Abstract
Objective
To describe the epidemiology of invasive fungal infection in very low birthweight (VLBW: <1500 g) infants in the United Kingdom.
Design
National prospective surveillance study between February 2003 and February 2004 using the British Paediatric Surveillance Unit reporting system reconciled with cases identified through routine laboratory reporting to the Health Protection Agency (England, Wales, and Northern Ireland), the Scottish Centre for Infection and Environmental Health, and the UK Mycology Reference Laboratory.
Results
Ninety four confirmed cases of invasive fungal infection were identified during the surveillance period giving an incidence of estimated annual incidence of 10.0 (95% confidence interval (CI) 8.0 to 12.0) cases per 1000 VLBW live births. Eighty one (86%) of the infants were of extremely low birth weight (ELBW: <1000 g), incidence 21.1 (95% CI 16.5 to 25.7) per 1000 ELBW live births. Candida species, predominantly C albicans and C parapsilosis, were isolated in 93% of cases. Most organisms were isolated from the bloodstream and urinary tract. Death occurred in 41% of the infected infants before 37 weeks postconceptional age.
Conclusions
The incidence of invasive fungal infection in VLBW and ELBW infants in the United Kingdom is lower than reported in previous studies from tertiary centres in North America and elsewhere. The associated late neonatal and post‐neonatal death rates are substantially higher than expected in infants without invasive fungal infection. These data may inform decisions about the evaluation and use of antifungal infection control strategies.
Keywords: very low birth weight, candida, surveillance, fungal infection
Invasive fungal infection is an important cause of morbidity and mortality in very low birthweight (VLBW: <1500 g) infants.1,2 The previously reported estimates of incidence of invasive fungal infection in VLBW infants are between 3% and 6%.3,4,5,6,7,8 Mortality of 25–40% is higher than associated with invasive bacterial infection. However, these estimates are from studies in tertiary centres and may overstate the true disease burden because of referral and ascertainment biases.
The clinical presentation of invasive fungal and bacterial infection is similar, and this may cause diagnostic delay.6 Diagnosis and treatment may be further delayed because of difficulty in culturing the organisms from blood, cerebrospinal fluid, or urine.9 A high index of suspicion and the use of additional laboratory and clinical tests may be needed to confirm the suspected diagnosis.
Given the high mortality and the difficulty in establishing an early diagnosis, recent research attention has focused on the effect of strategies to prevent invasive fungal infection in VLBW infants.10 Evidence exists from Cochrane systematic reviews that prophylactic antifungal therapy reduces the risk of invasive fungal infection in VLBW infants.11,12 However, in some of the randomised trials included in these reviews, the incidence of invasive fungal infection in the control group was extremely high.13,14 The applicability of these findings to settings with lower incidence has been questioned, as very many more infants than suggested from the trial data would need to receive prophylaxis to prevent a single extra case of infection. In addition, there is concern that widespread prophylactic use of antifungal agents, particularly fluconazole, may drive the emergence of antifungal resistant Candida species.
We have undertaken a national prospective surveillance study to describe the clinical epidemiology of invasive fungal infection in VLBW infants in the United Kingdom. These data may be used to inform the future evaluation and use of antifungal prophylaxis and other strategies to prevent invasive fungal infection in VLBW infants.
Methods
We identified cases of invasive fungal infection in VLBW infants through independent prospective national surveillance schemes.
Notifications to the British Paediatric Surveillance Unit (BPSU). Each month between February 2003 and February 2004 we asked all consultant paediatricians in the United Kingdom to report whether or not they had seen any new cases (table 1)
Reports of deep fungal infections in infants aged less than 3 months from microbiology laboratories to the Communicable Disease Surveillance Centre, Health Protection Agency (England, Wales, and Northern Ireland) and the Scottish Centre for Infection and Environmental Health
Fungal isolates from deep infections in infants aged less than 3 months referred to the UK Mycology Reference Laboratory for characterisation and/or susceptibility testing
Table 1 Case definition of invasive fungal infection in very low birthweight infants.
| One or more of the following: |
| • culture of fungus from a sterile site |
| • blood (peripheral site, not via an indwelling catheter) |
| • central intravascular catheter (“long line”) tip |
| • urine (suprapubic aspirate or aseptic “in‐out” urinary catheter sample) |
| • cerebrospinal fluid |
| • bone or joint |
| • peritoneal or pleural space |
| • pathognomonic findings on ophthalmological or renal ultrasound examination |
| • autopsy diagnosis of invasive fungal infection |
We sought basic clinical, and microbiological details of cases and resolved any discrepancies in case definition with the reporting clinicians. We pooled and reconciled cases using the infant's date of birth, sex, and hospital of care to avoid duplication.
