Abstract
Objectives
To (a) study the prevalence of hearing impairment in a cohort of very low birthweight (VLBW) infants and (b) evaluate the effectiveness of transient evoked otoacoustic emissions (TEOAE) as a first stage in‐hospital hearing screening tool in this population.
Study design
The study group was a cohort of 346 VLBW infants born in 1998–2000 at The Sheba Medical Center. The prevalence of hearing impairment in the study group was compared with that of all other newborn infants participating in a universal newborn hearing screening programme during the same period. To evaluate the effectiveness of TEOAE, a control group of 1205 healthy newborns who had no known risk factors for hearing impairment was selected. The results and follow up of hearing screening for these infants were examined retrospectively.
Results
Only one VLBW infant (0.3%) was diagnosed with bilateral sensory‐neural hearing loss. In addition, nine infants (2.7%) were diagnosed with conductive hearing loss. Bronchopulmonary dysplasia and low Apgar score were the most significant factors for predicting the occurrence of conductive hearing loss. The percentage of VLBW infants who successfully passed the in‐hospital TEOAE screening was 87.2, compared with 92.2% in the full term control group. No false negative cases were detected on follow up.
Conclusions
The study shows a low incidence of sensory‐neural hearing loss in a cohort of VLBW infants and a relatively high incidence of conductive hearing loss. TEOAE screening was found to be an effective first stage in‐hospital hearing screening tool in this population.
Keywords: hearing impairment, transient evoked otoacoustic emissions, very low birth weight, screening
The survival rate for very low birthweight (VLBW) infants (birth weight ⩽1500 g) has increased substantially over the last few decades because of recent improvements in obstetrical and neonatal care in developed countries.1,2 This increased survival rate of VLBW infants has led to an increase in the proportion of handicapped infants in this population.3 One possible handicap is sensory‐neural hearing loss (SNHL). It is generally agreed that the incidence of SNHL in preterm infants is much higher (2–4 in 100 live births) than in healthy, full term newborns (1–3 in 1000 live births).4 Among preterm infants (<37 weeks gestation), the population of VLBW is considered at high risk of hearing impairment. Reports in the literature, however, are contradictory concerning the incidence of SNHL in this population. Some studies reported a very high incidence of 4–9.7%,5,6,7,8,9,10 whereas others reported a much lower incidence of 0.7–1.5% for SNHL.11,12,13,14
The importance of universal newborn hearing screening (UNHS) in identifying hearing impaired infants as early as possible is already well recognised.4,15,16,17,18,19 Transient evoked otoacoustic emissions (TEOAE) have been established as a reliable method for UNHS in full term infants.20,21,22,23 Not as much evidence exists, however, for using this method with VLBW infants as a first stage screening tool before discharge home. The few studies using TEOAE screening in VLBW infants reported contradictory findings.24,25,26
On the basis of the 1993 recommendations of the American National Institutes of Health (NIH),15 the Speech and Hearing Center in collaboration with the Neonatal Department at the Chaim Sheba Medical Center embarked on a pioneer UNHS project in 1997. All babies cared for at the medical centre's well baby nursery (WBN), intermediate care unit, and neonatal intensive care unit (NICU) are tested using TEOAE as a first stage hearing screening before hospital discharge. The purpose of this study was therefore twofold: (a) to report the prevalence of hearing impairment in a large cohort of VLBW infants and compare it with that of all other newborn infants participating in our UNHS programme during the same period; (b) to evaluate the effectiveness of TEOAE as a screening tool in VLBW infants, compared with a control group of full term neonates.
Materials and methods
Subjects
During the three year period from 1 January 1998 through 31 December 2000, a total of 24 096 infants were born at the Chaim Sheba Medical Center, cared for in the WBN, intermediate care unit, and the NICU, and survived to discharge. Of these, 376 were VLBW infants (1.56%), of whom 346 survived to discharge. The cohort comprised all 346 VLBW infants who were cared for in the NICU and survived to discharge. The results of screening and follow up for these infants were retrospectively examined. The prevalence of hearing impairment in the study group was compared with that of all other newborns participating in our UNHS programme during the same period, including infants admitted to the NICU with birth weight >1500 g and WBN infants. To evaluate the effectiveness of TEOAE screening in VLBW infants, we compared their results with those of 1205 near or full term healthy newborns cared for in the WBN. The control group was selected from the WBN, using random number generation, by choosing three to four newborns born on the same birth dates as each of the VLBW infants participating in the study group.
