Abstract
Ventilator induced lung injury continues to occur at an unacceptably high rate, which is inversely related to gestational age. Although the “new BPD” may not be entirely avoidable in the extremely premature infant, recognition of risk factors and adoption of an appropriate ventilatory strategy, along with continuous real time monitoring, may help to minimise lung damage. This paper will review the pathogenesis of ventilator induced lung injury and strategies that may mitigate it.
Keywords: bronchopulmonary dysplasia, chronic lung disease, preterm, respiratory disease syndrome, ventilator induced lung injury
Bronchopulmonary dysplasia (BPD) was first described in 1967 by Northway et al,1 in a group of 13 infants who were mechanically ventilated for respiratory distress syndrome (RDS). These infants ranged from 1474 to 3204 g birth weight and 30 to 39 weeks gestational age. Most had required high concentrations of supplemental oxygen and high airway pressures. Their chest radiographs revealed overinflation and cystic emphysema, and pulmonary histopathology consisted of interstitial and alveolar oedema, small airway disease, extensive inflammation, and fibrosis.1 Over the ensuing decade, the mechanisms of chronic lung disease (CLD) became better understood. In 1975, Philip described the aetiology of BPD as “oxygen plus pressure plus time.”2 Over the next 25 years, advances in neonatal medicine extended survival to infants born considerably more immature than those described by Northway et al, and the characteristics of affected infants changed dramatically. The “old BPD” described by Northway and Philip has been replaced by the “new BPD” described by Jobe.3 The disorder is now seen primarily in very low birthweight infants requiring modest ventilator support and supplemental oxygen. It is characterised radiographically by diffuse haziness and a fine, lacy pattern, and histopathologically by decreased alveolarisation, minimal small airway disease, and less inflammation and fibrosis. There is no universally accepted definition of CLD, and thus its incidence has been variously reported, generally between 30% and 40% among very low birthweight infants.
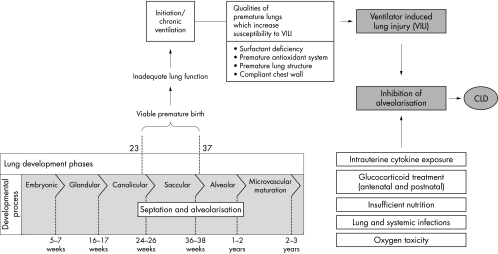
Although the past quarter century has seen dramatic advances in the treatment of RDS, including sophisticated mechanical ventilators and surfactant replacement therapy, the prevalence of CLD remains quite high. Although the characteristics of CLD have changed, and it may not be entirely avoidable, ventilator induced lung injury remains a significant component of it. Understanding the pulmonary injury sequence (fig 1) is important to the realisation that some lung damage may be minimised by using lung protective strategies. Ventilator induced lung injury itself is multifactorial.4,5 Various connotations have been used to describe its individual components, which may be inter‐related and act synergistically. Volutrauma refers to the damage caused by overdistension of the lung by the delivery of too much gas. Barotrauma, or excessive pressure, may damage airway epithelium and disrupt alveoli. Atelectotrauma refers to the damage caused by the continual opening and closing (the cycle of recruitment and subsequent de‐recruitment) of lung units. Biotrauma is a collective term to describe the injurious effects of infection and inflammation (and oxidative stress) on the developing lung. Rheotrauma refers to injury caused by inappropriate airway flow (fig 2). If excessive, turbulence, inefficient gas exchange, inadvertent positive end expiratory pressure (PEEP), and lung overinflation may occur; if inadequate, it may lead to flow starvation (air hunger) and increased work of breathing. Lung protective strategies seek to minimise ventilator induced lung injury by avoiding or ameliorating the damaging effects of these components.
Figure 1 The pulmonary injury sequence. The diagram illustrates the effect of ventilator induced injury and other factors on lung development and their relation to chronic lung disease (CLD). Reproduced from Attar MA, Donn SM. Mechanism of ventilator‐induced lung injury in premature infants. Semin Neonatol 2002;7:353–60, with permission from Elsevier.
