Abstract
The immediate prenatal and postnatal consequences of reduced fetal growth have long been known. The longer term associations between reduced birth weight and adult disease risk are also now well established. Reduced fetal growth is usually detected late in gestation, and the assumption has been that this is the time when factors regulating fetal growth have their greatest effect. However, recent evidence suggests that both the growth trajectory of the fetus and its adaptive responses to the prenatal and postnatal environment may be determined in the period around the time of conception.
Keywords: birth weight, twins, nutrition, developmental origins of health and disease, periconceptional nutrition
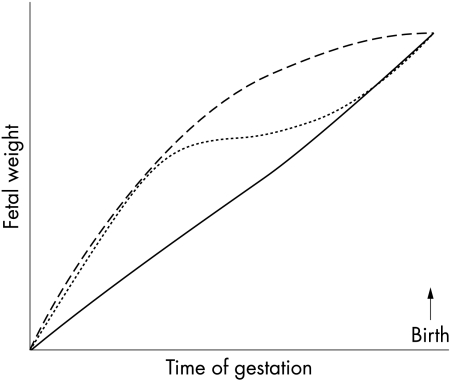
When considering the associations between birth weight and health outcomes, it is important to remember that birth weight is a single, cross sectional summative measure taken at the end of a long period during which growth is rapid but not linear. The same birth size can be obtained by quite different intrauterine growth trajectories (fig 1). Thus taking a single measure of growth at the time of birth may mask the true associations between fetal growth trajectory and the outcomes of interest. A simple clinical example is to compare two babies born at an appropriate weight for gestational age of 3200 g. One is the infant of Oriental parents whose first baby weighed 2900 g, and the other the infant of Polynesian parents whose previous babies all weighed around 4000 g. It seems likely that the Oriental baby has had normal intrauterine growth, the increase in birth weight compared with the sibling reflecting the well known phenomenon of lower birth weight in firstborns. In contrast, the Polynesian baby has probably suffered intrauterine growth restriction, and is more likely to experience the neonatal complications associated with this impaired growth.
Figure 1 Different fetal growth trajectories resulting in similar birth weights. The graph shows three exaggerated hypothetical growth trajectories: rapid growth in the first half of pregnancy with slowing thereafter (long dashed line); a similar initial trajectory followed by a period of slow growth before there is intrauterine catch‐up growth (short dashed line); initially slower growth with acceleration in the last half of gestation (solid line).
The distinction between fetal growth and birth weight can be clearly demonstrated in experimental animals. In fetal sheep, daily increase in chest circumference can be measured directly using surgically implanted growth catheters. Mellor and Murray1 showed that growth rate in late gestation fetal sheep slowed during nine days of maternal undernutrition, but recovered upon refeeding. However, if undernutrition was continued for 19 days, fetal growth rate remained reduced despite maternal refeeding. In the clinical setting, there are a few case reports suggesting that when human fetal growth has been compromised by severe maternal malnutrition, fetal growth trajectory can be increased in the second half of gestation by the initiation of maternal total parenteral nutrition, resulting in an increase in fetal abdominal circumference from an average of the 2nd to the 33rd centile.2 Thus there may be a variety of changes in fetal growth trajectory, including slowing followed by later acceleration or catch‐up, but resulting in a birth weight within the accepted normal range.
However, the fact that birth weight may fall in the normal range does not exclude other possible long term consequences as a result of intrauterine adaptations made during the period of undernutrition. To investigate this possibility, we undernourished pregnant sheep for 10 or 20 days in late gestation (term = 145 days) and followed the offspring to adulthood at 3 years of age. Consistent with the findings of Mellor and Murray,1 only offspring of ewes undernourished for 20 days were lighter than controls at birth. However, the offspring of ewes undernourished for only 10 days, whose birth weights were not different from those of controls, showed accentuated adrenocorticotrophic hormone and cortisol responses to stimulation with corticotrophin releasing hormone and arginine vasopressin at 3 years of age.3 In contrast, glucose tolerance at five and 36 months of age in all offspring was inversely related to birth weight, independent of the nutritional group in late gestation.4 These data illustrate two important points: firstly, intrauterine environmental insults that may affect fetal growth trajectory can result in altered postnatal physiology without an effect on birth weight;5 secondly, the same environmental insult may affect different physiological systems in different ways and presumably via different pathways.
