Abstract
Background
Newborns of 30–34 weeks gestation comprise 3.9% of all live births in the United States and 32% of all premature infants. They have been studied much less than very low birthweight infants.
Objective
To measure in‐hospital outcomes and readmission within three months of discharge of moderately premature infants.
Design
Prospective cohort study including retrospective chart review and telephone interviews after discharge.
Setting
Ten birth hospitals in California and Massachusetts.
Patients
Surviving moderately premature infants born between October 2001 and February 2003.
Main outcome measures
(a) Occurrence of assisted ventilation during the hospital stay after birth; (b) adverse in‐hospital outcomes—for example, necrotising enterocolitis; (c) readmission within three months of discharge.
Results
With the use of prospective cluster sampling, 850 eligible infants and their families were identified, randomly selected, and enrolled. A total of 677 families completed a telephone interview three months after hospital discharge. During the birth stay, these babies experienced substantial morbidity: 45.7% experienced assisted ventilation, and 3.2% still required supplemental oxygen at 36 weeks. Readmission within three months occurred in 11.2% of the cohort and was higher among male infants and those with chronic lung disease.
Conclusions
Moderately premature infants experience significant morbidity, as evidenced by high rates of assisted ventilation, use of oxygen at 36 weeks, and readmission. Such morbidity deserves more research.
Keywords: assisted ventilation; outcomes, prematurity; morbidity; intensive care
Much of the recent neonatal outcomes literature focuses on babies weighing <1500 g at birth, who are usually <33 weeks gestation and who have very high mortality and morbidity.1 Less is known about premature infants at higher gestations. Rates of several outcomes (mortality,2 cerebral palsy,3,4,5 respiratory syncytial virus infection,6 short term hospital morbidity,7 and readmission8,9) in these infants are lower than in very premature infants but higher than in term infants.
Newborns born at 30–346/7 weeks gestation constitute 3.9% of all infants and 32% of all premature infants in the United States.10 Neonatal mortality in 30–34 week gestation infants in the United States is 18.5 per 1000 live births, substantially higher than the 6.9/1000 rate found in 35–36 week gestation infants and the 2.5/1000 rate found in term infants, but considerably lower than the 285.3/1000 rate in babies <30 weeks gestation.10
Moreover, these infants make up a substantial proportion of admissions to neonatal intensive care units (NICUs). In an internal data review for the year 2003 in the Kaiser Permanente Medical Care Program, 27% of all NICU admissions were 30–34 week babies. Similarly, for the years 2002 through 2004, 38% of all NICU admissions at the Brigham and Women's Hospital in Boston were in this gestational age range and constituted 77% of all admissions <35 weeks.
To address this gap in the literature, we prospectively examined birth stay outcomes and three month follow up of a cohort of 30–34 week infants who survived the birth stay in California and Massachusetts. This report will focus on overall in‐hospital outcomes and readmission in the first three months after discharge from the NICU. Other findings are reported elsewhere.11,12,13,14,15,16
Methods
Study population and sampling strategy
Our target population was infants born at 30–346/7 weeks gestation discharged alive from 10 hospitals in Massachusetts and California between 30 October 2001 and 28 February 2003. These centres (described in an appendix which is available on request from the corresponding author) were selected because of previous participation in our studies, including one on healthy, moderately premature infants17 and previous discharge information indicating that they could recruit 100 infants over the 15 month period. Admission to the NICU is mandatory for all babies born at <35 weeks gestation at these 10 centres.
At three sites where the volume of admissions was very high, sampling algorithms were developed to ensure that the final sample size of 100 was evenly distributed over the enrolment period. Despite the prior information, in four sites enrolment was slower than expected, and we reduced the target size to 60–65 infants so that all sites were collecting data throughout the same period.
Study sample and enrolment procedures
Infants eligible for the study included those admitted to the study sites within the target gestational age range as determined by the best obstetrician defined estimate. Infants with major malformations or chromosomal disorders were excluded. Where potential ambiguities existed—for example, with respect to anomalies—the decision to exclude an infant was made by consensus among the investigators.
At least once a week during the enrolment period, trained research assistants visited each site or contacted designated hospital staff to ascertain admission of potentially eligible infants. Using the admission log at each site and computerised tracking files, research assistants identified potentially eligible infants and followed their hospital course until 34 weeks postmenstrual age (PMA). At that point parents were given a study packet and asked to participate in the follow up interview.
Parents whom we were unable to contact before discharge or who failed to respond were contacted by mail nine weeks after discharge (a time frame mandated by institutional review boards) and invited to participate in the telephone survey.
Data collection
Data for this report were obtained from the NICU admission logs at each site, maternal and infant medical records, and a telephone survey of parents three months after hospital discharge. The basic data collection protocol was based on the Kaiser Permanente neonatal minimum data set,18 the score for neonatal acute physiology, version II,19 and previous studies.20,21,22,23,24 Trained research assistants entered data directly into laptop computers, and encrypted data were sent to the Boston data coordinating centre.