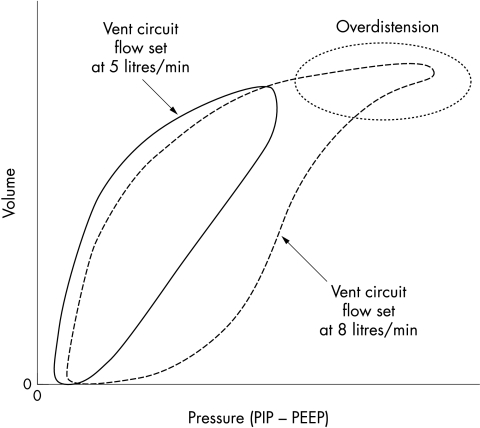
Figure 2 Pressure volume loops showing the effects of ventilator circuit flow on the elastic load. Note the overdistension occurring at the higher flow rate, which can be normalised when flow is decreased. PIP, Positive inspiratory pressure; PEEP, positive end expiratory pressure. Courtesy of V K Bhutani, MD.
Lung protective strategies
Lung protective strategies, in fact, begin even before the institution of mechanical ventilation or continuous positive airway pressure (CPAP). It is now a world wide practice to administer antenatal corticosteroids to women at risk of delivering preterm infants, generally before 32 weeks completed gestation. Antenatal corticosteroid therapy has dramatically reduced the incidence and severity of RDS and thus CLD,6 although it may be argued that improved survival of extremely preterm infants resulting from this has shifted the overall incidence of CLD. Similarly, there is little doubt about the impact of surfactant replacement therapy on the treatment of RDS resulting in increased survival,7 but without a concomitant reduction in CLD. Thus it would appear that efforts to bring about improvement in the incidence and severity of CLD must be approached by using appropriate ventilatory strategies aimed at reducing or avoiding ventilator induced lung injury.
Respiratory distress syndrome
RDS is a disorder of the premature lung. Although surfactant replacement therapy can compensate for the biochemical abnormalities to a large extent, the morphological abnormalities must be addressed. The lung has insufficient alveolarisation and thus diminished functional surface area for gas exchange. There is an increased distance from the alveolus to its adjacent capillary, and deposition of fibrin in the air spaces; both conspire to decrease gas diffusion. Pulmonary arteriolar muscularisation leads to elevated pulmonary vascular resistance and diminished pulmonary blood flow, often accompanied by right to left shunting.
Table 1 lists the goals of mechanical ventilation. These should be kept in mind no matter what device, mode, or modality is chosen. Ventilatory strategies range from the least invasive (CPAP) to the most invasive (extracorporeal membrane oxygenation), which is occasionally used in the larger premature infant with intractable respiratory failure.8
Table 1 Goals of mechanical ventilation.
| • Overcome alveolar atelectasis |
| • Achieve adequate pulmonary gas exchange |
| • Decrease the patient work of breathing |
| • Maximise patient comfort |
| • Avoid ventilator induced lung injury |
Continuous positive airway pressure
First introduced into neonatal practice by Gregory and colleagues in 1971,8a CPAP is a form of continuous distending pressure used to maintain some degree of alveolar inflation during expiration. According to the Law of LaPlace, the increased radius of curvature requires less pressure to overcome the surface tension promoting its collapse, and thus decreases the work of breathing. CPAP as a primary strategy for the treatment of RDS was popularised by Wung and colleagues, who showed a dramatic reduction in CLD compared with units where mechanical ventilation is often used.9 Wung et al emphasised the dependence on spontaneous breathing, the avoidance of sedatives and skeletal muscle relaxants, and the acceptance of a wider range of blood gases and pH. Although this remains an attractive hypothesis, there is still much controversy surrounding the use of CPAP. Randomised clinical trials are only now finally in progress. The best means of providing CPAP are yet to be determined. The relation of CPAP and surfactant replacement therapy is also in need of evaluation. Does the use of CPAP and the avoidance of intubation delay the effectiveness of surfactant in those infants who eventually require it? How do the work of breathing and energy expenditure during CPAP compare with a brief course of mechanical ventilation? What are the long term neurological outcomes for infants treated by primary CPAP as advocated by Wung et al? These issues may be resolved by the present trials but remain unanswered for now.