Importance of altered body proportions at birth
It is increasingly well recognised that different body proportions at birth may reflect different intrauterine growth trajectories. Traditional divisions of clinical intrauterine growth restriction into “symmetrical” and “asymmetrical” patterns, thought to reflect early or late onset fetal undernutrition respectively, are not supported by more careful examination of large datasets.6 However, the fetus clearly can and does make adaptations to adverse intrauterine environments that result in differential organ growth. For example, when oxygen supply is limited, studies in fetal sheep have shown that there is a redistribution of fetal cardiac output away from “non‐essential” organs such as carcass, gut, and skin to the “essential” organs such as brain, adrenals, and heart.7 Ultrasound studies of human growth restricted fetuses suggest that similar changes occur.8 These changes may help explain brain, and thus head, sparing and also the increased risk of necrotising enterocolitis in growth restricted babies.
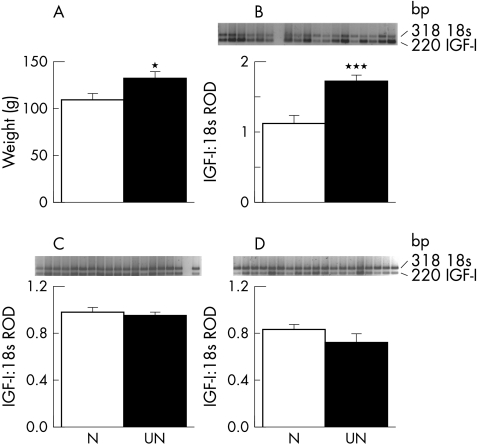
In fetal sheep, the proportion of umbilical venous blood flow that goes to the liver, rather than bypassing the liver via the ductus venosus, does not appear to be affected by hypoxaemia.9 However, when the ductus venosus was either stented or occluded, fetal hepatic weight and concentrations of insulin‐like growth factor (IGF)‐I mRNA were affected by the proportion of umbilical venous blood flow that passed through the liver. In fetuses with stents in the ductus venosus, causing more blood to bypass the liver, liver weight and hepatic IGF‐I mRNA concentrations were decreased; the converse was true when the ductus venosus was occluded with an embolisation coil for one week in late gestation.10 Intriguingly, detailed prospective ultrasound studies of human fetuses have reported perhaps analogous associations between shunting of umbilical venous blood flow through the ductus venosus and maternal nutritional status. In fetuses of mothers who were healthy but were slim or who had an “imprudent” diet before becoming pregnant, there was reduced shunting of umbilical venous blood through the ductus venosus and increased hepatic blood flow.11 The authors hypothesised that this may reflect the need for increased hepatic nutrient conversion, a phenomenon well described in fetal sheep.12,13 We have data from nutritional studies in sheep linking these two strands of evidence. Fetal sheep of ewes moderately undernourished from 60 days before until 30 days after mating had increased liver weights, but no difference in birth weight, more than three months later, compared with fetuses of ewes well nourished throughout pregnancy.14 Consistent with this, IGF‐I mRNA concentrations, measured by reverse transcriptase polymerase chain reaction, were significantly increased in the liver, but not placenta or skeletal muscle, in the fetuses of periconceptionally undernourished ewes (fig 2).
Figure 2 Fetal liver weight (A) and insulin‐like growth factor (IGF)‐I mRNA concentrations in fetal liver (B), muscle (C), and placenta (D), measured by reverse transcriptase polymerase chain reaction, in singleton ovine fetuses from ewes exposed to either good nutrition throughout pregnancy (N; n = 10) or a period of moderate periconceptional undernutrition from 60 days before to 30 days after mating, designed to reduce maternal body weight by 10–15% (UN; n = 12). Data are mean (SEM). *p<0.05, ***p<0.001 v N fetuses. RoD, Relative optical density.