The telephone interview at three months after discharge captured information on resource use after discharge, readmission, parent perception of infant health, satisfaction with NICU care, and breast feeding. Trained interviewers using computer assisted telephone interviewing software administered the survey. Questions on illness, readmission, planned and unplanned medical visits, and early intervention were adapted from the one year survey used in the evaluation of the Robert Wood Johnson national perinatal regionalization demonstration program.25
Statistical analysis
Because sampling fractions were not equal across study sites, all statistical analyses involving the entire cohort (as opposed to site specific calculations) used analytical methods in which the data for each site were weighted by the site's sampling fraction. For analyses involving outcomes after discharge, which were based on interview data, each site's data were weighted by the proportion of infants actually followed up at that centre. Not all study hospitals routinely collected data on maternal and infant race, but this information was obtained consistently from all interviews. As race was not available in the chart review dataset but was available in the interview dataset, we compared the results of tables and multivariate models with and without race. There were no major differences in the results, so all of the tables in this report include race.
Initial selection of variables for multivariate models was based on the results of bivariate comparisons, biological plausibility, and results of previous studies.8,9,26,27 Variables were retained in the final multivariate models if they reached statistical significance in preliminary models or if they were clinically important.
All analyses were conducted using Stata statistical software, version 8 (Stata, College Station, Texas, USA) and controlled for clustering due to multiple births.
Human subjects
The eight institutional review boards with jurisdiction over the 10 sites approved this study. Except for one site, which required written consent for all parts of the study, collection of medical record data was approved for all eligible infants, and parental permission for the interview was required.
Results
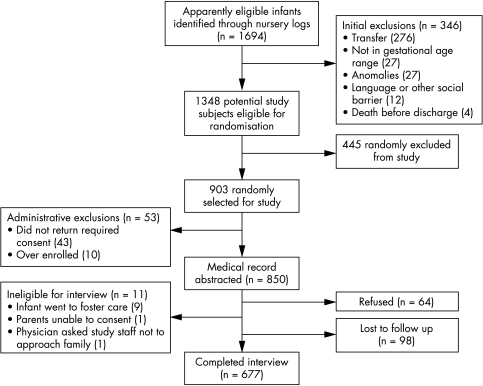
Figure 1 summarises study enrolment. Of the 1694 infants of 30–34 weeks gestation identified at the 10 study hospitals, 346 were found to be ineligible, leaving 1348 potential study subjects. Of these 1348 infants, 903 were randomly selected on the basis of the centre's designated sampling fraction. Of the ineligible infants, 276 (80%) were transferred to another hospital where we could not track their outcomes, 27 (8%) did not meet the gestational age criteria of 30–346/7 weeks, 27 (8%) had a major congenital anomaly, 12 (3%) were ineligible because of parent language barriers or other social reasons, and four (1%) died before discharge. Of the remaining 903 eligible infants, 43 parents did not provide the required consent for the chart review, and 10 infants enrolled in excess of site specific goals were excluded, giving a total cohort of 850 infants for the examination of hospital based morbidity.
Figure 1 Overview of identification, sampling, and study enrolment.
The parents of 64 (8%) of these 850 infants refused the three month interview, and interviews were not attempted for social reasons—for example, infants entering foster care—for 11 (1%). An additional 98 (12%) were lost to follow up. Thus interviews were completed for 677 (80%) of the eligible parents. Compared with the infants whose families completed the telephone survey, those whose families did not were not significantly different with respect to sex, birth weight, gestational age, proportion who were small for gestational age, the score for neonatal acute physiology, version II, assisted ventilation status, or chronic lung disease status.
Table 1 summarises cohort characteristics. The number of enrolled births listed is 1250 (not 850) because of weighting given different sampling fractions in the medical centres. Almost two thirds of the babies were 33–34 weeks gestation, and almost half experienced some form of assisted ventilation. The proportion of infants who received antenatal steroids was 66%, with considerable variation by gestational age, ranging from 45% among 34 week infants to 88% among 30 week infants. Mean PMA at discharge was 36.2 weeks. Most mothers had completed high school, and their mean age was 31 years. Annual income was at least $50 000 in 60% of the families.
