between means 14.9 ml (95% CI −0.6 to 30.3) (p = 0.059). Lung function was adjusted in two stages for the significant birth variables, birth weight standard deviation score, days ventilated, and oxygen dependency at discharge, and then for the birth variables plus the significant anthropometric variables, length and weight at measurement. These analyses show that the difference between male and female infants in mean Raw was halved (13%) and became non‐significant. Similarly, the difference in FRCHe (10%) was non‐significant after adjustment, but the differences between male and female infants for FRCpleth and sGaw were similar in size and remained significant after each of the two stages of adjustment (15% and 26% respectively; table 2).
Figure 1 Plot of functional residual capacity (FRC) against length. Individual data are given. The solid and dotted lines represent the data of FRCpleth and FRCHe according to sex.
Table 2 Lung function results in male (n = 42) and female (n = 34) infants before and after adjustment.
| Variable | Male | Female | Difference* | Adjusted difference† | Adjusted difference‡ | R2 (total)¶ (%) | R2 (sex)§ (%) |
|---|---|---|---|---|---|---|---|
| FRCpleth (ml) | 244.2 (50.8) | 213.6 (34.5) | 30.6 (9.8 to 51.5) | 32.4 (8.0 to 56.8) | 37.8 (13.7 to 61.9) | 19.3 | 10.7 |
| FRCpleth (ml/kg) | 29.0 (6.6) | 23.7 (4.6) | 5.3 (2.5 to 8.0) | 4.0 (0.9 to 7.2) | 3.6 (0.5 to 6.8) | 37.6 | 17.2 |
| Raw (kPa/litre/s) | 3.79 (1.58) | 2.81 (1.01) | 0.98 (0.31 to 1.64) | 0.50 (−0.25 to 1.24) | 0.36 (−0.40 to 1.12) | 23.4 | 11.1 |
| sGaw (1/kPa.s) | 1.28 (0.45) | 1.93 (0.61) | −0.65 (−0.90 to −0.40) | −0.52 (−0.81 to −0.23) | −0.51 (−0.80 to −0.21) | 34.9 | 27.9 |
| FRCHe (ml) | 212.4 (45.6) | 201.3 (35.0) | 11.1 (8.3 to 30.6) | 11.8 (−10.7 to 34.4) | 19.9 (−1.8 to 41.6) | 18.5 | 1.8 |
| FRCHe (ml/kg) | 25.2 (5.5) | 22.0 (5.1) | 3.2 (0.7 to 5.7) | 2.4 (−0.5 to 5.2) | 2.1 (−0.9 to 5.0) | 32.8 | 8.7 |
Values are mean (SD) or mean (95% confidence interval).
*Male − female results.
†Difference between male and female infants adjusted for birthweight standard deviation score, days ventilated, and oxygen dependence at discharge.
‡Difference between male and female infants adjusted for birthweight standard deviation score, days ventilated, oxygen dependence at discharge, and length and weight at time of measurement, except where lung function measure is already adjusted for weight.
¶R2 (total) is the proportion of variability explained by model 2.
§R2 (sex) is the proportion of variability explained by sex alone.
For FRCpleth, Raw, and sGaw, at least half of the total variability was explained by sex (table 2).
Sensitivity analyses were also performed with adjustment for maternal smoking in pregnancy and after birth and for race, even though these were not significant in the unifactorial analyses. The sizes of effect of sex for all the lung function measures were hardly changed after this further adjustment. This therefore shows that the observed effects of sex are not explained by smoking in pregnancy or after birth, or race (data not shown).