Denominator data
We used data from the Office of National Statistics (England and Wales), Information Statistics Division (Scotland), and Government Health Statistics (Northern Ireland) to estimate the number of VLBW infants live born in the United Kingdom during the study period. Complete figures were available from Northern Ireland for the study time period. For Scotland and England and Wales, we estimated the number of live births from the data that were available for January to December 2003.
Research ethics committee approval
The Scottish Multi‐centre Research Ethics Committee and the Ethics Committee of the Health Protection Agency approved the study.
Results
The response rate of UK consultant paediatricians to BPSU surveillance requests during 2003 was 92% (http://bpsu.inopsu.com/). We received reports of cases from paediatricians in 56 neonatal units. These identified 86 confirmed cases. Thirty six of these were also identified through microbiological surveillance reports to the Communicable Disease Surveillance Centre and the UK Mycology Reference Laboratory. Eight cases that were not reported to the BPSU were identified through microbiological surveillance by these agencies. Therefore 94 cases were identified in total.
About 9425 VLBW infants were live born in the United Kingdom during the study period. The estimated incidence of invasive fungal infection is therefore 10.0 (95% confidence interval (CI) 8.0 to 12.0) cases per 1000 liveborn VLBW infants.
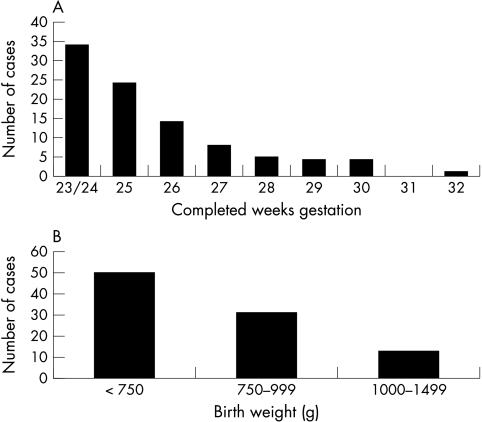
The median gestational age at birth of infants with invasive fungal infection was 25 weeks (range 23–32) (fig 1A). The median birth weight was 720 g (range 420–1460) (fig 1B). Eighty one (86%) of the patients were extremely low birth weight (ELBW: <1000 g). About 3837 ELBW infants were live born in the United Kingdom during the study period, giving an estimated annual incidence of 21.1 (95% CI 16.5 to 25.7) per 1000 liveborn ELBW infants.
Figure 1 Gestational age (A) and birth weight (B) distribution of very low birthweight infants with invasive fungal infection.
Incidence adjusted for early neonatal mortality
Early neonatal mortality (before seven days after birth) reported by the Office for National Statistics for 2002 was 132 per 1000 live births for VLBW infants and 280 per 1000 live births for ELBW infants. When the estimated number of infants who survive beyond the first week after birth is used as the denominator and only those cases identified more than six days after birth as the numerator, the adjusted estimates of incidence are 10.0 (95% CI 7.9 to 12.1) per 1000 VLBW infants and 25.7 (95% CI 19.7 to 31.7) per 1000 ELBW infants.
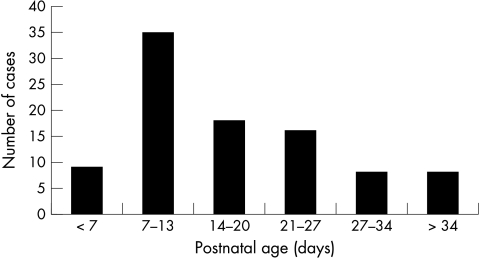
The median age at diagnosis was 14 days (range 0–106) (fig 2). Two cases had evidence of early onset (congenital) infection, with fungal sepsis identified within three days of birth. Other clinical features of the population, including exposure to putative risk factors for invasive fungal infection, are detailed in table 2.