Table 1 Characteristics of 30–346/7 week gestation premature infants in the study.
| Entire sample* | Range across sites† | |
|---|---|---|
| Enrolled births | 1250 | 60–100 |
| Maternal characteristics | ||
| Mean age (years) | 31.1 | 28.6–33.8 |
| Completed high school (%) | 73.3 | 56.7–86.0 |
| Annual income <$30 000 (%)‡ | 12.5 | 1.1–29.2 |
| Annual income $30 000–49 999 (%)‡ | 12.0 | 3.9–24.7 |
| Annual income ⩾$50 000 (%)‡ | 60.0 | 44.1–80.9 |
| White (%) | 58.5 | 26.0–86.1 |
| African‐American (%) | 14.2 | 0.0–35.1 |
| Asian (%) | 13.4 | 3.5–31.3 |
| Hispanic (%) | 10.3 | 0.0–26.5 |
| Antenatal steroids | ||
| All gestations | 66.0 | 61.5–72.0 |
| 30 weeks | 88.3 | 50.0–100 |
| 31 weeks | 84.8 | 70.0–100 |
| 32 weeks | 80.4 | 73.9–95.0 |
| 33 weeks | 73.1 | 54.2–85.7 |
| 34 weeks | 44.7 | 23.3–59.1 |
| Infant characteristics | ||
| Sex (% male) | 50.3 | 43.3–58.5 |
| Mean gestational age (weeks) | 33.1 | 32.9–33.5 |
| 33–346/7 weeks (%) | 61.9 | 53.0–76.7 |
| 30–326/7 weeks (%) | 38.1 | 23.3–47.0 |
| SGA (%) | 6.6 | 3.0–15.4 |
| Multiple gestation (%) | 39.5 | 20.0–51.0 |
| Mean birth weight (g) | 1933 | 1807–2091 |
| ⩾2000 g birth weight (%) | 41.5 | 30.0–56.7 |
| 1500–1999 g (%) | 43.3 | 31.7–50.0 |
| <1500 g (%) | 15.2 | 5.0–24.6 |
| Mean SNAP‐II | 5.6 | 3.5–7.1 |
| Ever ventilated (%) | 45.7 | 18.3–59.0 |
| nCPAP only (%) | 19.1 | 8.0–31.0 |
| IMV without nCPAP (%) | 4.1 | 0.0–9.0 |
| Ever transported (%) | 14.4 | 0.0–58.3 |
| Received surfactant (%) | 24.6 | 6.7–29.0 |
| Mean PMAD (weeks) | 36.2 | 35.5–36.6 |
*Results are weighted on the basis of different sampling fractions.
†Unweighted data.
‡14% of all respondents did not provide income information.
SGA, Small for gestational age; SNAP‐II, score for neonatal acute physiology, version II; nCPAP, nasal continuous positive airways pressure; IMV, intermittent ventilation; PMAD, premenstrual age at discharge.
Table 2 summarises the in‐hospital outcomes of the study infants. The rates of these outcomes were generally higher in babies 30–326/7 weeks than in those 33–346/7 weeks gestation. There were no differences between the two gestational age ranges in rates of pneumothorax, necrotising enterocolitis, or PMA at discharge ⩾36 weeks. Results obtained using stratification by birth weight were similar to those obtained using gestational age. Mean PMA at discharge was slightly lower in babies ⩾2000 g birth weight (36.0 weeks) than in those <2000 g (36.3 weeks, p = 0.019). None of the 24.5% of infants screened by an ophthalmologist developed retinopathy of prematurity.
Table 2 Rates of key in‐hospital outcomes, stratified by gestational age and birth weight.
| Gestational age (weeks) | Birth weight (g) | All infants (n = 1250) | |||
|---|---|---|---|---|---|
| 30–326/7 (n = 476) | 33–346/7 (n = 774) | <2000 (n = 731) | ⩾2000 (n = 519) | ||
| LOAV ⩾72 hours | 21.3 | 3.4* | 14.0 | 4.8* | 10.2 |
| Pneumothorax | 1.3 | 1.7 | 1.5 | 1.6 | 1.6 |
| Oxygen in use at 28 days | 7.1 | 0.1* | 4.8 | 0.0* | 2.8 |
| Oxygen in use at 36 weeks PMA | 6.7 | 1.0* | 4.3 | 1.5* | 3.2 |
| Necrotising enterocolitis | 1.2 | 0.7 | 1.0 | 0.8 | 0.9 |
| Sepsis/meningitis | 4.9 | 0.8* | 3.1 | 1.4 | 2.4 |
| IVH, any | 0.6 | 0.0 | 0.4 | 0.0 | 0.2 |
| IVH, grade 3 or 4 | 1.2 | 0.0* | 0.8 | 0.0 | 0.4 |
| Retinopathy of prematurity | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Discharge at <36 weeks PMA | 45.7 | 53.9 | 45.2 | 58.7* | 50.8 |
| Discharge at 36–38 weeks PMA | 41.6 | 38.6 | 43.8 | 34.0 | 39.8 |
| Discharge at ⩾38 weeks PMA | 12.7 | 7.4 | 11.0 | 7.2 | 9.4 |
Values are percentages. Statistical comparisons are between 30–326/7 week infants and 33–346/7 week infants and between infants <2000 g and infants ⩾2000 g birth weight. Asterisks indicate comparisons that reached significance at the p<0.05 level.
LOAV, Length of assisted ventilation; IVH, intraventricular haemorrhage; PMA, postmenstrual age.