Altered body proportions at birth have also been associated with altered physiology many years after birth in humans. For example, babies with a low ponderal index (PI) at birth had raised glucose concentrations after a glucose tolerance test at 7 years of age. The mean 60 minute glucose concentration in children with a PI at birth in the lowest quartile was 8.5 mM, compared with 7.9 mM in those with a PI in the highest quartile.15 Others have found a similar association between PI at birth and insulin tolerance or type 2 diabetes at 50–60 years of age.16 A decreased abdominal circumference at birth has also been associated with raised serum triacylglycerol concentrations at 50 years of age.17
Growth of the fetus relative to the placenta also seems to be important. Babies born small but with a relatively large placenta are less likely to show catch‐up growth in the 18 months after birth,18 and have an increased risk of hypertension in adult life.19 Evidence that undernutrition around the time of conception in rats can alter the number of cells in the inner cell mass and trophectoderm raises the intriguing possibility that apportioning of cells at this early blastocyst stage may influence the relative sizes of fetus and placenta.20
Fetal growth trajectory may be determined in early pregnancy
Fetal growth
In late gestation, most of the important fetal growth factors, such as IGF‐I, insulin, and the thyroid hormones, are nutritionally regulated. Thus fetal growth is also principally regulated by fetal nutrition. Although it is important to remember that changes in maternal nutrition do not necessarily lead directly to changes in fetal nutrition, as the fetus lies at the end of a long supply line,21 it is readily apparent that, where there is significant maternal undernutrition, fetal substrate supply may not meet fetal demand, necessitating a slowing of the fetal growth trajectory. However, the effect of maternal nutrition on fetal growth in human pregnancy has been substantially ignored, perhaps in part because numerous studies of maternal nutrient supplementation during pregnancy have failed to show a clinically significant effect on birth weight. The Cochrane review of balanced protein/energy supplementation in pregnancy reports a mean benefit in birth weight of only 37 g.22 Failure of the supplements to reach the fetus along a limited supply line may be one explanation. Another may be that, in these women, who were entered into the trials because of concern about longstanding nutritional status, the intervention was too late and fetal growth trajectory had already been set much earlier in gestation. One study of over 4000 healthy women with a normal menstrual history and a first trimester ultrasound scan found that pregnancies in which there was a discrepancy of between two and six days between the measured and expected crown‐rump length on the first trimester scan had a 2–3‐fold increased risk of producing a growth restricted or preterm baby, suggesting that the growth trajectory and gestational length were already determined in the first trimester.23
Studies of the relation between maternal diet during pregnancy and birth weight also suggest that the early pregnancy period is important. Godfrey et al24 have reported that women who had a high carbohydrate and low dairy protein intake in early pregnancy but the reverse in late pregnancy had offspring with the highest birth weight. Similar relations have been reported between birth weight and maternal dietary intake, particularly of dairy products, protein, and fish, in very early pregnancy, but much weaker or absent relations with dietary intakes in late pregnancy.25 Others have reported that a history of an eating disorder in the mother, even if resolved before pregnancy, results in lower birth weight.26 Similarly, the birth weight of a baby is reported to be correlated with the pre‐pregnancy weight of the mother, and even more closely with the mother's own birth weight, again suggesting the importance of maternal nutritional status before or in very early pregnancy, in determining fetal growth trajectory.27
We have direct evidence from studies in singleton fetal sheep that fetal growth rate in late gestation is determined by nutritional status at the time of conception.28 Fetuses of ewes exposed to moderate undernutrition from 60 days before to 30 days after mating—that is, up to one month of a five month pregnancy—had reduced growth rates in late gestation and were thinner, but not lighter, at birth.14 Furthermore, fetal growth and metabolic responses to maternal nutritional restriction in late gestation were also determined by prior growth rate and periconceptional nutritional status. In response to maternal nutritional restriction in late gestation, fetuses of ewes undernourished in the periconceptional period, growing more slowly than fetuses of well nourished ewes, continue to grow at the same rate. In contrast, fetuses of ewes well nourished in the periconceptional period, growing more quickly in late gestation, slow their growth with maternal undernutrition in late gestation.28 Consistent with these findings, fetuses of periconceptionally well nourished ewes have raised arterial partial pressures of oxygen in response to a three day maternal fast in late gestation, suggesting reduced oxidative demand at the time of slowing of fetal growth. Fetuses of periconceptionally undernourished ewes did not have altered arterial partial pressure of oxygen, but did have raised lactate concentrations, suggesting continued growth with a shift to alternative fuel sources.14
Thus there is now good evidence that fetal growth trajectory in late gestation is determined much earlier, probably in the periconceptional period. The growth trajectory that is set may be predictive of the likely future intrauterine environment. This growth trajectory may be further modified by factors in late gestation, but the fetal growth response to later insults may itself be determined by the trajectory that was set in early gestation. The developmental trajectory of other physiological systems may be determined in a similar fashion as demonstrated below; the appropriateness or otherwise of these predictive responses to the postnatal environment may contribute to disease risk.29
Fetal development and gestation length
Evidence is now accumulating that reduced maternal nutrition in early pregnancy not only affects fetal growth rate in late gestation, but also has other effects on fetal development and even length of gestation.
