Abstract
Background
Infection with group B streptococci (GBS) is a serious neonatal disease. The GBS cell surface proteins α and Rib elicit protective immunity in animal models and have been suggested as potential antigens in a vaccine against human GBS disease.
Aims
To test the hypothesis that transplacentally transferred maternal antibodies to GBS proteins contribute to the protection of the neonate from GBS infection.
Methods
Thirty neonates with invasive infection were included in a case‐control study. IgG antibody concentrations were measured in sera from these neonates, their mothers, and from 60 non‐infected controls, neonates as well as mothers.
Results
A clear association was found between concentrations of antibody to proteins α and Rib in neonatal and maternal sera, indicating that transplacental transfer had occurred. Moreover, low concentrations of antibodies to α and Rib in neonatal sera were associated with invasive GBS infection caused by strains expressing the Rib protein. The odds ratio was 0.0007 (95% confidence interval 0.000 to 0.54) for antibodies to α and 0.002 (95% confidence interval 0.000 to 0.57) for antibodies to Rib.
Conclusion
These findings support the notion that antibodies to GBS surface proteins contribute to the protection against neonatal infection.
Keywords: group B streptococci, infection, antibody, protein α, protein Rib
Infection with group B streptococci (Streptococcus agalactiae; GBS) is an important cause of neonatal morbidity and mortality in many countries including Sweden.1,2,3,4,5 As many as a quarter of pregnant women may be colonised with GBS during late pregnancy,6,7 with a concomitant risk of about 1% for their children to develop invasive GBS infection during the neonatal period.8
Animal studies have shown that vaccine induced antibodies to the polysaccharide capsule8 and to proteins exposed on the bacterial surface of GBS confer protective immunity.9,10,11,12 Our work focused on protective GBS surface proteins, particularly α and Rib, which are expressed on the bacterial surface by most GBS strains that cause human invasive neonatal infection.12,13,14 Immunisation of mice with highly purified preparations of α and Rib efficiently protects against lethal infection with strains expressing the corresponding protein.13,15 The α and Rib proteins have been extensively characterised and belong to a family of streptococcal proteins with extremely repetitive sequence.16,17,18 Animal antisera raised against the purified α and Rib proteins show little or no cross reactivity, although the two proteins exhibit extensive amino acid residue identity.12,13
Antibodies to type‐specific capsular polysaccharide are present in human sera, and low concentrations of such antibodies have been associated with neonatal GBS infection.19,20,21,22 Antibodies to GBS cell surface proteins also occur naturally in human sera,9,23 and it has been suggested that low serum concentrations of such antibodies may favour occurrence of neonatal GBS infection.24,25 However, studies comparing serum concentrations of antibodies to GBS proteins in infected neonates and their mothers with those in non‐infected neonates are scarce.26,27
We hypothesised that transplacentally transferred antibodies to the α and Rib proteins contribute to the protection of the neonate from GBS disease. Our objectives in this study were to compare the concentrations of antibodies to α and Rib in sera from infected and non‐infected neonates and their respective mothers.
Subjects, materials, and methods
Study design
Cases were recruited from the neonatal wards at nine hospitals in the southern part of Sweden, covering a population of 1.9 million. During the study period, October 1995 to December 1998, there were about 20 000 live births annually. In Sweden, almost all deliveries occur in hospital. Neonatal care is available for all newborns, and seven of the nine neonatal wards in the study area offer intensive care with respiratory support. General screening by culture for GBS in pregnant women is not carried out. A risk based approach is used for prevention of GBS disease. The recruitment and dimension of the study were based on the assumption of a prevalence of GBS carriage in late pregnancy of 25%.6,7 Control subjects were recruited from the neonatal ward at Lund University Hospital, the regional hospital of southern Sweden. Sera from female blood donors were used as reference representing non‐pregnant women. Analyses were performed in two steps. Firstly, comparisons were made between all cases and controls. In the next step, the cases were divided in two groups according to expression of protein α or Rib by the infecting strain and compared with the control group.
Cases
Neonates with culture positive invasive GBS infection (septicaemia and/or meningitis) within eight days of parturition and their mothers were defined as cases. Sera were collected from both neonates and mothers. The gestational age, birth weight, and sex of the neonates and the age of the mothers were recorded (table 1). The date of collection of neonatal and maternal sera was recorded; for two of the maternal sera this information was missing.
Table 1 Background characteristics of cases with invasive group B streptococcal infection and non‐infected controls.
| Characteristic | Cases (n = 30)* | Controls (n = 60)† | ||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Gestational age (weeks) | 39 | 26–42 | 34 | 24–43 |
| Birth weight (g) | 3320 | 940–4800 | 2165 | 465–4450 |
| Mother's age (years) | 28 | 21–40 | 30 | 15–41 |
*20 male and 10 female.
†33 male and 27 female.
Controls
Neonates with a non‐infectious diagnosis such as prematurity or transient tachypnoea and their mothers were recruited as controls. Inclusion criteria were no clinical signs of infection, negative blood cultures, and concentration of C reactive protein less than 11 mg/l in the neonates. Furthermore, the mothers had to show no signs of infection or have been treated with antibiotics before or during delivery. The gestational age, birth weight, and sex of the neonates and the age of the mothers were recorded (table 1).