Table 3 shows rates of readmission within three months of discharge. The overall readmission rate was 11.3% and varied considerably across centres (6.0–18.2%). In unadjusted comparisons, infants who experienced assisted ventilation for at least 72 hours, African‐American infants, male infants, and infants with chronic lung disease (whether defined as oxygen use at 28 days of age or as oxygen use at 36 weeks PMA) were more likely to be readmitted.
Table 3 Readmission within three months of discharge in the study cohort.
| Infant group | Readmission rate | Range across sites |
|---|---|---|
| All infants (n = 1250) | 11.3 | 6.0–18.2 |
| Singleton birth (n = 735) | 12.7 | 6.7–22.2 |
| Multiple gestation birth (n = 515) | 9.2 | 0–15.8 |
| 30–32 weeks (n = 494) | 12.5 | 6.1–32.0 |
| 30–32 weeks, received antenatal steroids (n = 422) | 12.3 | 5.3–31.8 |
| 30–32 weeks, no antenatal steroids (n = 72) | 13.3 | 0.0–33.3 |
| 33–34 weeks (n = 756) | 10.5 | 2.2–17.9 |
| 33–34 weeks, received antenatal steroids (n = 422) | 11.3 | 0.0–27.8 |
| 33–34 weeks, no antenatal steroids (n = 334) | 9.4 | 0.0–14.8 |
| <2000 g (n = 745) | 11.4 | 5.7–23.8 |
| 2000+ g (n = 505) | 11.0 | 5.3–21.4 |
| Male infants (n = 638) | 14.4 | 4.9–20.0 |
| Female infants (n = 612) | 8.0 | 0.0–15.6 |
| White infants (n = 731) | 11.1 | 5.0–13.6 |
| African‐American infants (n = 177) | 14.1 | 0.0–38.5 |
| Asian infants (n = 167) | 10.6 | 0.0–33.3 |
| Hispanic infants (n = 129) | 9.8 | 0.0–50.0 |
| Infants with LOAV ⩾72 hours (n = 133) | 13.6 | 0.0–25.0 |
| Infants with oxygen in use at 28 days (n = 36) | 39.8 | 0.0–100.0 |
| Infants with oxygen in use at 36 weeks PMA (n = 37) | 31.0 | 0.0–50.0 |
| Mother did not complete high school (n = 92) | 9.4 | 0.0–66.7 |
| Mother completed high school (n = 1158) | 11.4 | 6.4–18.9 |
| Annual income <$30 000 (n = 159)* | 9.8 | 0.0–28.6 |
| Annual income $30 000–49 999 (n = 151) | 9.4 | 0.0–60.0 |
| Annual income ⩾50 000 (n = 744) | 11.8 | 3.2–17.4 |
Values are percentages.
LOAV, Length of assisted ventilation; PMA, postmenstrual age.
*14% of all respondents did not provide income information.
Table 4 shows the results of a multivariate model that included infant sex, gestational age, chronic lung disease (defined as oxygen use at 36 weeks PMA), birth weight, race, and a combined hospital adverse outcome variable that included the occurrence of at least one of the following outcomes: sepsis/meningitis, pneumothorax, prolonged length of stay, or necrotising enterocolitis. Only male sex was a statistically significant predictor for readmission (adjusted odds ratio 1.93; 95% confidence interval 1.10 to 3.41). Having a birth weight ⩾2000 g was not protective. Incorporating maternal education and/or family income did not result in any substantial change in the odds ratios.
Table 4 Multivariate analysis for readmission.
| AOR | 95% CI | |
|---|---|---|
| Infant sex | ||
| Male | 1.93 | 1.10 to 3.41 |
| Female (ref) | ||
| Gestational age | ||
| 30–31 weeks | 0.78 | 0.35 to 1.75 |
| 32–33 weeks | 0.68 | 0.35 to 1.35 |
| 34 weeks (ref) | ||
| Bronchopulmonary dysplasia | ||
| Yes | 2.95 | 0.82 to 10.56 |
| No (ref) | ||
| Birth weight | ||
| <2000 g | 1.09 | 0.56 to 2.12 |
| ⩾2000 g (ref) | ||
| Race/ethnicity | ||
| African‐American | 1.49 | 0.62 to 3.55 |
| White (ref) | ||
| Other | 0.93 | 0.48 to 1.81 |
| Combined hospital adverse outcome | ||
| Yes | 1.53 | 0.65 to 3.59 |
| No (ref) |
Method used was logistic regression, with the dichotomous outcome of interest being any readmission during the three months after discharge from the birth stay. Of the 1250 infants in the cohort, 141 (11.3%) were admitted within three months of discharge. The model controls for clustering due to multiple births to the same mother. The adjusted odds ration (AOR) and 95% confidence interval (CI) were estimated from the logistic regression coefficients. Combined hospital adverse outcome is the occurrence of at least one of the following during the birth stay: pneumothorax, prolonged length of stay (discharge at >38 weeks postmenstrual age), sepsis/meningitis, or necrotising enterocolitis.