We have reported that periconceptional undernutrition (once again lasting only to the end of the first month of a five month pregnancy) in sheep leads to precocious activation of the fetal hypothalamic‐pituitary‐adrenal axis and preterm birth.30,31 These fetuses also had altered glucose and insulin responses to a glucose tolerance test and an arginine stimulation test, consistent with accelerated pancreatic maturation.32
There is now some evidence that poor maternal nutrition in humans in early pregnancy may also affect gestation length. In women in the Gambia, where there are seasonal periods of famine and plenty, gestational length is shortest eight to nine months after the peak period of famine.33 In rural India, gestational length is about a week shorter than in Southampton.34 The Southampton women's study, an ongoing large prospective study of women of child bearing age that includes detailed nutritional assessment, should provide further information on the influence of early nutritional status and pregnancy outcome.
Long term consequences
Maternal nutrition around the time of conception has also been shown to have long term consequences for the lifetime health of the offspring, independent of any effects on size at birth. Nutritional restriction in early pregnancy in sheep results in increased fat mass in offspring, associated with increased leptin mRNA concentrations and an accentuated leptin response to catecholamines.35 In rats, a maternal low protein diet only during the preimplantation period (0–4.5 days after mating) results in lower cell numbers in the inner cell mass and trophectoderm, reduced birth weight, and hypertension in the offspring.20
Similar data are beginning to accumulate on human pregnancy. In a cohort of Jamaican women followed prospectively throughout pregnancy, the blood pressure of their 10–12 year old children correlated most closely with maternal triceps skinfold thickness at 15 weeks gestation, suggesting that growth trajectory, and the postnatal consequences associated with reduced growth trajectory, were already determined at the end of the first trimester.36
Data from the Dutch hunger winter at the end of the second world war provide more direct evidence of the effects of severe maternal undernutrition in different trimesters of pregnancy on later disease risk. Offspring of women exposed to famine in early gestation were of normal birth weight, but had abnormalities in lipid profile and an increased risk of coronary heart disease in later life.37,38 In contrast, offspring of women exposed to famine in late gestation were lighter at birth and had an increased risk of glucose intolerance later in life, especially if they became obese.39 Once again, such data suggest that at least some aspects of postnatal physiology are determined by maternal nutritional status in early pregnancy, but that the mechanisms and timing may be different for different organ systems.
Fetal growth trajectory in twins
Twins are born both smaller and earlier than singletons.40,41 Clearly therefore, in experimental studies of fetal growth, it is critical to consider the effects of the presence of multiple fetuses on the outcomes of interest. Similarly, in investigations of the consequences of altered fetal growth on in utero or postnatal physiology, the potential confounding effects of co‐twins must be considered. Unfortunately, many animal studies have included mixtures of singletons and twins, making the results sometimes difficult to interpret.
Although twin growth rate in humans has been documented ultrasonographically to be reduced in late gestation, the supposition that this reduced rate of growth and the shortened gestational length are secondary to constraints of uterine size are without supporting data. We speculate that fetal growth trajectory in twins is also set early in gestation, although it may then be modified by late gestational factors similar to those acting on singletons. There are some human data to support this hypothesis. In multiple pregnancies in which fetal number was reduced early in pregnancy, either spontaneously or medically, fetal size and gestation length are related to the initial number of fetuses, rather than the number present at delivery.42,43 Similarly, recent human ultrasound data and animal postmortem data suggest that growth in twins may diverge from that in singletons as early as the first trimester.44,45
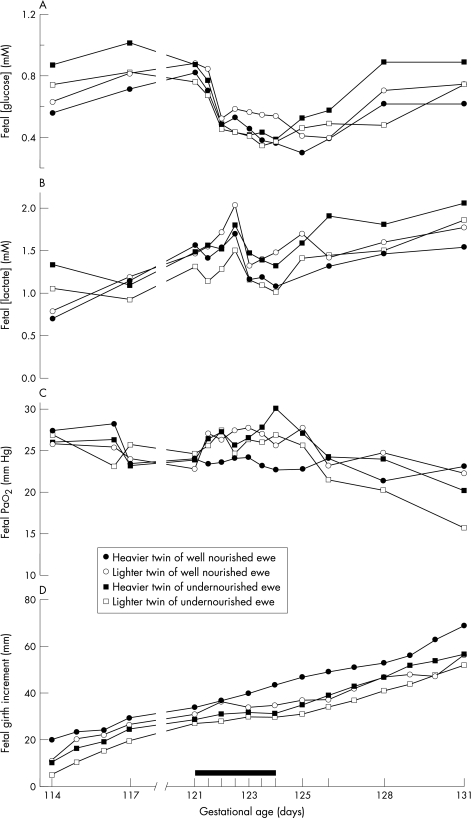
To test the hypothesis that fetal growth trajectory in twins is determined early in gestation, we studied both singleton and twin fetuses of ewes that were either well nourished throughout pregnancy or were undernourished from 60 days before to 30 days after mating. Ewes carrying both twins and singletons underwent an additional brief, three day fast in late gestation during which detailed measurements of fetal growth and metabolites were made. We divided the twins into the heavier and lighter twins in each pair, on the basis of postmortem data obtained at the end of the experiment. We considered that, in the animals that had been well nourished throughout pregnancy, the heavier twin in each pair could be considered to be the twin with optimal growth. However, consistent with our hypothesis that reduced growth in twins is already determined early in gestation, the heavier of the well nourished twin pairs showed a growth and metabolic profile in response to a maternal fast that was similar to that of singletons undernourished in the periconceptional period.46 Interestingly, fetal metabolic responses to the maternal fast were determined by an interaction between twin growth status—that is, heavier or lighter twin—and periconceptional nutritional status—that is, well fed or undernourished (fig 3).46 From these data, we suggest that twinning per se does indeed result in a signal or cue to the developing embryo that affects the fetal growth trajectory set early in gestation. The fact that growth and metabolic responses to a late gestation fast in twins are determined by an interaction between periconceptional nutritional status and twin growth status, defined as being the heavier or lighter twin, suggests that other factors also play a role.