TEOAE testing
TEOAE screening was performed with an ILO88 OAE analyser (Otodynamics Ltd, Hatfield, Hertfordshire, UK; version 4.2) in the Quickscreen mode of stimulation using click stimuli at a peak level of 78–85 dBpeSPL (dB peak equivalent sound pressure level). At least 50 low noise samples were collected for each ear before the test ended. If the pass criterion was not met at that point, the presentation of stimuli continued up to the point where the pass criterion was met or up to 500 samples. Infants were tested in their open crib in a quiet room in the nursery by audiologists and staff members who had received extensive training and were familiar with TEOAE equipment.
TEOAE scoring criteria
The protocol had a most conservative criterion to prevent false negative occurrences (based on the Rhode Island Hearing Assessment Project27).
Pass: the results should meet the following criteria in two ears:
General reproducibility should be ⩾50%.
Reproducibility and signal to noise ratio by frequency bands should be as shown in table 1.
Table 1 Pass criteria for transient evoked otoacoustic emissions (TEOAE) screening.
| Frequency band (kHz) | Signal to noise ratio (dB) | Reproducibility (%) |
|---|---|---|
| 1.5 | ⩾+3 | ⩾50 |
| 2.0 | ⩾+6 | ⩾70 |
| 3.0 | ⩾+6 | ⩾70 |
| 4.0 | ⩾+6 | ⩾70 |
Refer: no emissions are present or the results do not meet the aforementioned criteria, in one or two ears.
Auditory brainstem response (ABR) testing
ABR testing was conducted using a Biologic Navigator‐Pro Evoked potential system or an Amplaid MK 12 using alternating click stimuli at a presentation rate of 21 per second. Responses were averaged over 2048 sweeps. At least two runs were collected for each intensity. The initial intensity was 85 dB HL (hearing level). Peak absolute latencies of ABR waves I, III, and V as well as interpeak latencies of waves I–III and I–V were calculated at an intensity of 85 dB HL. ABR thresholds were defined as the lowest intensity at which wave V could still be detected. Testing was performed during natural sleep or under sedation by certified audiologists, according to the protocol at our Speech and Hearing Center.
ABR analysis
ABR results were classified as:
Pass: thresholds ⩽30 dBnHL in both ears.
Refer: thresholds >30 dBnHL in one or two ears or abnormal ABR recording defined as prolonged brainstem transmission time (BTT) of waves I–V—that is, more than 2 SDs above the mean value according to the norms in our clinic despite normal thresholds.
VLBW screening protocol
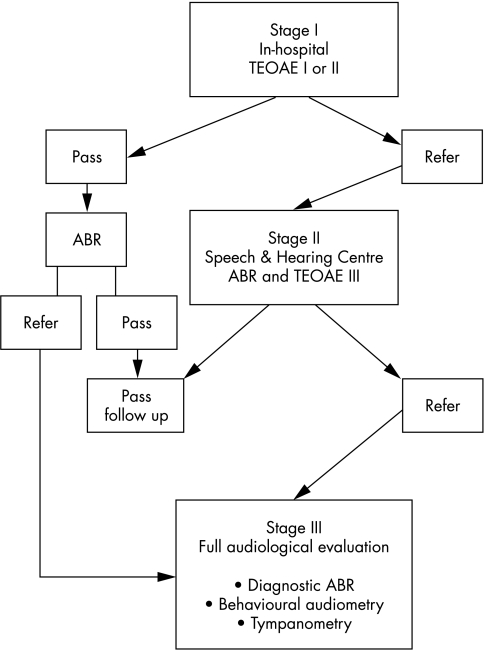
The VLBW infants hearing screening protocol was conducted in three stages (fig 1).
Figure 1 Very low birthweight infants hearing screening protocol as conducted in three stages. ABR, Auditory brainstem response; TEOAE, transient evoked otoacoustic emissions.
First stage: in‐hospital screening. The initial TEOAE test was usually performed three to four days before discharge. Infants who failed the initial test in one or both ears were re‐examined using TEOAE on the following day. Infants who failed the in‐hospital TEOAE tests or were discharged before completing the screening were referred to our Speech and Hearing Center two weeks after discharge from the hospital for the second stage of the screening.
Second stage: outpatient screening. An air conduction, click evoked ABR for threshold detection and a third TEOAE test were performed in natural sleep at the Speech and Hearing Center. Infants who failed these tests were referred to their paediatrician or otolaryngologist for an otoscopic examination and immediately referred to the third stage of the programme.
Third stage. A full audiological evaluation was performed at our centre, including diagnostic ABR under sedation to click and tonal stimuli including air and bone conduction, behavioural audiometry, and tympanometry.
All VLBW infants who passed the in‐hospital TEOAE screening were also referred for an air conduction, click evoked ABR within a month after discharge and for audiological follow ups at regular intervals until the age of 3 years, in accordance with the Joint Committee on Infant Hearing 1994 Position Statement.16
WBN screening protocol
The WBN hearing screening protocol was similar to the VLBW protocol except for stage II where an air conduction, click evoked ABR was performed only in those patients who failed TEOAE III.