Permissive hypercapnia
The concept of permissive hypercapnia, allowing an elevated arterial carbon dioxide tension, was based on a retrospective observation of Kraybill et al in 1989, in which infants with the highest levels of carbon dioxide had the lowest incidence of CLD.10 The rationale for permissive hypercapnia as a protective lung strategy is that it may decrease volutrauma, decrease the duration of positive pressure ventilation, reduce alveolar ventilation, reduce complications associated with hypocapnia, and increase oxygen unloading from haemoglobin at the tissue level.
To date, there have been two prospective, controlled trials: Mariani et al11 and Carlo et al,12 both published in 1999. Although there was a reduction in the duration of positive pressure ventilation, it was not associated with a decrease in the incidence of CLD. Although this is an attractive hypothesis, further study is necessary. Parenthetically, permissive hypercapnia is also difficult to achieve with newer modes of ventilation such as patient triggered ventilation (PTV).
Conventional mechanical ventilation
It is not surprising that the early experience with intermittent mandatory ventilation would result in CLD. It is likely that patient‐ventilator asynchrony resulted in complications, including inefficient gas exchange, gas trapping and air leaks, and irregular cerebral blood flow velocity. The advent of miniature transducers and sophisticated microprocessor based mechanical ventilators ushered in the era of PTV and the development of newer modes of ventilation, including synchronised intermittent mandatory ventilation, assist‐control, and pressure support ventilation.13,14 It was hoped that by eliminating asynchronous breathing, these complications could be eliminated and the incidence of CLD reduced. PTV uses a signal derived from the patient, which serves as a measure of spontaneous breathing and synchronises the delivery of a mechanical breath to it. Using an airway flow signal, a breath may also be terminated synchronously. Avoidance of asynchronous breathing may result in the need for less pressure, facilitate weaning by making pressure the primary weaning variable, and significantly reduce gas trapping and air leaks. In addition, the advent of pressure support ventilation enabled variable levels of synchronised support during the weaning phase.
Unfortunately, trials of PTV have thus far been disappointing. Most of the studies have been underpowered to address the true impact on CLD, although short term benefits, such as decreased ventilator days and air leaks, have been described.15 Similar to permissive hypercapnia, the concept of PTV makes good sense, but additional investigation is warranted.
Volume targeted ventilation has received recent attention. With this modality, the clinician selects a volume of gas to be delivered to the patient, and the ventilator pressure is varied to deliver this volume. Theoretically, this should result in a consistent delivery of tidal volume, regardless of the patient's lung compliance, and thus volume delivery will be controlled, or limited, reducing volutrauma. A meta‐analysis of randomised controlled trials shows potential advantages of volume controlled ventilation in terms of reducing complications and duration of assisted ventilation.16 In addition, a recently completed randomised controlled trial also showed a significant reduction in time for weaning and duration of ventilation in infants 600–1000 g treated with volume controlled ventilation.17 The concept of targeting tidal volume is growing in neonatal medicine, and further studies are in progress.
Another novel technique is proportional assist ventilation. This form of ventilation is based on the individual pulmonary mechanics and the elastic and resistive loading and unloading of respiratory musculature to servo‐regulate the ventilator (adaptive ventilation). Schulze et al18 published a small clinical trial in 2001 showing lower mean and peak transpulmonary pressures compared with conventional ventilation. This may be a way to reduce barotrauma, and further study seems advisable.
High frequency ventilation
High frequency ventilation emerged in the 1980s as an alternative to conventional tidal ventilation. Two forms have been in use: high frequency jet ventilation (HFJV) and high frequency oscillatory ventilation (HFOV). The basic concept of all high frequency ventilation is that tiny gas volumes, less than the anatomical deadspace, are moved in and out of the lung at rapid rates. Minute ventilation then becomes a function of the square of tidal volume, and ventilation can be accomplished at lower pressure. HFOV differs from HFJV in that it also creates active exhalation, where gas is actively withdrawn from the lung during expiration. Frequencies used during HFJV are typically 150–660 breaths per minute, whereas those used during HFOV (with its even smaller tidal volumes) are 480–900 breaths per minute.