Figure 2 Postnatal age (days) at diagnosis of invasive fungal infection in very low birthweight infants.
Table 2 Clinical exposures of cases.
| Exposure | Number of cases |
|---|---|
| Parenteral nutrition | 91 |
| Central vascular access | 89 |
| Mechanical ventilation | 90 |
| Any antibiotics | 92 |
| • Aminoglycosides | 80 |
| • Vancomycin/teicoplanin | 75 |
| • Cephalosporins | 64 |
| Postnatal steroids | 18 |
| H2 receptor antagonists | 16 |
| Peritoneal dialysis | 3 |
Table 3 details the organ involvement and sites of isolation of fungi during the clinical course. Fungi were isolated from multiple sites in 42 (45%) cases. Of the 27 infants who did not have a fungus isolated from blood culture, 13 had a diagnosis based only on another single positive culture or pathognomonic finding (table 4).
Table 3 Organ and system involvement and sites of isolation of fungus.
| Site of infection or site of isolation of organism | Number of cases |
|---|---|
| Peripheral blood | 67 |
| Urine | 26 |
| Radiographic evidence of pneumonia* | 26 |
| Umbilical line tip | 21 |
| Percutaneous central line tip | 17 |
| Skin abscess | 17 |
| Ultrasound finding of renal “fungal balls” | 9 |
| Meningitis† | 7 |
| Peritonitis | 7 |
| Osteomyelitis | 2 |
| Endocarditis | 3 |
| Ophthalmitis | 2 |
| Postmortem diagnosis only | 3 |
*In addition to the 26 infants with chest radiographic evidence of pneumonia, reporting clinicians were unable to attribute chest radiographic changes to infection as opposed to respiratory distress in a further 11 infants.
†34 infants did not have a cerebrospinal fluid examination.
Table 4 Cases diagnosed on the basis of a single clinical finding or fungus isolation.
| Site of infection or site of isolation of organism | Number of cases |
|---|---|
| Urine | 6 |
| Cerebrospinal fluid | 2 |
| Peritoneum | 2 |
| Umbilical line tip* | 1 |
| Ophthalmitis | 1 |
| Ultrasound finding of renal “fungal balls” | 1 |
*This patient also had fungus isolated on two “clean catch” urine specimens.
Mycology
Candida species were isolated in 87 (93%) of the cases. Most were identified as C albicans (50; 53%) or C parapsilosis (23; 24%). Three isolates were identified as C glabrata and one each as C lusitanaei and C migosa. We do not know the species of nine Candida isolates. The remaining five fungal isolates were: two Malassezia species, one Aspergillus species, one Rhizopus species, and one unidentified yeast. Infants infected with C albicans, C parapsilosis, or other Candida species did not differ significantly in median birth weights, gestational age, or age at diagnosis.
Antifungal resistance patterns were unknown or not determined in 53 (56%) cases. Of the remainder, only one case involving an antifungal resistant organism was reported (C glabrata resistant to fluconazole).
Thirty three (35%) infants had microbiological evidence of surface colonisation with Candida species before the diagnosis of invasive fungal infection. Thirty six (38%) cases had received antifungal prophylaxis, most commonly with oral and/or topical nystatin. Nine infants had received fluconazole as prophylaxis.
Antifungal therapy
Seventy four (78%) infants were treated with amphotericin B, usually in a lipid complex formulation. Fluconazole was also commonly prescribed (46%). Forty seven (50%) infants received more than one drug either simultaneously or sequentially. Flucytosine was never prescribed as monotherapy but always used in combination with amphotericin B. Antifungal therapy was started before confirmation of the diagnosis in 21 (22%) cases. In seven cases, clinicians ceased drug administration because of suspected drug toxicity, mainly renal toxicity attributed to amphotericin B (both lipid complex and non‐lipid complex formulations), and suspected bone marrow or renal toxicity attributed to flucytosine.