Figure 3 Metabolic and growth responses to a three day maternal fast in late gestation twin fetuses of ewes that were either well nourished or undernourished from 60 days before to 30 days after mating. The experiment included 10 well nourished and nine undernourished twin pairs; this figure is a representative example of the responses seen in the groups as a whole. (A) Fetal arterial glucose concentrations; (B) fetal arterial lactate concentrations; (C) fetal arterial oxygen tension (Pao2), and (D) increment in fetal chest girth. The duration of the maternal fast is indicated by the solid black bar; term = 145 days. Note the complex interaction in fetal glucose and lactate responses during the late‐gestation fast between maternal periconceptional nutritional status and twin growth status. Before the late‐gestation fast, the undernourished twin pair have higher glucose and lactate concentrations, consistent with use of lactate as an alternative fuel source; during the maternal fast, concentrations of glucose fall and lactate rise, but the lighter twin of the periconceptionally well nourished ewe has the highest, and the lighter twin of the periconceptionally undernourished ewe the lowest, lactate and glucose concentrations. During the maternal fast, the heavier twin of the periconceptionally well nourished ewe maintains its growth rate and there is no change in Pao2; in the other three fetuses growth rate slows and Pao2 rises, consistent with reduced oxidative demand.
In cases of human twinning where there is discordant intrauterine growth, it is not known what determines individual growth trajectories in a twin pair, but the fact that birth weight discrepancy also occurs in monozygotic twins suggests that it is environmental rather than genetic.47 Twin growth status within a twin pair often correlates with each twin's placental mass, suggesting that at least some of the discordant growth may relate to placental supply.48 There are some observational data that may support this, although it is difficult to establish a causal relation.49 This proposal would suggest that twins set a reduced growth trajectory in the periconceptional period by virtue of being a twin, that this trajectory can be further modified by maternal nutritional status in this period, and may also be affected at a later stage in gestation by relative placental supply.
If reduced fetal growth trajectory in twins is indeed determined in early gestation, then an association between twinning per se and later disease risk might be expected. The literature on the association between birth weight in twins and later disease risk is conflicting, and it has been argued that this is evidence against any association between fetal growth and postnatal physiology.50 However, there are many reasons why twin studies might not show the expected association,51 not least of which are the lack of a singleton control group and the comparison of outcomes between the heavier and lighter co‐twins, thereby making the assumption that both twins are not affected. Clearly, if twin growth trajectory is determined early in gestation, this assumption is incorrect. Indeed, a recent study from our institution reported that insulin sensitivity was greatly reduced in prepubertal children who were twins compared with singleton controls.52 Likewise, the incidence of diabetes or glucose intolerance in a population based study of adult twins from Denmark was 35%, much higher than in the general population.53
Recent twin studies, separating within‐pair and between‐pair regression analyses,54 rather than using a simple paired difference analysis, indicate that there are significant associations between birth weight in twins and postnatal outcomes such as blood pressure and diabetes.55,56 Using a similar approach, we have recently shown that adrenocorticotrophic hormone and cortisol responses to a combined corticotrophin releasing hormone plus arginine vasopressin challenge in postpubertal female sheep is related to birth weight in both singletons and twins, with similar regression coefficients, but much larger effect sizes, in twins.57 The strength of the association increased in a within‐pair analysis, suggesting that factors specific to the growth of each fetus, rather than to maternal factors, were important.