Hearing impairment
Hearing impairment was defined as bilateral or unilateral SNHL or conductive hearing loss (CHL), averaging 35 dB or more in the 500–4000 Hz frequency region.17 The prevalence of hearing impairment in the VLBW cohort was compared with that of all WBN newborns and NICU >1500 g born during the same period at our medical centre.
Data analysis
The pass rates in the VLBW group and the WBN control group were compared using a two sample proportion test based on corrected z statistics. The proportion of subjects with SNHL and CHL was compared between the different groups using Fisher's exact test. A χ2 test and logistic regression were used to assess the effect of risk factors for hearing impairment on the prevalence of CHL after adjustment for gestational age. The results of the logistic regression were expressed in odds ratios (ORs) and 95% confidence intervals (CIs).
The research was carried out in accordance with the principles of the Declaration of Helsinki. The institutional review board of the hospital approved the study.
Results
During the three year period, 346 VLBW infants survived to discharge and participated in the study. Their mean (SD) gestational age at birth was 30 (2.7) weeks (range 24–37), and their mean (SD) birth weight was 1144 (242) g (range 515–1495). The mean chronological age at first testing of the VLBW group was 55.24 (29.7) days (range 16–180). Table 2 summarises the characteristics of the VLBW cohort.
Table 2 Characteristics of the very low birthweight (VLBW) cohort.
| Number of infants | Percentage | |
|---|---|---|
| VLBW infants | 376 | 100.0 |
| Mortality | 30 | 8.0 |
| Caesarean section | 256 | 68.1 |
| Part of multiplicity | 223 | 59.3 |
| Male infants | 176 | 46.8 |
| Small for gestational age | 116 | 30.9 |
| Birth weight <1000 g | 122 | 32.4 |
| Birth weight <750 g | 36 | 9.6 |
A total of 1205 infants from the WBN served as controls for the effectiveness of TEOAE screening. Their mean (SD) gestational age at birth was 39.7 (1.5) weeks (range 35–43), and their mean (SD) birth weight was 3253 (472) g (range 1695–4735). The mean chronological age at first testing of the control group was 30.3 (26.1) hours (range 4–240).
Prevalence of hearing impairment in VLBW group
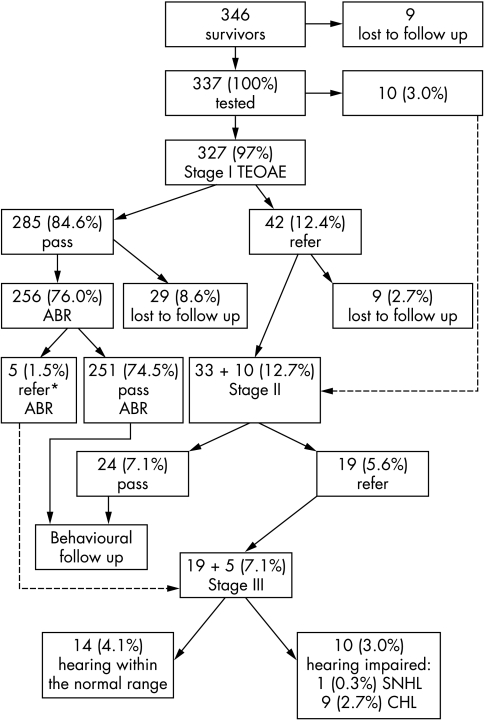
Figure 2 presents in detail the TEAOE screening results of the VLBW infants. During the three year project, 346 VLBW infants survived to discharge. A total of 337 infants (who comprised 97.4% of the survivors) were tested. Of these, 327 infants had an initial TEOAE screening test. In 10 additional cases (2.9%), an ABR test was performed immediately as an initial test at the outpatient clinic because of modifications in the protocol following their clinical situation.
Figure 2 The transient evoked otoacoustic emissions (TEOAE) screening results of the very low birthweight infants. Percentages refer to the entire cohort. *Prolonged brainstem transmission time despite normal ABR thresholds. ABR, Auditory brainstem response; CHL, conductive hearing loss; SNHL, sensory‐neural hearing loss.
At the end of the full audiological evaluation (stage III), 10 infants (3.0%) were found to have a hearing impairment. Of these, only one infant (0.3%) was found to have bilateral moderate to severe SNHL. This child was born after 30 weeks of gestation (780 g), suffered from respiratory distress syndrome, developed bronchopulmonary dysplasia (BPD), and required a total of 23 days of antibiotic treatment (including aminoglycosides and vancomycin). Nine infants (2.7%) were identified with CHL (four with unilateral CHL and five with bilateral CHL).