HFJV is used in tandem with a conventional ventilator, which provides PEEP and can be used for sigh breaths to help recruit lung volume and avoid atelectasis. It has been used primarily as a rescue therapy and for management of pulmonary interstitial emphysema. One study conducted by Keszler et al in 1997, in which HFJV was used as a primary strategy, showed a reduction in the incidence of CLD and a lower need for home oxygen therapy.19
HFOV has a wider evidence base, although the results are no more compelling. The trial of Gerstmann et al20 showed improved survival without an increase in CLD for babies treated with HFOV compared with conventional intermittent mandatory ventilation. Two German studies failed to show a reduction in CLD. Rettwitz‐Volk et al21 found no differences in any of the tested outcome variables, and Thome et al22 found a shorter time to extubation but no differences in the incidence of CLD, intraventricular haemorrhage, or death. The study of Moriette et al23 showed a decreased need for surfactant but no decrease in CLD. Two trials published in 2002 had conflicting results. The study of Courtney et al24 showed a small reduction in the incidence of CLD in babies weighing 601–1200 g, but the control arm of the trial used synchronised intermittent mandatory ventilation rather than assist‐control. The UKOS trial of Johnson et al25 found no reduction in the incidence of CLD in infants of 23–28 weeks gestation when HFOV was compared with time cycled, pressure limited intermittent mandatory ventilation. Although the strategies used in these two studies were different, possibly accounting for the discrepant conclusions, HFOV cannot be recommended as a primary strategy for RDS on the basis of the evidence to date.26
Continuous monitoring techniques
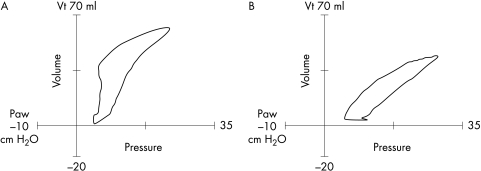
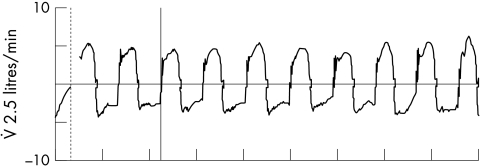
Monitoring of the ventilated infant has changed dramatically during the era of mechanical ventilation. Intermittent blood gas sampling and once or twice daily radiography have given way to continuous monitoring techniques, which can assess oxygenation, ventilation, and pulmonary mechanics (and cardiac function) on a breath to breath and beat to beat basis. Real time pulmonary graphics and waveforms enable detection of events such as hyperinflation (fig 3) and gas trapping (fig 4) before they become clinically evident. Monitoring has been shown to be a useful adjunct to weaning infants from mechanical ventilation. Although there are no randomised controlled trials, these techniques can be used to customise strategies for each patient, such as determining the optimal PEEP, adjusting how the gas flow is delivered (rise time), and judging synchrony.27
Figure 3 Pressure‐volume loops. (A) The loop shows hyperinflation, with an upper inflection point on the inspiratory limb. (B) The loop has been normalised by reducing the peak inspiratory pressure. Vt, Tidal volume; Paw, peak airways pressure.
Figure 4 Flow waveform indicative of gas trapping. On each waveform the decelerating expiratory flow never returns to baseline (zero flow state) before initiation of the next breath. Thus more gas is entering the lung than exiting.
Use of the newer ventilatory modalities such as synchronised intermittent mandatory ventilation with pressure support ventilation requires an understanding of pulmonary graphics. However, it also enhances the ability of the clinician to choose ventilator settings that produce the best results in the individual patient.
Optimising mechanical ventilation in the preterm infant
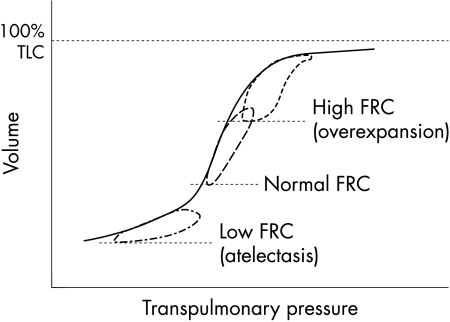
Application of the basic principles of mechanical ventilation and choosing the proper strategy should optimise pulmonary gas exchange and reduce lung injury. Avoidance of the “mechanical” elements of ventilator induced lung injury—barotrauma, volutrauma, atelectotrauma, and rheotrauma—can be accomplished by ventilating the lung close to the normal functional residual capacity. This occurs at the mid‐portion of the inflationary limb of the pressure‐volume relation (fig 5), where compliance is greatest and the zones of lung injury can be avoided. The use of higher pressures results in increased functional residual capacity, lower compliance, lung overexpansion, and both barotrauma and volutrauma. The use of pressures below the “safe zone” results in decreased functional residual capacity, lower compliance, alveolar collapse, and atelectotrauma.