Conclusions
Although birth weight provides some useful information about the sum of fetal growth, it does not give any information about the trajectory a fetus followed to attain a given size. Other measures of size at birth may add additional information. However, longitudinal measures of growth during pregnancy are optimum in the study of fetal growth and its associations with postnatal physiology and are possible in animal experiments. There is accumulating evidence that factors in the periconceptional period, including maternal nutritional history, the prevailing maternal nutritional, hormonal and metabolic environment, and the presence of other fetuses, can modify fetal growth trajectory. These factors may also influence other aspects of fetal development, including the length of gestation, and long term physiological status after birth. We suggest that the reduced fetal growth trajectory in twins is also determined, at least to some degree, in the periconceptional period, although it may be further modified later in gestation, and that this altered trajectory, is associated with physiological changes that persist into postnatal life. Further research is needed to identify the periconceptional signals or cues that influence fetal growth trajectory and to narrow down the time window during which they operate. Studies of twins, using appropriate singleton controls if possible and separating out the within‐pair from the between‐pair effects, may provide an additional route to understanding the regulation of fetal growth. Such studies may, in the future, make it possible to provide better information on nutritional status to women of child bearing age.
Acknowledgements
Our work referred to in this article was all approved by the Animal Ethics Committee of the University of Auckland, and was funded by the Health Research Council of New Zealand, the New Zealand Lottery Health Trust Board, and the National Research Centre for Growth and Development. We acknowledge all the members of the Fetal Growth Group at the Liggins Institute who contributed to this work.
Abbreviations
IGF - insulin‐like growth factor
PI - ponderal index
Footnotes
Competing interests: none declared
References
- 1.Mellor D J, Murray L. Effects on the rate of increase in fetal girth of refeeding ewes after short periods of severe undernutrition during late pregnancy. Res Vet Sci 198232377–382. [PubMed] [Google Scholar]
- 2.Caruso A, De Carolis S, Ferrazzani S.et al Pregnancy outcome and total parenteral nutrition in malnourished pregnant women. Fetal Diagn Ther 199813136–140. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield F H, Oliver M H, Giannoulias C D.et al Brief undernutrition in late‐gestation sheep programs the hypothalamic‐pituitary‐adrenal axis in adult offspring. Endocrinology 20031442933–2940. [DOI] [PubMed] [Google Scholar]
- 4.Oliver M H, Breier B H, Gluckman P D.et al Birth weight rather than maternal nutrition influences glucose tolerance, blood pressure, and IGF‐I levels in sheep. Pediatr Res 200252516–524. [DOI] [PubMed] [Google Scholar]
- 5.Hanson M. Birth weight and the fetal origins of adult disease. Pediatr Res 200252473–474. [DOI] [PubMed] [Google Scholar]
- 6.Kramer M S, McLean F H, Oliver M.et al Body proportionality and head and length “sparing” in growth‐retarded neonates: a critical reappraisal. Pediatrics 198984717–723. [PubMed] [Google Scholar]
- 7.Bocking A D, Gagnon R, White S E.et al Circulatory responses to prolonged hypoxemia in fetal sheep. Am J Obstet Gynecol 19881591418–1424. [DOI] [PubMed] [Google Scholar]
- 8.Al‐Ghazali W, Chita S K, Chapman M G.et al Evidence of a redistribution of cardiac output in asymmetrical growth retardation. Br J Obstet Gynaecol 198996697–704. [DOI] [PubMed] [Google Scholar]
- 9.Edelstone D I, Rudolph A M, Heymann M A. Effects of hypoxemia and decreasing umbilical flow liver and ductus venosus blood flows in fetal lambs. Am J Physiol 1980238H656–H663. [DOI] [PubMed] [Google Scholar]
- 10.Tchirikov M, Kertschanska S, Sturenberg H J.et al Liver blood perfusion as a possible instrument for fetal growth regulation. Placenta 200223(suppl A)S153–S158. [DOI] [PubMed] [Google Scholar]
- 11.Haugen G, Hanson M, Kiserud T.et al Fetal liver‐sparing cardiovascular adaptations linked to mother's slimness and diet. Circ Res 20059612–14. [DOI] [PubMed] [Google Scholar]