Mortality
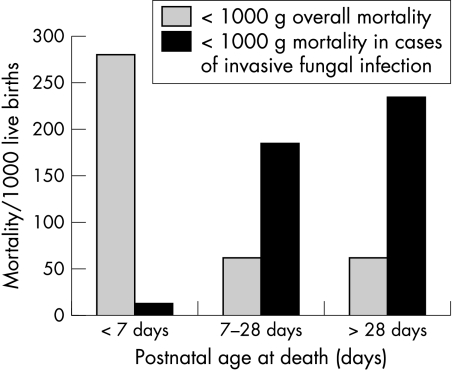
Thirty nine (41%) VLBW infants died before 37 weeks postconceptional age. Thirty five (43%) ELBW infants died. Late neonatal and post‐neonatal mortality for ELBW infants with invasive fungal infection was much higher than overall mortality for all ELBW infants in 2002 (fig 3). None of the infants with evidence of fungal meningitis died before 37 weeks postconceptional age. Mortality for infants infected with C albicans and C parapsilosis was not significantly different.
Figure 3 Mortality (early neonatal, late neonatal, post‐neonatal) per 1000 for infants with invasive fungal infection compared with all liveborn extremely low birthweight infants (Office for National Statistics 2002).
Discussion
This national prospective surveillance study estimated the incidence of invasive fungal infection to be about 1% in VLBW infants and about 2% in ELBW infants in the United Kingdom. The vast majority of cases were due to “late onset” infection acquired in hospital. We identified only two cases of fungal infection diagnosed within the first three days of birth. Although “early onset” or congenital invasive fungal infection has been described, it is extremely rare.15,16
Overall, most liveborn ELBW or VLBW infants who die do so within the early neonatal period and do not survive to acquire late onset nosocomial infection. We calculated adjusted incidences allowing for reported rates of early neonatal mortality. After adjustment, the incidence of invasive fungal infection was 2.6% in ELBW infants, still substantially lower than the incidence of 5–15% reported in other studies.2,3,4,5,6,7,8,14
Validity of case ascertainment
This population based, prospective surveillance study is less likely to have been affected by referral bias than studies restricted to tertiary centres, where the smallest and sickest infants are cared for. We undertook surveillance of the whole UK population of VLBW infants to avoid problems with clustering or temporal variation that may bias estimates of incidence from smaller studies. As our study included all liveborn VLBW infants born in the United Kingdom during the study period, we were able to provide a more precise estimate of incidence than previous smaller studies.
Some clinicians may not have reported cases but this is unlikely to be a major source of under‐ascertainment as we were able to identify unreported cases through independent laboratory sources. We may have failed to identify some cases where the diagnosis of invasive fungal infection was not confirmed by the infant's clinician. The diagnostic sensitivity of blood culture for invasive fungal infection is low (about 50%) and may be even lower in infants receiving prophylactic systemic antifungal treatment.9 About 30% of the reported cases did not have a fungus isolated from blood culture but had evidence of invasive fungal infection from culture of urine, cerebrospinal fluid, or a central intravascular line tip, or from other findings on clinical examination. It is also possible that cases in which an infant died but did not undergo postmortem examination remained undetected.
What is already known on this topic
Invasive fungal infection, mainly due to Candida species, is an increasingly common cause of mortality and morbidity in VLBW infants
The incidence is highest in the lowest birth weight and gestational age categories
We do not think that over‐ascertainment is a major problem in this study. We obtained sufficient identifying data to remove duplicate reports. We discussed any discrepancies in the case definition with the reporting clinician and resolved disagreements by consensus. Adhering to the a priori case definition reduced the likelihood of false positive microbiological diagnoses. The case definition did not include diagnoses based on culture of blood drawn from indwelling intravascular catheters as these may represent contamination rather than true bloodstream infection.
Although it is also possible that cases diagnosed solely on the basis of a fungal culture from a removed umbilical or percutaneous central line tip may be due to colonisation rather than true infection, all but one of these infants had other evidence of invasive fungal infection from deep cultures (mainly positive blood cultures from peripheral samples) or from clinical findings.