Discussion
We have shown that male compared with female very prematurely born infants had significantly different lung function at 1 year corrected age and that significant differences in FRCpleth and sGaw remained after adjustment for differences in birth and current size. Adjustment for size at birth is important, as we20 and others have shown that intrauterine growth retardation adversely effects infant lung function; in particular, airways resistance is significantly related to intrauterine growth retardation. Results of previous studies3,21,22,23,24,25,26 are consistent with diminished airway function in male compared with female infants and children. Our data highlighting a lower sGaw in the very premature infants are compatible with these findings and suggest that the effect of sex on lung function occurs relatively early in gestation. Previous studies, however, have not reported a difference in forced vital capacity between the sexes2 or sex related differences in functional residual capacity early in life,1,27,28 but those studies included healthy infants and thus the population was very different from that studied here. Several studies29,30 have shown that, even in the absence of neonatal respiratory disease, preterm delivery is associated with altered lung function. Examination of 32 healthy preterm infants (gestational age 25–33 weeks) after birth at a mean PCA of 40 weeks revealed that, compared with healthy full term infants, they had lower specific compliance and impaired gas mixing efficiency. This suggests that preterm birth per se affects alveolarisation and formation of elastic tissues in the lungs.29 In another study,30 24 preterm infants had significantly reduced maximum expiratory flow at functional residual capacity at 1 year compared with those determined at three weeks after birth, suggesting that preterm delivery is associated with altered airway development during early infancy. Lung volumes have been reported to be greater in older boys than similarly aged girls,31 and hence it was suggested2 that, if such a difference is present in infants, it may be too small to be detected by the numbers evaluated using the rapid thoracic compression technique. In this study, we determined lung volumes by both helium gas dilution and a plethysmographic technique and found that lung volumes related to body weight were significantly larger in the boys than the girls. The difference in lung volume between the sexes was greater for FRCpleth than FRCHe and remained significant after adjustment. In addition, we found that the boys had evidence of greater gas trapping—that is, the difference between FRCpleth and FRCHe was larger. Greater gas trapping is compatible with poorer airway function. We interpret our results as showing that the sex differences in lung volume are due to hyperinflation in the boys and not to small lung volumes in the girls.
What is already known on this topic
Male compared with female children born at term may be disadvantaged by having poorer respiratory function and more respiratory illnesses
In prematurely born infants, male sex is a significant risk factor for troublesome respiratory symptoms and treatment requirement in infancy
What this study adds
In very prematurely born infants, lung function at 1 year (corrected for prematurity) was significantly worse in boys than girls, and this effect was not entirely explained by differences in birth variables or current size
These results are consistent with a greater proportion of prematurely born male infants being symptomatic in infancy and suggest such infants will have greater ongoing respiratory morbidity
The strengths of this study are that (a) it is the first to examine the influence of sex on lung function of very premature infants at follow up and (b) a comprehensive lung function assessment was made. A potential weakness, however, is that the study population was originally recruited to determine the effect of two different methods of respiratory support.8 No significant differences in the lung function results were found with regard to the randomised mode of respiratory support,9 and the proportions of male and female infants supported by high frequency oscillation ventilation who had lung function measurements at follow up were similar (table 1). Hence, we feel it was appropriate to assess the effect of sex on lung function using this study population.
The lungs of boys are about one week more immature than those of girls, as demonstrated by lower lecithin/sphingomyelin ratios in amniotic fluid and a later appearance of phosphatidylglycerol.32 Male fetuses are exposed to higher concentrations of androgen and Mullerian inhibiting substance, which adversely affect surfactant production.33,34 Boys are more likely to develop respiratory distress syndrome,35 suffer a more severe course, and die from respiratory distress syndrome.36 It is therefore not surprising that we showed that the male infants required significantly longer mechanical support and a greater proportion were oxygen dependent at both 36 weeks PMA and discharge home (table 1). Nevertheless, regression analysis revealed that the effect of male sex on lung function was independent of the duration of ventilation and requirement for supplementary oxygen at either 36 weeks PMA or discharge and other factors.
More of the boys had mothers who smoked in pregnancy or after birth, but these differences did not reach statistical significance (table 1). Nevertheless, as maternal smoking is known to be associated with impaired airway development and function,37,38 we performed a sensitivity analysis adjusting for maternal smoking and race. These analyses showed that the sizes of the effect of sex for all of the lung function measures were hardly changed after this further adjustment. Thus the observed effects of sex on lung function were not explained by smoking in pregnancy or after birth, or race.
It has been reported that diminished airway function at the end of the first year appears to be related to impaired airway development during early life.37 In infants born at or near term (>35 weeks gestation), sGaw during end expiration was significantly diminished in infants who had diminished sGaw during end expiration eight weeks after birth.37 We only measured our study population on one occasion, at a corrected age of 1 year, so cannot comment on their earlier lung function. It would be interesting to perform serial measurements during infancy in very premature infants to determine whether male infants have poorer airway function even in the first weeks after birth and are therefore predisposed to diminished airway function at 1 year and a greater tendency to have troublesome respiratory symptoms.