Discussion
We have conducted a prospective descriptive study of in‐hospital outcomes and readmissions within three months of discharge in surviving infants born at 30 to 346/7 weeks gestation. These infants experience considerable in‐hospital morbidity, and a substantial fraction are readmitted within three months of discharge.
Resource limitations precluded us from incorporating a term comparison group, but a limited number of denominator based studies for ⩾37 week babies suggest that the morbidity experienced by infants in our cohort is substantially higher. In our cohort, 45.7/1000 experienced assisted ventilation, which is more than 4 times higher than the 10.4/1000 rate found in term infants by Wilson et al.28 With respect to pneumothorax, three studies29,30,31 have reported rates ranging from 0.17 to 0.70/1000 live births; in contrast, the rate in our cohort was 16/1000. Chen et al32 reported a sepsis/meningitis rate of 1.6/1000 in term infants, also substantially lower than the 24.0/1000 rate in our cohort. If one only considers grade 3 or 4 intraventricular haemorrhages, the rate in our cohort (4.0/1000) is much higher than that reported by Jocelyn et al33 in term infants (0.49/1000). The difference may not be as great with respect to having any intraventricular haemorrhage, as some studies, which did not report haemorrhage grades, have documented such events in as many as 5% of all term infants.34,35 A recent article by Wang et al,7 which compared outcomes in near term (35–366/7 weeks) and term infants, also supports the notion that moderate degrees of prematurity are also associated with substantial morbidity.
Recent reports of readmission among term infants36,37,38 use a four to six week window rather than our three month interval. In these studies, readmission rates range from 19.0 to 39.5/1000. In addition, we were able to make comparisons with the readmission rate at three months in the ⩾37 week gestational age cohort of the Kaiser Permanente Medical Care Program in 2002, where this rate was 43.0/1000. Thus it seems reasonable to infer that 30–34 week gestation infants, whose readmission rate in the three months after hospital discharge was 113.0/1000, are at much higher risk than term infants.
Our analyses show that characteristics such as an infant's sex and clinical variables—for example, chronic lung disease—have strong associations with in‐hospital outcomes. We also found that male sex was a predictor for readmission in the three months after discharge, a finding that we cannot explain. In previous studies,39,40 we have reported an increased risk of severe (⩾25 mg/dl) jaundice in male infants ⩾36 weeks gestation. However, in another study involving readmission within two weeks of discharge in infants of all gestations, the increased risk in male infants was not limited to jaundice.9
Of importance is the fact that a birth weight of ⩾2000 g is not protective against assisted ventilation or readmission. Although a large body of evidence clearly shows that the key determinant for neonatal mortality and morbidity is gestational age, and although ultrasound gestational age assignment is becoming more widespread—for example, in the Kaiser Permanente Medical Care Program all pregnant women have at least one ultrasound—use of birth weight as a predictor remains quite common, in both the literature and clinical practice. Our study shows that babies whose weights might lead many clinicians to consider them to be “big premies” nonetheless experience considerable morbidity.
Our study does have important limitations. The first is that our sample of hospitals may not be representative. For example, at our study sites, admission to a special care setting is mandatory for babies <35 weeks gestation, a policy that may not be universal. It is conceivable that moderately premature infants in other locations could have better or worse outcomes than the ones we describe in this report. It is also possible that, as we excluded infants who were transferred (who, at these medical centres, are most likely to be babies who could safely be sent for convalescence at smaller hospitals), our sample may be relatively enriched with sicker moderately premature infants. Given the relatively low frequency of certain events—for example, only 3.2% of the cohort had chronic lung disease—our study may not have the power to detect associations between events occurring during the birth stay and subsequent outcome. Because this study was planned as an exploration of the factors affecting outcomes of moderately premature infants, we did not collect detailed utilisation data—for example, actual follow up services provided to families and infants—and we also have limited data on the obstetric management experienced by these infants. Clearly, future studies should capture such data because of its established impact on readmission rates.9 Future collaborative studies could address these issues.
It is very clear that, for many important neonatal outcomes, two important gradients exist. The first is a quantitative gradient, with an exponential decrease in the numbers of babies as gestational age decreases. However, adverse outcomes follow a gradient in the opposite direction, with increasing rates as gestational age decreases. Thus, for certain outcomes, the absolute burden to society may be greater from infants who are not as premature because there are more such infants. Consideration of these gradients suggests that a broader conceptual shift needs to be made in perinatal medicine and epidemiology. Such a shift needs to consider issues involving the care of infants as well as the study of the care infants receive.