Figure 5 Pressure‐volume relation. The best compliance is seen at the steepest slope of the curve, where ventilation occurs at normal functional residual capacity (FRC). TLC, Total lung capacity.
Careful attention must be given to airway and circuit flow. Flow may be defined as the time rate of volume delivery. Excessive flow in the ventilator circuit will cause a greater delivery of gas volume at the same inspiratory time, contributing to an increased elastic load and overdistension. Gas trapping and inadvertent PEEP may occur if flow is too high or if there is inadequate expiratory time for the lung to empty. Conversely, if flow is set too low, gas volumes may be too small and air hunger may develop leading to tachypnoea and increased work of breathing.
Monitoring of tidal volume delivery, irrespective of whether the target variable is pressure or volume, has taken on increased significance in recent years owing to our ability to finally measure volumes at the proximal airway. Provision of a physiological tidal volume during conventional ventilation seems prudent.
Conclusion
Clinical investigation has not yet defined the best way to avoid lung injury in preterm infants requiring mechanical ventilation. The reality is that some CLD may be inevitable in those infants delivered at a time when the lung is still in the saccular phase of development. Nevertheless, clinicians must try to avoid inflicting further damage during this time.
The ideal mode of ventilation should maintain adequate and consistent tidal volume and minute ventilation at low airway pressures. It should be able to respond quickly to sudden or unpredictable changes in pulmonary mechanics or patient demand. It should provide the lowest possible work of breathing for the baby.
The ideal ventilator is one that achieves all of the goals of mechanical ventilation. It should provide a variety of modes and modalities that can ventilate even the most challenging pulmonary diseases. It must have monitoring capabilities to adequately assess ventilator and patient performance and interaction. It must also have safety features and alarms that offer lung protective strategies. Finally, it should be operated by an experienced clinician who continues to ask the right questions and seek the right answers.
Abbreviations
BPD - bronchopulmonary dysplasia
CLD - chronic lung disease
CPAP - continuous positive airway pressure
HFJV - high frequency jet ventilation
HFOV - high frequency oscillatory ventilation
PEEP - positive end expiratory pressure
PTV - patient triggered ventilation
RDS - respiratory distress syndrome
Footnotes
Competing interests: none declared
References
- 1.Northway W H, Rosan R C, Porter D Y. Pulmonary disease following respirator therapy of hyaline membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967276357–368. [DOI] [PubMed] [Google Scholar]
- 2.Philip A G S. Oxygen plus pressure plus time: the etiology of bronchopulmonary dysplasia. Pediatrics 19755544–50. [PubMed] [Google Scholar]
- 3.Jobe A H. The new BPD: an arrest of lung development. Pediatr Res 199946641–643. [DOI] [PubMed] [Google Scholar]
- 4.Attar M A, Donn S M. Mechanism of ventilator‐induced lung injury in premature infants. Semin Neonatol 20027353–360. [DOI] [PubMed] [Google Scholar]
- 5.Dreyfuss D, Saumon G. Ventilator‐induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 1998157294–323. [DOI] [PubMed] [Google Scholar]
- 6.Crowley P. Antenatal corticosteroid therapy: a meta analysis of the randomized trials 1972–1994. Am J Obstet Gynecol 1995173322–325. [DOI] [PubMed] [Google Scholar]
- 7.Soll R F, Morley C J. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2001(2)CD000510. [DOI] [PubMed]
- 8.Donn S M, Sinha S K. Invasive and non invasive neonatal mechanical ventilation. Respir Care 200348426–441. [PubMed] [Google Scholar]