In conclusion, male compared with female very premature infants have poorer lung function at 1 year corrected age. Diminished lung function at that age in premature infants is predictive of ongoing respiratory morbidity.6 It is therefore important to determine whether these male infants are at increased risk of such morbidity during the preschool years.
Acknowledgements
MRT and LM were supported by the Medical Research Council. We thank Mrs D Gibbons for secretarial assistance.
Abbreviations
FRCHe - functional residual capacity assessed using a helium gas dilution technique
FRCpleth - functional residual capacity measured by whole body plethysmography
Pao - pressure at the airway opening
PMA - postmenstrual age
Raw - airways resistance
sGaw - specific conductance
Footnotes
Competing interests: none declared
References
- 1.Hanrahan J P, Tager I B, Castile R G.et al Pulmonary function measures in healthy infants: variability and size correction. Am Rev Respir Dis 19901411127–1135. [DOI] [PubMed] [Google Scholar]
- 2.Jones M, Castile R, Davis S.et al Forced expiratory flows and volumes in infants. Am J Respir Crit Care Med 2000161353–359. [DOI] [PubMed] [Google Scholar]
- 3.Lum S, Hoo A‐F, Dezateux C.et al The association between birthweight, sex and airway function in infants of non‐smoking mothers. Am J Respir Crit Care Med 20011642078–2084. [DOI] [PubMed] [Google Scholar]
- 4.Ho A ‐ F, Henschen M, Dezateux C.et al Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med 1998158700–705. [DOI] [PubMed] [Google Scholar]
- 5.Greenough A, Limb E, Marston L.et al Risk factors for respiratory morbidity in infancy after very premature birth. Arch Dis Child Fetal Neonatal Ed 200590F320–F323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giffin F, Greenough A, Yuksel B. Relationship between lung function results in the first year of life and respiratory morbidity in early childhood in patients born prematurely. Pediatr Pulmonol 199418290–294. [DOI] [PubMed] [Google Scholar]
- 7.Yuksel B, Greenough A. Relationship of symptoms to lung function abnormalities in preterm infants at follow up. Pediatr Pulmonol 199111202–206. [DOI] [PubMed] [Google Scholar]
- 8.Johnson A H, Peacock J L, Greenough A.et al High frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med 2002347633–642. [DOI] [PubMed] [Google Scholar]
- 9.Thomas M, Thomas M, Rafferty G.et al Pulmonary function at follow up of very preterm infants from the UK oscillation study. Am J Resp Crit Care Med 2004169868–872. [DOI] [PubMed] [Google Scholar]
- 10.Frey U, Stocks J, Coates A.et al Specifications for equipment used for infant pulmonary function testing. ERS/ATS task force on standards for infant respiratory function testing. Eur Respir J 200016731–740. [DOI] [PubMed] [Google Scholar]
- 11.Thomas M, Greenough A, Blowes R.et al Airway resistance estimation by best fit analysis in very premature infants. Physiol Meas 200223279–285. [DOI] [PubMed] [Google Scholar]
- 12.Stocks J, Levy N M, Godfrey S. A new apparatus for the accurate measurement of airway resistance in infancy. J Appl Physiol 197743155–159. [DOI] [PubMed] [Google Scholar]
- 13.Stocks J, Marchal F, Kraemer R.et al Plethysmographic assessment of functional residual capacity and airway resistance. In: Stocks J, Sly PD, Tepper RS, et al, eds. Infant respiratory function testing. New York: John Wiley & Sons, Inc, 1996190–240.
- 14.Stocks J, Godfrey S, Bearsmore C.et al Plethysmographic measurements of lung volume and airway resistance. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J 200117302–312. [DOI] [PubMed] [Google Scholar]
- 15.Yuksel B, Greenough A. Functional residual capacity to thoracic gas volume (FRC:TGV) ratio in healthy neonates. Respir Med 199589429–433. [DOI] [PubMed] [Google Scholar]
- 16.Tepper R S, Merth I T, Newth C J.et al Measurement of functional residual capacity in infants by helium dilution and nitrogen washout techniques. In: Stocks J, Sly PD, Tepper RS, et al, eds. Infant respiratory function testing. New York: John Wiley & Sons, Inc, 1996165–189.