In addition, five VLBW infants out of the entire cohort (1.5%) were found to have abnormal ABR results despite normal ABR thresholds and normal TEOAEs. In four of these infants, BTT (I–V interval) was prolonged bilaterally and in one case unilaterally. On the other hand, no cases of auditory neuropathy, defined as infants who successfully passed TEOAE screening but had a severely abnormal or absent ABR, were detected in our cohort.
Although 47 out of 346 infants (13.6%) were lost on follow up, none of these babies was identified in a national survey through Israeli rehabilitation centres for hearing impaired children.
A statistical analysis revealed that the prevalence of permanent SNHL in the VLBW cohort was found to be non‐significantly higher than that of the WBN group (0.3% v 0.1% respectively), but lower than that of the NICU >1500 g population (although the difference did not reach statistical significance because of the small number of hearing impaired babies in the VLBW group—that is, only one baby; 0.3% v 0.99% respectively). The prevalence of CHL was found to be significantly higher in the VLBW cohort than in the WBN and NICU >1500 g groups (p<0.001) (table 3).
Table 3 Prevalence, 95% confidence interval, and number of infants with permanent SNHL and CHL in the population born between 1 January 1998 and 31 December 2000 at the Chaim Sheba Medical Center and cared for in the WBN, intermediate care unit, and the NICU and surviving to discharge.
| VLBW (A) | NICU >1500 g (B) | WBN (C) | p Value | |||
|---|---|---|---|---|---|---|
| A v B | A v C | C v B | ||||
| No of newborns | 346 | 1011 | 22739 | |||
| Prevalence of SNHL | 1 (0.3) | 10 (0.99) | 24 (0.1) | NS | NS | <0.0001 |
| 95% CI | −0.28 to 0.86% | 0.38 to 1.6% | 0.063 to 0.15% | |||
| Prevalence of CHL | 9 (2.7) | 5 (0.5) | 14 (0.06) | <0.001 | <0.0001 | <0.001 |
| 95% CI | 0.92 to 4.28% | 0.06 to 0.93% | 0.03 to 0.09% | |||
Prevalence values are number (%).
CHL, Conductive hearing loss; CI, confidence interval; NICU, neonatal intensive care unit; SNHL, sensory‐neural hearing loss; VLBW, very low birthweight; WBN, well baby nursery.
Screening results of TEOAE control group
Of the 1205 WBN infants who had an initial TEOAE screening test, 1111 infants (92.2%) passed the in‐hospital screening. At the end of the full audiological evaluation (stage III), two infants were diagnosed with CHL (0.16%). No SNHL cases were diagnosed in this control group.
Risk factors for hearing impairment
Table 4 summarises the risk factors for hearing impairment, based on the Joint Committee for Infant Hearing Position Statement (1994),16 found in our VLBW cohort. As only one child was identified with SNHL, we analysed the relation between the different risk factors for hearing impairment and the prevalence of CHL. Table 4 presents the comparison of the prevalence of transient CHL between the groups of infants with and without risk factors for hearing impairment (n = 337). A multivariate regression analysis that included all risk factors for hearing impairment was implemented. BPD and low Apgar score were found to be the significant factors for predicting the occurrence of CHL (OR = 15.12, 95% CI = 1.47 to 157.1, p<0.05; OR = 12.0, 95% CI = 1.93 to 74.88, p<0.01 respectively).
Table 4 Comparison of prevalence of transient CHL between the groups of infants with and without risk factors for hearing impairment (n = 337).
| Risk factor | Infants with risk factor | Infants without risk factor | p Value | ||
|---|---|---|---|---|---|
| Total | No with CHL | Total | No with CHL | ||
| Apgar 0–4 at 1 min and/or 0–6 at 5 min* | 42 | 4 | 272 | 5 | <0.01 |
| RDS | 215 | 8 | 122 | 1 | NS |
| BPD (O2 >28 days) | 52 | 4 | 285 | 5 | 0.01 |
| Mechanical ventilation ⩾5 days | 145 | 6 | 192 | 3 | NS |
| Ototoxic drugs ⩾10 days | 154 | 6 | 183 | 3 | NS |
| Bilirubin >13 mg%* | 16 | 0 | 298 | 9 | NS |
| IVH (grades 3–4) | 8 | 0 | 329 | 9 | NS |
| PVL | 21 | 0 | 316 | 9 | NS |
| Neurological symptoms* | 42 | 1 | 272 | 8 | NS |
| In utero infection* | 2 | 0 | 312 | 9 | NS |
| Proven sepsis (early & late) | 60 | 3 | 277 | 6 | NS |
| Familial history of SNHL* | 11 | 0 | 303 | 9 | NS |
*Only 314 infants with known data.