Implications for antifungal prophylaxis
Evidence from Cochrane reviews suggests that prophylactic antifungal therapy reduces the incidence of invasive fungal infection in VLBW infants. However, the general applicability of the data has been questioned because of the high incidence of invasive fungal infection in the control or placebo groups of some of the included trials.13,14 With regard to fluconazole prophylaxis, the Cochrane review estimated that treating eight (95% CI 5 to 20) VLBW infants with prophylactic fluconazole would prevent one extra case of invasive fungal infection.14 However, based on the incidence estimates in the UK population of infants from this surveillance study, we would need to treat 125 VLBW infants (or 45 ELBW infants) to prevent one extra case of invasive fungal infection.
There is concern that such widespread use may lead to the emergence of antifungal resistance. Although we did not find evidence that drug resistance is a common problem in fungal isolates in VLBW infants in the United Kingdom at present, changes in practice such as more widespread use of prophylactic antifungal therapy might alter this situation. It is essential to maintain microbiological surveillance systems to detect changes in the epidemiology of invasive fungal infection in high risk groups including VLBW infants.
Mortality
Almost half of the infants diagnosed with invasive fungal infection died before 37 weeks postconceptional age. Although the late neonatal and post‐neonatal mortality in ELBW infants with invasive fungal infection was much higher than would be expected in the absence of invasive fungal infection, we cannot attribute all deaths to infection. In some cases it may be that infants who were receiving maximum intensive care were already critically unwell and likely to die even before acquiring invasive fungal infection. Unexpectedly, none of the infants with confirmed fungal meningitis died before 37 weeks postconceptional age. However, we cannot be sure that all cases of meningitis were identified, as 40% of infected infants did not have cerebrospinal fluid examination to exclude meningitis, and this group may have included infants with meningitis who were too unwell and clinically unstable to tolerate lumbar puncture.
What this study adds
In the United Kingdom, about 1% of VLBW infants and 2% of ELBW infants acquire invasive fungal infection, lower than in previously reported studies from tertiary centres in North America and elsewhere
Late neonatal and post‐neonatal mortality are much higher than expected in infants without infection
Antifungal treatment
The antifungal treatment regimens used in the United Kingdom are similar to those reported from North America.17 At present there is insufficient evidence to favour one antifungal agent over another for treating VLBW infants with invasive fungal infection.18 Currently the clinical choice of therapy may be affected by concerns about the risk of toxicity associated with the various drugs. In some patient groups—for example, cancer patients with neutropenia—evidence exists that renal toxicity is higher with conventional amphotericin B than with lipid complex formulations or with azole drugs.19,20 However, there are only a few observational data on the relative risks of toxicity of antifungal drugs in VLBW infants.21,22
In this study, antifungal treatment was administered before the diagnosis was confirmed in less than one quarter of the cases. Given the difficulty and delay in achieving a definite diagnosis of invasive fungal infection in VLBW infants, and the high mortality, it has been proposed that early empirical antifungal therapy for suspected invasive infection could improve outcomes for VLBW infants.23 This strategy has not yet been evaluated prospectively. Although early empirical antifungal treatment is part of management algorithms for suspected infection in other immunocompromised populations—for example, cancer patients with neutropenia—there is only limited evidence that this approach improves clinical outcomes.24 Continuing efforts to develop better methods for rapid diagnosis of invasive fungal infection may help clinicians to target earlier antifungal treatment.
Acknowledgements
We thank all clinicians who responded via the various surveillance mechanisms and especially those who provided clinical details of reported cases. We thank Richard Lynn and colleagues at the British Paediatric Surveillance Unit, Elizabeth Johnson at the UK Mycology Reference Laboratory, and Alison Balfour at the Royal Hospital for Sick Children, Glasgow.