- 17.Morris M G, Gustafsson P, Tepper R.et al The bias flow nitrogen washout technique for measuring the functional residual capacity in infants. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Eur Respir J 200117529–536. [DOI] [PubMed] [Google Scholar]
- 18.Bland J M, Altman D G. Statistical methods for assessing the agreement between two methods of clinical measurement. Lancet 1986I307–310. [PubMed] [Google Scholar]
- 19.Cole T J, Freeman J V, Preece M A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalised likelihood. Stat Med 199817407–429. [PubMed] [Google Scholar]
- 20.Greenough A, Yuksel B, Cheeseman P. Effect of inutero growth retardation on lung function at follow up of prematurely born infants. Eur Respir J 200424731–733. [DOI] [PubMed] [Google Scholar]
- 21.Tager I B, Hanrahan J P, Tosteson T D.et al Lung function pre and postnatal smoke exposure and wheezing in the first year of life. Am Rev Respir Dis 1993147817. [DOI] [PubMed] [Google Scholar]
- 22.Stocks J, Henschen M, Hoo A F.et al The influence of ethnicity and gender on airway function in preterm infants. Am J Respir Crit Care Med 19971561855–1862. [DOI] [PubMed] [Google Scholar]
- 23.Clarke J R, Salmon B, Silverman M. Bronchial hyperresponsiveness in the neonatal period as a risk factor for wheezing in infancy. Am J Respir Crit Care Med 19951511434–1440. [DOI] [PubMed] [Google Scholar]
- 24.Martinez F D, Morgan W J, Wright A L.et al Diminished lung function as a predisposing factor for respiratory illness in Infants. N Engl J Med 19883191112–1117. [DOI] [PubMed] [Google Scholar]
- 25.Hubbert M, Lanniga N, Raven J.et al Gender differences in lung growth. Pediatr Pulmonol 199519129–134. [DOI] [PubMed] [Google Scholar]
- 26.Gold D, Rotnizky R A, Damokosh A.et al Race and gender differences in respiratory illness prevalance and their relationship to environmental exposures in children 7 to 14 years of age. Am Rev Respir Dis 199314810–14. [DOI] [PubMed] [Google Scholar]
- 27.Tepper R S, Morgan W J, Cota K.et al Physiological growth and development of the lung during the first year of life. Am Rev Respir Dis 1986134513–519. [DOI] [PubMed] [Google Scholar]
- 28.Tepper R S, Resiter T. Forced expiratory flows and lung volumes in normal infants. Pediatr Pulmonol 199315357–361. [DOI] [PubMed] [Google Scholar]
- 29.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med 200216583–87. [DOI] [PubMed] [Google Scholar]
- 30.Hoo A ‐ F, Dezateaux C, Henschen M.et al Development of airway function in infancy after preterm delivery. J Pediatr 2002141652–658. [DOI] [PubMed] [Google Scholar]
- 31.Quanjer P H, Stocks J, Polcan G.et al Compilation of reference values for lung function measurements in children. Eur Respir J 19894184S–261S. [PubMed] [Google Scholar]
- 32.Fleisher B, Kulovich M V, Hallman M.et al Lung profile: sex differences in normal pregnancy. Obstet Gynaecol 198566327–330. [PubMed] [Google Scholar]
- 33.Catlin E A, Powell S M. Manganaro TF, et al. Sex‐specific fetal lung development and Mullerian inhibiting substance. Am Rev Respir Dis 1990141466–470. [DOI] [PubMed] [Google Scholar]
- 34.McMillan E M, King G M, Adamson I Y. Sex hormones influence growth and surfactant production in fetal lung explants. Exp Lung Res 198915167–169. [DOI] [PubMed] [Google Scholar]
- 35.Perelman R H, Palta M, Kirby R.et al Discordance between male and female deaths due to the respiratory distress syndrome. Pediatrics 198678238–244. [PubMed] [Google Scholar]
- 36.Khoury M J, Marks J S, McCarthy B J.et al Factors affecting the sex differential in neonatal mortality: the role of respiratory distress syndrome. Am J Obstet Gynecol 1985151777–782. [DOI] [PubMed] [Google Scholar]
- 37.Dezateaux C, Stocks J, Wade A M.et al Airway function at one year: association with premorbid airway function, wheezing and maternal smoking. Thorax 200156680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dezateaux C, Stocks J, Dundas I.et al Impaired airway function and wheezing in infancy. The influences of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med 1999159403–410. [DOI] [PubMed] [Google Scholar]