CHL, Conductive hearing loss; RDS, respiratory distress syndrome; BPD, bronchopulmonary dysplasia; IVH, intraventricular haemorrhage; PVL, periventricular leucomalacia; SNHL, sensory‐neural hearing loss.
Effectiveness of TEOAE screening
To evaluate the effectiveness of TEOAE screening in VLBW infants, we analysed the screening results of those infants who underwent only TEOAE tests as in‐hospital screening compared with a control group of healthy newborns. Before discharge from the hospital, 285 VLBW infants (out of 327; 87.2%) passed the in‐hospital screening successfully, compared with 1111 (out of 1205; 92.2%) in the WBN control group. This lower percentage of pass rates in the VLBW group than in the WBN control group was significant (χ2 = 8.09, p<0.05).
A significant relation was found between the number of risk factors per child and the rate of failure in TEOAE testing performed before discharge from the hospital—that is, the more risk factors, the higher chance of failure (χ2 = 9.27, p = 0.02). A multivariate regression analysis revealed that the combination of three or more risk factors per child significantly increased the chance of failure in the first TEOAE (OR = 3.75, 95% CI = 1.39 to 10.16, p = 0.009).
Discussion
This study shows a low incidence (0.3%) of SNHL in a population of all surviving VLBW infants born at our centre in 1998–2000. On the other hand, a high incidence (2.7%) of CHL was found in the studied population, with BPD and low Apgar scores being the most significant factors for predicting the occurrence of CHL. The prevalence of permanent SNHL in our VLBW cohort was found to be unexpectedly lower than that of the NICU >1500 g population (0.3% v 0.99% respectively), and higher than that of the WBN and intermediate care unit group (0.3% v 0.1% respectively). In this study, TEOAE was found to be an effective first stage in‐hospital screening tool for VLBW infants, with a pass rate of 87.2% compared with 92.2% in our WBN control group.
In Israel, all infants identified with significant hearing loss requiring amplification and rehabilitation are referred to one of the national rehabilitation centres for the hard of hearing. None of the 47 babies who were lost to follow up was later identified with hearing loss in need of rehabilitation. Furthermore, no late onset cases were identified, as all babies in our country are routinely screened at the age of 7 months in the well baby clinics, thus those diagnosed with hearing impairment would have been referred for rehabilitation.
In addition, 1.5% of our VLBW cohort was found to have abnormal ABR results (prolongation of BTT I–V) despite normal ABR thresholds and normal TEOAEs.
This study of VLBW infants, although retrospectively analysed, represents a large cohort at one centre over a three year period, without major changes in NICU practices and with three to six years of national follow up. This relatively low incidence of SNHL found in our study is consistent with other studies that also reported a low incidence of SNHL in VLBW infants (0.7–1.5%),11,12,13,14,28,29 but not consistent with earlier studies that reported a high incidence of SNHL (up to 9.7%) in the same population.5,6,7,8,9 Further support for our findings was found in a recently published population based national report on the developmental outcome of 1104 surviving VLBW infants born in Israel between 1995 and 1996 with an incidence of 0.4% with severe hearing loss.30
The incidence of other major neurological sequelae in our cohort is less than 10% (to be reported elsewhere) and is in accordance with the previously reported rate of handicaps.3 It should be noted, however, that about 30% of our VLBW population were small for gestational age, making this cohort more mature in terms of gestational age. The large discrepancy in the incidence of SNHL in VLBW infants reported in the literature over the last few decades (from 2–3 per 1000 to 9.7 per 100) may also originate from different factors, such as changes in both prenatal and perinatal care over the last 30 years,8,13 population recruitment including differences in birth weight and gestational age distribution,10 survival rate, cohort selection, and appropriateness for gestational age, as well as different classifications of hearing impairment, etc.