Abbreviations
ELBW - extremely low birth weight
VLBW - very low birth weight
Footnotes
Competing interests: this study was partly supported by an educational grant provided by Pfizer UK Ltd. The company had no role in the collection, analysis, and interpretation of data, or in the writing of the report and the decision to submit the paper for publication
Funding: partly supported by an educational grant provided by Pfizer UK Ltd
References
- 1.Benjamin D K, Jr, Poole C, Steibach W J.et al Neonatal candidemia and end‐organ damage: a critical appraisal of the literature using meta‐analytic techniques. Pediatrics 2003112634–640. [DOI] [PubMed] [Google Scholar]
- 2.Kossoff E H, Buescher E S, Karlowicz M G. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr Infect Dis J 199817504–508. [DOI] [PubMed] [Google Scholar]
- 3.Stoll B J, Hansen N, Fanaroff A A.et al Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002110285–291. [DOI] [PubMed] [Google Scholar]
- 4.Makhoul I R, Sujov P, Smolkin T.et al Epidemiological, clinical, and microbiological characteristics of late‐onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 200210934–39. [DOI] [PubMed] [Google Scholar]
- 5.Saiman L, Ludington E, Pfaller M.et al Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey Study Group. Pediatr Infect Dis J 200019319–324. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin D K, Jr, Ross K, McKinney R E., Jret al hen to suspect fungal infection in neonates: a clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase‐negative staphylococcal bacteremia. Pediatrics 2000106712–718. [DOI] [PubMed] [Google Scholar]
- 7.Lopez Sastre J B, Coto Cotallo G D, Fernandez Colomer B. Grupo de Hospitales Castrillo. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases, Am J Perinatol 200320153–163. [DOI] [PubMed] [Google Scholar]
- 8.Johnsson H, Ewald U. The rate of candidaemia in preterm infants born at a gestational age of 23–28 weeks is inversely correlated to gestational age. Acta Paediatr 200493954–958. [PubMed] [Google Scholar]
- 9.Schelonka R L, Moser S A. Time to positive culture results in neonatal Candida septicemia. J Pediatr 2003142564–565. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman D. Strategies for prevention of neonatal invasive candidiasis. Semin Perinatol 200327414–424. [DOI] [PubMed] [Google Scholar]
- 11.Austin N C, Darlow B. Prophylactic oral antifungal agents to prevent systemic candida infection in preterm infants. Cochrane Database Syst Rev 20042 [DOI] [PubMed] [Google Scholar]
- 12.McGuire W, Clerihew L, Austin N. Prophylactic intravenous antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev 20031 [DOI] [PubMed] [Google Scholar]
- 13.Sims M E, Yoo Y, You H.et al Prophylactic oral nystatin and fungal infections in very‐low‐birthweight infants. Am J Perinatol 1988533–36. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman D, Boyle R, Hazen K C.et al Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med 20013451660–1666. [DOI] [PubMed] [Google Scholar]
- 15.Melville C, Kempley S, Graham J.et al Early onset systemic Candida infection in extremely preterm neonates. Eur J Pediatr 1996155904–906. [DOI] [PubMed] [Google Scholar]
- 16.Stoll B J, Hansen N, Fanaroff A A.et al Changes in pathogens causing early‐onset sepsis in very‐low‐birth‐weight infants. N Engl J Med 2002347240–247. [DOI] [PubMed] [Google Scholar]
- 17.Rowen J L, Tate J M. Management of neonatal candidiasis. Neonatal Candidiasis Study Group. Pediatr Infect Dis J 1998171007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clerihew L, McGuire W. Systemic antifungal drugs for invasive fungal infection in preterm infants. Cochrane Database Syst Rev 20041 [DOI] [PubMed] [Google Scholar]
- 19.Johansen H K, Gotzsche P C. Amphotericin B lipid soluble formulations vs amphotericin B in cancer patients with neutropenia. Cochrane Database Syst Rev 20024 [DOI] [PubMed] [Google Scholar]
- 20.Johansen H K, Gotzsche P C. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database Syst Rev 20024 [DOI] [PubMed] [Google Scholar]
- 21.Adler‐Shohet F, Waskin H, Lieberman J M. Amphotericin B lipid complex for neonatal invasive candidiasis. Arch Dis Child Fetal Neonatal Ed 200184F131–F133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huttova M, Hartmanova I, Kralinsky K.et al Candida fungemia in neonates treated with fluconazole: report of forty cases, including eight with meningitis. Pediatr Infect Dis J 1998171012–1015. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin D K, Jr, De Long E R, Steibach W J.et al Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics 2003112543–547. [DOI] [PubMed] [Google Scholar]
- 24.Gøtzsche P C, Johansen H K. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database Syst Rev 20022 [DOI] [PubMed] [Google Scholar]