- 12.Cetin I, Fennessey P V, Quick A N J.et al Glycine turnover and oxidation and hepatic serine synthesis from glycine in fetal lambs. Am J Physiol 1991260E371–E378. [DOI] [PubMed] [Google Scholar]
- 13.Vaughn P R, Lobo C, Battaglia F C.et al Glutamine‐glutamate exchange between placenta and fetal liver. Am J Physiol 1995268E705–E711. [DOI] [PubMed] [Google Scholar]
- 14.Oliver M H, Hawkins P, Harding J E. Periconceptional undernutrition alters growth trajectory, endocrine and metabolic responses to fasting in late gestation fetal sheep. Pediatr Res 200557591–598. [DOI] [PubMed] [Google Scholar]
- 15.Law C M, Gordon G S, Shiell A W.et al Thinness at birth and glucose tolerance in seven‐year‐old children. Diabet Med 19951224–29. [DOI] [PubMed] [Google Scholar]
- 16.Lithell H O, McKeigue P M, Berglund L.et al Relation of size at birth to non‐insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ 1996312406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker D J, Martyn C N, Osmond C.et al Growth in utero and serum cholesterol concentrations in adult life. BMJ 19933071524–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding J E, McCowan L M. Perinatal predictors of growth patterns to 18 months in children born small for gestational age. Early Hum Dev 20037413–26. [DOI] [PubMed] [Google Scholar]
- 19.Barker D J, Bull A R, Osmond C.et al Fetal and placental size and risk of hypertension in adult life. BMJ 1990301259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong W Y, Wild A E, Roberts P.et al Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 20001274195–4202. [DOI] [PubMed] [Google Scholar]
- 21.Bloomfield F H, Harding J E. Experimental aspects of nutrition and fetal growth. Fetal and Maternal Medicine Revues 19981091–107. [Google Scholar]
- 22.Kramer M S. Balanced protein/energy supplementation in pregnancy (Cochrane review). Cochrane Library. Oxford: Update Software, 2003
- 23.Smith G C, Smith M F, McNay M B.et al First‐trimester growth and the risk of low birth weight. N Engl J Med 19983391817–1822. [DOI] [PubMed] [Google Scholar]
- 24.Godfrey K, Robinson S, Barker D J.et al Maternal nutrition in early and late pregnancy in relation to placental and fetal growth. BMJ 1996312410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell E A, Robinson E, Clark P M.et al Maternal nutritional risk factors for small for gestational age babies in a developed country: a case‐control study. Arch Dis Child Fetal Neonatal Ed 200489F431–F435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kouba S, Hallstrom T, Lindholm C.et al Pregnancy and neonatal outcomes in women with eating disorders. Obstet Gynecol 2005105255–260. [DOI] [PubMed] [Google Scholar]
- 27.Ounsted M, Scott A, Moar V A. Constrained and unconstrained fetal growth: associations with some biological and pathological factors. Ann Hum Biol 198815119–129. [DOI] [PubMed] [Google Scholar]
- 28.Harding J E. Periconceptual nutrition determines the fetal growth response to acute maternal undernutrition in fetal sheep of late gestation. Prenat Neonatal Med 19972310–319. [Google Scholar]
- 29.Gluckman P D, Hanson M A. Living with the past: evolution, development, and patterns of disease. Science 20043051733–1736. [DOI] [PubMed] [Google Scholar]
- 30.Bloomfield F H, Oliver M H, Hawkins P.et al A periconceptional nutritional origin for non‐infectious preterm birth. Science 2003300606. [DOI] [PubMed] [Google Scholar]
- 31.Bloomfield F H, Oliver M H, Hawkins P.et al Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic‐pituitary‐adrenal axis in late gestation. Endocrinology 20041454278–4285. [DOI] [PubMed] [Google Scholar]
- 32.Oliver M H, Hawkins P, Breier B H.et al Maternal undernutrition during the periconceptual period increases plasma taurine levels and insulin response to glucose but not arginine in the late gestational fetal sheep. Endocrinology 20011424576–4579. [DOI] [PubMed] [Google Scholar]
- 33.Rayco‐Solon P, Fulford A J, Prentice A M. Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. Am J Clin Nutr 200581134–139. [DOI] [PubMed] [Google Scholar]
- 34.Yajnik C S, Fall C H, Coyaji K J.et al Neonatal anthropometry: the thin‐fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord 200327173–180. [DOI] [PubMed] [Google Scholar]
- 35.Symonds M E, Budge H, Stephenson T.et al Experimental evidence for long‐term programming effects of early diet. Adv Exp Med Biol 200556924–32. [DOI] [PubMed] [Google Scholar]