Newer technology used in modern NICUs, careful implementation of new treatments, better infection control and oxygen supplementation, as well as strict measurement of serum drug concentrations tend to protect VLBW infants from hearing impairment despite the increase in the survival rate of smaller infants. The prevalence of SNHL found in our universal hearing screening programme indicates that being treated in the NICU rather than being VLBW seems to increase the risk of permanent hearing loss by three (0.99% v 0.3% respectively). These results are in accordance with other studies, which also concluded that the combination of risk factors and the general status of the neonates rather than the effects of low birth weight per se are critical in the development of SNHL.13,31,32,33 Some studies also focus on the relation of perinatal and postnatal complications associated with hearing impairment in low birthweight infants.28,34
It is generally agreed that newborns admitted to the NICU have a high incidence of transient middle ear effusions.35,36,37,38,39 Undiagnosed CHL in this particular age of the first year of life can influence speech and language development. In this study, an incidence of 2.7% CHL was found in the VLBW cohort. This incidence was significantly higher than that of NICU infants whose birth weight was >1500 g (27 per 1000 v 5 per 1000 respectively). It should be noted that the incidence of CHL in this study refers only to the outcome of our in‐hospital hearing screening programme and not to other CHL cases which might have developed later on in childhood. These results are in agreement with previous studies which also reported a high incidence of conductive hearing impairment in VLBW infants.9,11,40 BPD and low Apgar score were found to be the most significant unrelated factors for predicting the occurrence of CHL. BPD is known as a risk factor for CHL, as it usually involves prolonged periods of mechanical ventilation, which might cause Eustachian tube dysfunction, predisposing infants to middle ear effusions.9,11,25,38,39
What is already known on this topic
VLBW infants are at increased risk of sensory‐neural hearing loss
What this study adds
This study shows a low prevalence of sensory‐neural hearing loss in a group of VLBW infants, which needs confirmation
The rate of conductive hearing impairment was high as expected
TEOAE was found to be an effective first stage screening tool in our VLBW cohort, with a pass rate of 87.2% compared with 92.2% in our WBN control group. These results are in accordance with other studies which reported pass rates of 84–92.8% using TEOAE in high risk preterm babies.24,41,42 On the other hand, other studies reported much lower pass rates of 54–59% in TEOAE screening in VLBW infants.25,26 Differences in pass rates reported in the literature are influenced by several factors. Preterm infants tend to suffer from noisy breathing and/or middle ear effusion or dysfunction, resulting in a high failure rate and requiring further or repeated examinations.26,36,37,43,44 Furthermore, TEOAE testing of NICU infants who are still on monitors or have nasogastric tubes is difficult. According to our protocol, TEOAE screening takes place two to three days before discharge from the hospital, when the infants are already free of nasogastric tubes and most often without oxygen supplementation, as also recommended by Kok et al.43 This may have contributed to the higher pass rates in our cohort. Other factors that might have affected the pass rates include the protocol used, the pass criteria, and the equipment used.
TEOAE seems therefore to be a feasible, rapid, and effective method for first stage screening of VLBW infants before discharge home, with ABR testing on the first follow up. The relative convenience of using TEOAE in the NICU before discharge is important for early diagnosis and treatment, and in our opinion should be implemented routinely in the NICU. TEOAE should, however, be interpreted cautiously in those infants with risk factors such as hyperbilirubinaemia, hypoxia, and neurological abnormalities known to increase the risk of auditory neuropathy (or retrocochlear damage).45 In our cohort, 1.5% of the VLBW infants were found to have abnormal ABR results—that is, prolonged BTT—despite normal ABR thresholds and normal TEOAEs. Similar findings were reported by Jiang et al.46 This finding supports routine implementation of TEOAE as a quick, easily performed first stage screening tool before discharge from hospital (thus reaching all babies), but a diagnostic ABR should also be routinely performed in this population.
In summary, a low rate of SNHL was found in our cohort of VLBW infants. On the other hand, VLBW infants should be carefully monitored for their high incidence of CHL. Further follow up studies should be conducted to confirm this trend, particularly with increased survival of extremely low birthweight infants.
Acknowledgements
We are grateful to Ela August and Orit Visoker from the Department of Communication Disorders at Tel Aviv University for data collection, Ester Shabtai for the statistical analysis, and the National Rehabilitation Centers for the Hard‐of‐Hearing for their help in follow ups.