- 8a. Gregory G A, Kitterman J A, Phibbs R H.et al Treatment of the idiopathic respiratory distress syndrome with continuous positive airway pressure. N Engl J Med 1971284133–140. [DOI] [PubMed] [Google Scholar]
- 9.Wung J T, Koons A H, Driscoll J M., Jret al Changing incidence of brochopulmonary dysplasia. J Pediatr 197995845–847. [DOI] [PubMed] [Google Scholar]
- 10.Kraybill E N, Runyun D K, Bose C L.et al Risk factors for chronic lung disease in infants with birth weights of 751 to 1000 grams. J Pediatr 1989115115–120. [DOI] [PubMed] [Google Scholar]
- 11.Mariani G, Cifuentes J, Carlo W A. Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 19991041082–1088. [DOI] [PubMed] [Google Scholar]
- 12.Carlo W A, Stark A R, Bauer C.et al Effects of minimal ventilation in a multicenter randomized controlled trial of ventilator support and early corticosteroid therapy in extremely low birthweight infants. Pediatrics 1999104738–739. [Google Scholar]
- 13.Greenough A. Update on patient‐triggered ventilation. Clin Perinatol 200128533–546. [DOI] [PubMed] [Google Scholar]
- 14.Donn S M, Sinha S K. Newer modes of mechanical ventilation for the neonate. Curr Opin Pediatr 20011399–103. [DOI] [PubMed] [Google Scholar]
- 15.Greenough A, Milner A D, Dimitriou G. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst Rev 2004(3)CD000456. [DOI] [PubMed]
- 16.McCallion N, Davis P G, Morley C J. Volume‐targeted versus pressure‐limited ventilation in the neonate. Cochrane Database Syst Rev 2005(3)CD003666. [DOI] [PubMed]
- 17.Singh J, Sinha S K, Donn S M.et al A randomized controlled trial of volume control ventilation [abstract]. Pediatr Res 2005571546A [Google Scholar]
- 18.Schulze A, Gerhardt T, Musante G.et al Proportional assist ventilation in low birth weight infants with acute respiratory disease: a comparison to assist/control and conventional mechanical ventilation. J Pediatr 1999135339–344. [DOI] [PubMed] [Google Scholar]
- 19.Keszler M, Modanlou H D, Brudno D S.et al Multi‐center controlled clinical trial of high‐frequency jet ventilation in preterm infants with uncomplicated respiratory distress syndrome. Pediatrics 1997100593–599. [DOI] [PubMed] [Google Scholar]
- 20.Gerstmann D R, Minton S D, Stodard R A.et al The Provo multicenter early high frequency oscillatory high frequency trial: Improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics 1996981044–1057. [PubMed] [Google Scholar]
- 21.Rettwitz‐Volk W, Veldman A, Roth B.et al A prospective, randomized, multicenter trial of high‐frequency oscillatory ventilation compared with conventional ventilation in preterm infants with respiratory distress syndrome receiving surfactant. J Pediatr 1998132249–254. [DOI] [PubMed] [Google Scholar]
- 22.Thome U, Kossel H, Lipowsky G.et al Randomized comparison of high‐frequency ventilation with high‐rate intermittent positive pressure ventilation in preterm infants with respiratory failure. J Pediatr 199913539–46. [DOI] [PubMed] [Google Scholar]
- 23.Moriette G, Paris‐Llado J, Walti H.et al Prospective randomized Multicenter comparison of high‐frequency oscillatory ventilation and conventional ventilation in preterm infants with respiratory distress syndrome. Pediatrics 2001107363–372. [DOI] [PubMed] [Google Scholar]
- 24.Courtney S E, Durand D J, Asselin J M.et al High‐frequency oscillatory ventilation versus conventional mechanical ventilation for very‐low‐birth‐weight infants. N Engl J Med 2002347643–652. [DOI] [PubMed] [Google Scholar]
- 25.Johnson A H, Peacock J L, Greenough A.et al High‐frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med 2002347633–642. [DOI] [PubMed] [Google Scholar]
- 26.Stark A R. High‐frequency oscillatory ventilation to prevent bronchopulmonary dysplasia: are we there yet? N Engl J Med 2002347682–684. [DOI] [PubMed] [Google Scholar]
- 27.Bhutani V K. Clinical applications of pulmonary function and graphics. Semin Neonatol 20027391–399. [DOI] [PubMed] [Google Scholar]