In the presence of widespread practice variation and absence of specific, evidence based guidelines, which should include rational discharge planning strategies, it is conceivable that both clinicians and insurers may use tacit or “de facto” guidelines that treat these infants on the basis of their birth weight as well as presumed asymptomatic status. This could lead some infants to be treated—inappropriately—using guidelines defined for term infants. Professional societies such as the Royal College of Paediatrics and Child Health and the American Academy of Pediatrics should take an active role in defining gestational age specific guidelines for moderately premature infants. Such guidelines will probably need to go into greater detail with respect to gestational age ranges—that is, not simply group all babies <37 weeks into a single category.
The fact that so many moderately premature infants require some form of assisted ventilation suggests that assessment of the value of specific obstetric interventions aimed at prolonging pregnancy should take these infants' outcomes into account. For example, not all obstetricians would attempt tocolysis at 33–34 weeks gestation. Another important issue that needs to be re‐examined is the risk‐benefit ratio of elective caesarean delivery for moderately premature infants. A recent randomised trial to prevent respiratory distress in term infants born by elective caesarean section provides a good example of the sort of questions that need to be addressed in moderately premature infants.41
What is already known on this topic
Premature infants are at higher risk of mortality and morbidity than term infants
Very low birthweight and very premature infants are at the highest risk of adverse outcomes
In developed nations, accurate gestational age measures have become more commonly available. Moreover, during analysis of any dataset, use of cut off points (particularly dichotomous ones) for continuous data entails information loss. Consequently, future studies on premature infants should: (a) base their cohorts on gestational age criteria, not birth weight; (b) report outcomes for specific weeks of gestation; (c) whenever possible, include gestational age as a continuous outcome in multivariate models.
In conclusion, given that previous studies may not have considered the contribution of moderate prematurity to overall outcomes rates, it may be necessary to re‐examine certain existing assumptions about the costs and benefits of specific interventions. For example, if one bases certain obstetric interventions only on the outcomes experienced by very low birthweight infants, one may come to erroneous conclusions about the value or risk of such interventions among women presenting with preterm labour and moderate prematurity.
What this study adds
Moderately premature (30–34 weeks gestation) infants experience significant morbidity during their hospital stay after birth
Moderately premature infants have a very high readmission rate in the three months after discharge
Acknowledgements
This project was supported by the Agency for Healthcare Research and Quality (grant number 5 R01 HS 10131‐02, “Unstudied Infants: Low Risk Babies in a High Risk Place”). We thank Dr Joseph Selby, Director of the Kaiser Permanente Division of Research, for reviewing the manuscript, and Ms Kimberley Harris for formatting the manuscript. In Massachusetts, the Beth Israel Deaconess Medical Center, the Brigham and Women's, Lowell General, Newton‐Wellesley, and Winchester hospitals participated in the study, and the California sites were the Kaiser Foundation Hospitals in Hayward, Santa Clara, San Francisco, and Walnut Creek, and the Alta Bates Medical Center in Berkeley. In Massachusetts, we are very grateful for the assistance we received from the Beth Israel Deaconess Medical Center's Neonatologist in Chief, Dr DeWayne Pursley, as well as from the Nurse Manager, Jane Smallcomb; at the Winchester Hospital, we are grateful for the assistance we received from Dr Karen McAlmon, Medical Director, Special Care Nursery, and Ms Suzanne Murphy, Nurse Manager, Special Care Nursery; at the Lowell Hospital, we are grateful to Dr Mary Blackwell, Medical Director, Special Care Nursery, Dr Kim Chatson, Associate Director, Special Care Nursery, and Mary Beth Foley, Nurse Manager, Special Care Nursery; at the Newton‐Wellesley Hospital, we are grateful to Dr Richard Wilker, Chief of Neonatology, Ms Karen Ross, Nurse Manager, Special Care Nursery, and Ms Karen Mueller, Assistant Nurse Manager, Special Care Nursery; at the Brigham and Women's Hospital, we are grateful to Dr Steven Ringer, Director of the NICU, and Ms Marianne Cummings, Nurse Manager, NICU. We also wish to thank Rebecca Roberts and Chris Morgan for project support. In California, we thank the following neonatal intensive care unit directors for their support and assistance: Gilbert Duritz, MD, PhD, and Lee Reuben, MD, at the Alta Bates Medical Center; Jocelyn Alcantara, MD, Kaiser Permanente Hayward Medical Center; James Kantor, MD, and Carlos Botas, MD, Kaiser Permanente Medical Center San Francisco; Stephen Fernbach, MD, Kaiser Permanente Medical Center, Santa Clara; and Allen Fischer, MD, Kaiser Permanente Medical Center, Walnut Creek. We are also grateful for the assistance we received from Pamela Bigbee, RN, ICN Nurse Manager, and Rebecca Mitchell, RN, Assistant ICN Nurse Manager at the Kaiser Permanente Hayward Medical Center; Pat Keightley, RN, ICN Nurse Manager, and Nancy Taquino, RN, ICN Nurse Manager, at the Kaiser Permanente San Francisco Medical Center; Lisette Santos, RN, ICN Nurse Manager, and Bernadette Custodio, ICN Unit Assistant, at the Kaiser Permanente Santa Clara Medical Center; Shirley Dostal, RN, ICN Nurse Manager, Marilyn Taylor, RN, Assistant ICN Nurse Manager, Josie Franck, RN, Christine Retta, RN, and Noni Ettl, LCSW, at the Kaiser Permanente Walnut Creek Medical Center; and Katie Tobin, RN, Director, Women and Infants' Services, Amarjit Sandhu, MD, Peggy Lindsley, RN, BSN, NICU Nurse Manager, Alison Brooks, RN, MS, Ann Frost, RN, Sue Wittstock, RN, Gail Levine, MD, and Deborah Wilson, RN, NNP. We also thank Rachel Warder, BS, Diane Lott‐Garcia, Jeanne Holbrook, RN, and Christina Olguin at the Division of Research.