- 36.Godfrey K M, Forrester T, Barker D J.et al Maternal nutritional status in pregnancy and blood pressure in childhood. Br J Obstet Gynaecol 1994101398–403. [DOI] [PubMed] [Google Scholar]
- 37.Roseboom T J, van der Meulen J H, Osmond C.et al Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr 2000721101–1106. [DOI] [PubMed] [Google Scholar]
- 38.Roseboom T J, van der Meulen J H, Osmond C.et al Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 200084595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravelli A C, van der Meulen J H, Michels R P.et al Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998351173–177. [DOI] [PubMed] [Google Scholar]
- 40.Buckler J M, Green M A. Comparison of the early growth of twins and singletons. Ann Hum Biol 200431311–332. [DOI] [PubMed] [Google Scholar]
- 41.Alexander G R, Kogan M, Martin J.et al What are the fetal growth patterns of singletons, twins and triplets in the United States? Clin Obstet Gynecol 199841115–125. [DOI] [PubMed] [Google Scholar]
- 42.Alexander J M, Hammond K R, Steinkampf M P. Multifetal reduction of high‐order multiple pregnancy: comparison of obstetrical outcome with nonreduced twin gestations. Fertil Steril 1995641201–1203. [DOI] [PubMed] [Google Scholar]
- 43.Pinborg A, Lidegaard O, la Cour Freiesleben N.et al Consequences of vanishing twins in IVF/ICSI pregnancies. Hum Reprod 2005202821–2829. [DOI] [PubMed] [Google Scholar]
- 44.Iffy L, Lavenhar M A, Jakobovits A.et al The rate of early intrauterine growth in twin gestation. Am J Obstet Gynecol 1983146970–972. [DOI] [PubMed] [Google Scholar]
- 45.Bertolini M, Mason J B, Beam S W.et al Morphology and morphometry of in vivo‐ and in vitro‐produced bovine concepti from early pregnancy to term and association with high birth weights. Theriogenology 200258973–994. [DOI] [PubMed] [Google Scholar]
- 46.Bloomfield F H, Rumball C, Oliver M H.et al Periconceptional undernutrition and twin size both affect growth and metabolic responses of twin fetal sheep to an acute maternal fast in late gestation [abstract]. 3rd International Congress of Developmental Origins of Health and Disease. Toronto Pediatric Res 2005581107 [Google Scholar]
- 47.Baird J, Osmond C, MacGregor A.et al Testing the fetal origins hypothesis in twins: the Birmingham twin study. Diabetologia 20014433–39. [DOI] [PubMed] [Google Scholar]
- 48.Bleker O P, Wolf H, Oosting J. The placental cause of fetal growth retardation in twin gestations. Acta Genet Med Gemellol (Roma) 199544103–106. [DOI] [PubMed] [Google Scholar]
- 49.Bajoria R, Sooranna S R, Ward S.et al Placental transport rather than maternal concentration of amino acids regulates fetal growth in monochorionic twins: implications for fetal origin hypothesis. Am J Obstet Gynecol 20011851239–1246. [DOI] [PubMed] [Google Scholar]
- 50.Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet 2002360659–665. [DOI] [PubMed] [Google Scholar]
- 51.Morley R, Dwyer T, Carlin J B. Studies of twins: can they shed light on the fetal origins of adult disease hypothesis? Twin Res 20036520–525. [DOI] [PubMed] [Google Scholar]
- 52.Jefferies C A, Hofman P L, Knoblauch H.et al Insulin resistance in healthy prepubertal twins. J Pediatr 2004144608–613. [DOI] [PubMed] [Google Scholar]
- 53.Poulsen P, Vaag A A, Beck‐Nielsen H. Does zygosity influence the metabolic profile of twins? A population based cross sectional study. BMJ 1999319151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlin J B, Gurrin L C, Sterne J A.et al Regression models for twin studies: a critical review. Int J Epidemiol 2005341089–1099. [DOI] [PubMed] [Google Scholar]
- 55.Iliadou A, Cnattingius S, Lichtenstein P. Low birthweight and Type 2 diabetes: a study on 11 162 Swedish twins. Int J Epidemiol 200433948–953 discussion 953–944. [DOI] [PubMed] [Google Scholar]
- 56.Johansson‐Kark M, Rasmussen F, Stavola B D.et al Fetal growth and systolic blood pressure in young adulthood: the Swedish young male twins study. Paediatr Perinat Epidemiol 200216200–209. [DOI] [PubMed] [Google Scholar]
- 57.Bloomfield F H, Oliver M H, Harding J E. The importance of being a twin: within pair analysis of glucose tolerance and hypothalamic‐pituitary‐adrenal axis responsiveness. In: Medical Sciences Congress of New Zealand. Queenstown: C8, 2004