Abbreviations
ABR - auditory brainstem response
BPD - bronchopulmonary dysplasia
BTT - brainstem transmission time
CHL - conductive hearing loss
CI - confidence interval
NICU - neonatal intensive care unit
OR - odds ratio
SNHL - sensory‐neural hearing loss
TEOAE - transient evoked otoacoustic emissions
UNHS - universal newborn hearing screening
VLBW - very low birthweight
WBN - well baby nursery
Footnotes
Competing interests: none declared
References
- 1.Lorenz J M. Survival of the extremely preterm infant in North America in the 1990s. Clin Perinatol 200027255–262. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg R L, Rouse D J. Prevention of premature birth. N Engl J Med 1998339313–320. [DOI] [PubMed] [Google Scholar]
- 3.Msall M E, Tremont M R. Functional outcomes in self‐care, mobility, communication and learning in extremely low‐birth‐weight infants. Clin Perinatol 200027381–401. [DOI] [PubMed] [Google Scholar]
- 4.Erenberg A, Lemons J, Sia C.et al Newborn and infant hearing loss: detection and intervention. American Academy of Pediatrics. Task force on Newborn and Infant Hearing, 1998–1999. Pediatrics 1999103527–530. [DOI] [PubMed] [Google Scholar]
- 5.Abramovich S J, Gregory S, Slemick M.et al Hearing loss in very low birthweight infants treated with neonatal intensive care. Arch Dis Child 197954421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman I, Hirsch R P, Fria T J.et al Cause of hearing loss in the high‐risk premature infant. J Pediatr 198510695–101. [DOI] [PubMed] [Google Scholar]
- 7.Bradford B C, Baudin J, Conway M J.et al Identification of sensory neural hearing loss in very preterm infants by brainstem auditory evoked potentials. Arch Dis Child 198560105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grogaard J B, Lindstrom D P, Parker R A.et al Increased survival rate in very low birth weight infants (1500 grams or less): no association with increased incidence of handicaps. J Pediatr 1990117139–146. [DOI] [PubMed] [Google Scholar]
- 9.Salamy A, Eldredge L, Tooley W H. Neonatal status and hearing loss in high‐risk infants. J Pediatr 1989114847–852. [DOI] [PubMed] [Google Scholar]
- 10.Leslie G I, Kalaw M B, Bowen J R.et al Risk factors for sensorineural hearing loss in extremely premature infants. J Paediatr Child Health 199531312–316. [DOI] [PubMed] [Google Scholar]
- 11.Tudehope D, Smyth V, Scott J.et al Audiological evaluation of very low birthweight infants. J Paediatr Child Health 199228172–175. [DOI] [PubMed] [Google Scholar]
- 12.Veen S, Sassen M L, Schreuder A M.et al Hearing loss in very preterm and very low birthweight infants at the age of 5 years in a nationwide cohort. Int J Pediatr Otorhinolaryngol 19932611–28. [DOI] [PubMed] [Google Scholar]
- 13.Meyer C, Witte J, Hildmann A.et al Neonatal screening for hearing disorders in infants at risk: incidence, risk factors and follow‐up. Pediatrics 1999104900–904. [DOI] [PubMed] [Google Scholar]
- 14.Davis N M, Doyle L W, Ford G W.et al Auditory function at 14 years of age of very‐low‐birthweight children. Dev Med Child Neurol 200143191–196. [PubMed] [Google Scholar]
- 15.National Institutes on Deafness and Other Communication Disorders National Institutes of Health Consensus Statement: Early Identification of Hearing Impairment in Infants and Young Children. Rockville, MD: National Institutes on Deafness and Other Communication Disorders, 1993 [PubMed]
- 16.Joint Committee on Infant Hearing 1994 position statement. Pediatrics 1995100152–156. [PubMed] [Google Scholar]
- 17.Joint Committee on Infant Hearing Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Am J Audiol 200099–29. [PubMed] [Google Scholar]
- 18. European Consensus Statement on Neonatal Hearing Screening (Milan, 15–16 May, 1998)
- 19.Yoshinaga‐Itano C, Sedey A L, Coulter D K.et al Language of early‐ and later‐identified children with hearing loss. Pediatrics 19981021161–1171. [DOI] [PubMed] [Google Scholar]
- 20.Watkin P M. Neonatal otoacoustic emission screening and the identification of deafness. Arch Dis Child Fetal Neonatal Ed 199674F16–F25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finitzo T, Albright K, O'Neal J. The newborn with hearing loss: detection in the nursery. Pediatrics 19981021452–1460. [DOI] [PubMed] [Google Scholar]
- 22.Vohr B R, Carty L M, Moore P E.et al The Rhode Island Hearing Assessment Program: experience with statewide hearing screening (1993–1996). J Pediatr 1998133353–357. [DOI] [PubMed] [Google Scholar]
- 23.Spivak L, Dalzell L, Berg A.et al New‐York State universal newborn hearing screening demonstration project: inpatient outcome measures. Ear Hear 20002192–103. [DOI] [PubMed] [Google Scholar]