Abbreviations
NICU - neonatal intensive care unit
PMA - postmenstrual age
Footnotes
Competing interests: none declared
Presented at a platform session at the May 2004 meetings of the Society for Pediatric Research in San Francisco, California, and at Bethesda, MD at the NICHD Workshop on Optimizing Care and Long‐term Outcome of Near‐term Pregnancy and the Near‐term Newborn Infant in July of 2005.
References
- 1.Hack M, Fanaroff A A. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol 2000589–106. [DOI] [PubMed] [Google Scholar]
- 2.Kramer M S, Demissie K, Yang H.et al The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA 2000284843–849. [DOI] [PubMed] [Google Scholar]
- 3.Blair E, Stanley F J. Cerebral palsy in low‐birthweight infants: implications for perinatal care. Pediatr Perinat Epidemiol 19926298–310. [DOI] [PubMed] [Google Scholar]
- 4.Cummins S K, Nelson K B, Grether J K.et al Cerebral palsy in four northern California counties, births 1983 through 1985. J Pediatr 1993123230–237. [DOI] [PubMed] [Google Scholar]
- 5.Hagberg B, Hagberg G, Beckung E.et al Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–94. Acta Paediatr 200190271–277. [PubMed] [Google Scholar]
- 6.Boyce T G, Mellen B G, Mitchel E F., Jret al Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr 2000137865–870. [DOI] [PubMed] [Google Scholar]
- 7.Wang M L, Dorer D J, Fleming M P.et al Clinical outcomes of near‐term infants. Pediatrics 2004114372–376. [DOI] [PubMed] [Google Scholar]
- 8.Escobar G J, Joffe S J, Gardner M N.et al Rehospitalization in the first two weeks after discharge from the Neonatal Intensive Care Unit. Pediatrics 19991041–9. [DOI] [PubMed] [Google Scholar]
- 9.Escobar G J, Greene J, Hulac P.et al Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child 200590125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathews T. Infant mortality statistics from 2002 period linked birth/infant death data set: National Vital Statistics Report. Hyattsville, MD: National Center for Health Statistics, 2002 [PubMed]
- 11.Richardson D, Zupancic J, Escobar G J.et al The moderately preterm infant project: inter‐institutional practice variation [abstract]. Pediatr Res 200353382A [Google Scholar]
- 12.Eichenwald E, Escobar G J, Zupancic J.et al Inter‐NICU variation in discharge timing of moderately premature infants: earlier discharge does not affect 3‐month outcome [abstract]. Pediatr Res 200455372A [Google Scholar]
- 13.Blackwell M, McCormick M C, Zupancic J.et al Growth variation at discharge and 3 months post‐discharge in moderately premature infants [abstract]. Pediatr Res 200455442A [Google Scholar]
- 14.Blackwell M, McCormick M C, Zupancic J.et al Variation in nutritional support impacts growth outcomes of 30–35 week gestation infants in 10 California and Massachussetts NICUs [abstract]. Pediatr Res 200455442A [Google Scholar]
- 15.Blackwell M, McCormick M C, Zupancic J.et al Breastfeeding in 30 to 35 week infants from birth to 3 months after NICU discharge [abstract]. Pediatr Res 200455442A [Google Scholar]
- 16.Profit J, McCormick M C, Zupancic J.et al Influence of census and patient‐to‐nurse ratios on the decision to discharge moderately premature infants [abstract]. Pediatr Res 200455521A [Google Scholar]
- 17.Blackwell M T, Eichenwald E C, McAlmon K.et al Interneonatal intensive care unit variation in growth rates and feeding practices in healthy moderately premature infants. J Perinatol 200525478–485. [DOI] [PubMed] [Google Scholar]
- 18.Escobar G J, Fischer A, Kremers R.et al Rapid retrieval of neonatal outcomes data: the Kaiser Permanente Neonatal Minimum Data Set. Qual Manag Health Care 1997519–33. [PubMed] [Google Scholar]
- 19.Richardson D K, Corcoran J D, Escobar G J.et al SNAP‐II and SNAPPE‐II: simplified newborn illness severity and mortality risk scores. J Pediatr 200113892–100. [DOI] [PubMed] [Google Scholar]
- 20.Richardson D K, Gray J E, McCormick M C.et al Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics 199391617–623. [PubMed] [Google Scholar]
- 21.Richardson D K, Phibbs C S, Gray J E.et al Birth weight and illness severity: independent predictors of neonatal mortality. Pediatrics 199391969–975. [PubMed] [Google Scholar]
- 22.Eichenwald E C, Lloyd J S, Tran T.et al Inter‐NICU variation in discharge timing: effects of relationship between apnea and feeding management [abstract]. Pediatr Res 199945195A. [DOI] [PubMed] [Google Scholar]
- 23.Blackwell M, Petit K, McAlmon K.et al Inter‐NICU Variation in growth, feeding practices and gestational age at discharge (GAdisch) in healthy preterm infants [abstract]. Pediatr Res 199945238A [Google Scholar]
- 24.Escobar G J, Li D K, Armstrong M A.et al Neonatal sepsis workups in babies ⩾2000 grams at birth: a population‐based study. Pediatrics 2000106256–263. [DOI] [PubMed] [Google Scholar]
- 25.McCormick M C, Shapiro S, Starfield B H. The regionalization of perinatal services. Summary of the evaluation of a national demonstration program. JAMA 1985253799–804. [PubMed] [Google Scholar]
- 26.Cavalier S, Escobar G J, Fernbach S A.et al Postdischarge utilization of medical services by high‐risk infants: experience in a large managed care organization. Pediatrics 199697693–699. [PubMed] [Google Scholar]
- 27.Escobar G J, Gonzales V M, Armstrong M A.et al Rehospitalization for neonatal dehydration: a nested case‐control study. Arch Pediatr Adolesc Med 2002156155–161. [DOI] [PubMed] [Google Scholar]
- 28.Wilson A, Gardner M N, Armstrong M A.et al Neonatal assisted ventilation: predictors, frequency, and duration in a mature managed care organization. Pediatrics 2000105822–830. [DOI] [PubMed] [Google Scholar]
- 29.Boer H R, Andrews B F. Spontaneous pneumothorax in the neonate. South Med J 197770841–846. [DOI] [PubMed] [Google Scholar]
- 30.Al Tawil K, Abu‐Ekteish F M, Tamimi O.et al Symptomatic spontaneous pneumothorax in term newborn infants. Pediatr Pulmonol 200437443–446. [DOI] [PubMed] [Google Scholar]
- 31.Lee S K, McMillan D D, Ohlsson A.et al Variations in practice and outcomes in the Canadian NICU network: 1996–1997. Pediatrics 20001061070–1079. [DOI] [PubMed] [Google Scholar]
- 32.Chen K T, Ringer S, Cohen A P.et al The role of intrapartum fever in identifying asymptomatic term neonates with early‐onset neonatal sepsis. J Perinatol 200222653–657. [DOI] [PubMed] [Google Scholar]
- 33.Jocelyn L J, Casiro O G. Neurodevelopmental outcome of term infants with intraventricular hemorrhage. Am J Dis Child 1992146194–197. [DOI] [PubMed] [Google Scholar]
- 34.Sachs B P, Acker D, Tuomala R.et al The incidence of symptomatic intracranial hemorrhage in term appropriate‐for‐gestation‐age infants. Clin Pediatr (Phila) 198726355–358. [DOI] [PubMed] [Google Scholar]
- 35.Heibel M, Heber R, Bechinger D.et al Early diagnosis of perinatal cerebral lesions in apparently normal full‐term newborns by ultrasound of the brain. Neuroradiology 19933585–91. [DOI] [PubMed] [Google Scholar]
- 36.Marbella A M, Chetty V K, Layde P M. Neonatal hospital lengths of stay, readmissions, and charges. Pediatrics 199810132–36. [DOI] [PubMed] [Google Scholar]
- 37.Madlon‐Kay D J, DeFor T A, Egerter S. Newborn length of stay, health care utilization, and the effect of Minnesota legislation. Arch Pediatr Adolesc Med 2003157579–583. [DOI] [PubMed] [Google Scholar]
- 38.Martens P J, Derksen S, Gupta S. Predictors of hospital readmission of Manitoba newborns within six weeks postbirth discharge: a population‐based study. Pediatrics 2004114708–713. [DOI] [PubMed] [Google Scholar]
- 39.Newman T B, Escobar G J, Gonzales V M.et al Frequency of neonatal bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics 19991041198–1203. [PubMed] [Google Scholar]
- 40.Newman T B, Xiong B X, Gonzales V M.et al Prediction and prevention of extreme neonatal hyperbilirubinemia in a mature health maintenance organization. Arch Pediatr Adolesc Med 20001541140–1147. [DOI] [PubMed] [Google Scholar]
- 41.Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ 2005331662. [DOI] [PMC free article] [PubMed] [Google Scholar]