- 24.Gill A W, Gosling D, Kelly C.et al Predischarge screening of very low birthweight infants by click evoked otoacoustic emissions. J Paediatr Child Health 199834456–459. [DOI] [PubMed] [Google Scholar]
- 25.Meredith R, Stephens D, Hogan S.et al Screening for hearing loss in an at‐risk neonatal population using evoked otoacoustic emissions. Scand Audiol 199423187–193. [DOI] [PubMed] [Google Scholar]
- 26.Valkama A M, Laitakari K T, Tolonen E U.et al Prediction of permanent hearing loss in high‐risk preterm infants at term age. Eur J Pediatr 2000159459–464. [DOI] [PubMed] [Google Scholar]
- 27.Maxon A B, White K R, Behrens T R.et al Referral rates and cost efficiency in a universal newborn hearing screening program using transient evoked otoacoustic emissions. J Am Acad Audiol 19956271–277. [PubMed] [Google Scholar]
- 28.Van Naarden K, Decoufle P. Relative and attributable risks for moderate to profound bilateral sensorineural hearing impairment associated with lower birth weight in children 3 to 10 years old. Pediatrics 1999104905–910. [DOI] [PubMed] [Google Scholar]
- 29.Perrott S, Dodds L, Vincer M. A population‐based study of prognostic factors related to major disability in very preterm survivors. J Perinatol 200323111–116. [DOI] [PubMed] [Google Scholar]
- 30. In: Reychman B, Glasser S, Levitski O, Lerner‐Geva L. eds. In collaboration with the Israel Neonatal Network. Developmental assessment at two‐years of age of children born at very‐low‐birth‐weight in 1995–1996, Women and Children's Health Research Unit, Gertner Institute and the Israel Center for Disease Control, Ministry of Health, Publication No 3601 2003
- 31.Eavey R D, Bertero M C, Thornton A R.et al Failure to clinically predict NICU hearing loss. Clin Pediatr 199534138–145. [DOI] [PubMed] [Google Scholar]
- 32.Marlow E S, Hunt L P, Marlow N. Sensorineural hearing loss and prematurity. Arch Dis Child Fetal Neonatal Ed 200082F141–F144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruszewicz A, Pospiech I. Low birth weight as a risk factor of hearing loss. Scand Audiol Suppl 200152194–196. [DOI] [PubMed] [Google Scholar]
- 34.Borradori C, Fawer C L, Buclin T.et al Risk factors of sensorineural hearing loss in preterm infants. Biol Neonate 1997711–10. [DOI] [PubMed] [Google Scholar]
- 35.Balkany T J, Berman S A, Simmons M A.et al Middle ear effusions in neonates. Laryngoscope 197888398–405. [DOI] [PubMed] [Google Scholar]
- 36.Van Zanten B G, Kok M R, Brocaar M P.et al The click‐evoked oto‐acoustic emission, c‐EOAE, in preterm‐born infants in the post conceptional age range between 30 and 68 weeks. Int J Pediatr Otorhinolaryngol 199532S187–S197. [DOI] [PubMed] [Google Scholar]
- 37.Sutton G J, Gleadle P, Rowe S J. Tympanometry and otoacoustic emissions in a cohort of special care neonates. Br J Audiol 1996309–17. [DOI] [PubMed] [Google Scholar]
- 38.Engel J, Mahler E, Anteunis L.et al Why are NICU infants at risk for chronic otitis media with effusion? Int J Pediatr Otorhinolaryngol 200157137–144. [DOI] [PubMed] [Google Scholar]
- 39.Gray P H, Sarkar S, Young J.et al Conductive hearing loss in preterm infants with bronchopulmonary dysplasia. J Paediatr Child Health 200137278–282. [DOI] [PubMed] [Google Scholar]
- 40.Sauve R S, Singhal N. Long‐term morbidity of infants with bronchopulmonary dysplasia. Pediatrics 198576725–733. [PubMed] [Google Scholar]
- 41.Morlet T, Ferber‐Viart C, Putet G.et al Auditory screening in high‐risk pre‐term and full‐term neonates using transient evoked otoacoustic emissions and brainstem auditory evoked potentials. Int J Pediatr Otorhinolaryngol 19984531–40. [DOI] [PubMed] [Google Scholar]
- 42.Eshraghi A, Francois M, Narcy P. Evolution of transient evoked otoacoustic emissions in preterm newborns: a preliminary study. Int J Pediatr Otorhinolaryngol 199637121–127. [DOI] [PubMed] [Google Scholar]
- 43.Kok M R, Van Zanten G A, Brocaar M P.et al Click‐evoked otoacoustic emissions in very‐low‐birth‐weight infants: a cross‐sectional data analysis. Audiology 199433152–164. [DOI] [PubMed] [Google Scholar]
- 44.El‐Refaie A, Parker D J, Bamford J M. Otoacoustic emissions versus ABR screening: the effect of external and middle ear abnormalities in a group of SCBU neonates. Br J Audiol 1996303–8. [DOI] [PubMed] [Google Scholar]
- 45.Psarommatis I M, Tsakanikos M D, Kontorgianni A D.et al Profound hearing loss and presence of click‐evoked otoacoustic emissions in the neonate: a report of two cases. Int J Pediatr Otorhinolaryngol 199739237–243. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Z D, Brosi D M, Wilkinson A R. Hearing impairment in preterm very low birthweight babies detected at term by brainstem auditory evoked responses. Acta Paediatr 2001901411–1415. [DOI] [PubMed] [Google Scholar]