For comparisons of antibody concentrations in pregnant and non‐pregnant women, antibodies to the α and Rib proteins were also measured in sera from 100 female blood donors of about 30 years of age, randomly sampled from blood donors at the Lund University Hospital in 1992.
GBS isolates
GBS strains isolated from blood or cerebrospinal fluid of the infected neonates were serotyped at the Clinical Microbiology Laboratory, Lund University Hospital or Statens Serum Institut, Copenhagen, Denmark. Cell surface expression of proteins was analysed with rabbit antisera specific to α and Rib.12 Rabbit antiserum specific to Rib recognises both Rib and the closely related Rib‐like protein. It has been found that the Rib‐like protein is identical with the group A streptococcal R28 protein.18,28,29 As Rib and Rib‐like protein (R28) cross react and elicit cross protective immunity in animal studies,30 we have classified them as one antigen in this study (table 2).
Table 2 Characterisation of 30 group B streptococcal isolates from neonates with invasive infection by polysaccharide capsular type and cell surface expression of proteins.
| Capsular type (no of isolates) | Surface protein reacting with antibodies to | ||
|---|---|---|---|
| α (n = 14) | Rib (n = 14) | None* (n = 2) | |
| Ia (4) | 4 | – | – |
| Ib (6) | 6 | – | – |
| II (3) | 2 | 1 | – |
| III (7) | – | 6 | 1 |
| IV (2) | 2 | – | – |
| V (6) | – | 5† | 1 |
| NT (2) | – | 2 | – |
Protein preparations
The α and Rib proteins were isolated from streptococci by several purification steps to ensure that they were free of contaminating polysaccharides, as described previously.12,13
Enzyme linked immunosorbent assay (ELISA)
Concentrations of IgG antibodies to the α and Rib proteins were measured by ELISA. The wells of a microtitre plate (Maxisorp Nunc‐Immunoplate; Tamrolab, Molndal, Sweden) were coated with 100 μl of a solution of purified protein α or Rib in phosphate buffered saline (PBS) (0.75 μg/ml). The test sera were diluted 1:500 in PBSAT (PBS containing 0.02% azide and 0.05% Tween 20) and tested in duplicate. Goat anti‐human IgG conjugated to alkaline phosphatase (Sigma, St Louis, Missouri, USA) diluted 1:10 000 was used to detect bound antibodies. The plate was read in a microplate reader at 405 nm. For each serum, the background value for an uncoated well was subtracted. Antibody concentrations are presented as the absorbance value.
As sera with known concentrations of antibodies to the α and Rib proteins are not available, commercial γ globulin (Gammaglobulin 165 mg/ml, Pharmacia, Uppsala, Sweden) was used to standardise the ELISA. Optimal dilutions of the reagents were determined by checkerboard titration.31 Standard curves for γ globulin (in serial dilutions in PBSAT) against each of the two GBS proteins were determined. Appropriate dilutions for γ globulin were chosen to be included as control on each microtitre plate to eliminate inter‐assay variations.
Statistical methods
Background characteristics of the cases and controls were calculated using statistical descriptive methods including mean, median, range, and proportion. Linear relations of antibody concentrations to the α and Rib proteins in sera from cases and controls were estimated using Pearson correlation coefficient and corresponding confidence interval (CI). Associations between the concentrations of antibodies to protein α and Rib in sera from mothers and female blood donors were estimated using the Wilcoxon two sample test. Associations between invasive infection and antibody concentration were estimated by odds ratios (OR) and corresponding 95% CI, adjusted for sex and gestational age using logistic regression. Statistical significance was defined as p<0.05.
Ethics
This study was approved by the ethics committee of Lund University. All mothers gave written consent after oral and written information.
Results
Study subjects
Over the study period, 35 cases of neonatal GBS infection diagnosed by positive cultures from blood and/or cerebrospinal fluid were reported from the catchment area. Two term neonates were excluded because their infection occurred several weeks after birth, and for three neonates (two term and one preterm) the bacterial isolate or both the neonatal and the maternal sera were missing. The remaining 30 neonates with invasive GBS infection were included in the study (table 1); three of them had meningitis. Twenty one (70%) showed signs of illness within 24 hours and 27 (90%) within 72 hours of birth. Sixty non‐infected neonates were included in the control group. The gestational age of cases was higher and therefore their birth weight was higher. Sixty seven per cent of the infected neonates were boys compared with 55% of the controls. The ages of mothers of infected and non‐infected neonates were similar, median 28 and 30 years, mean 29 and 30 years, respectively. Twenty nine acute sera from the 30 infected neonates and 29 sera from their mothers were collected within seven days of admittance to the neonatal ward (median 1.5 days for the neonates and 3 days for their mothers). All sera from the control group were collected within five days of admittance.
Properties of GBS strains isolated from neonates
The 30 GBS strains collected from blood and/or cerebrospinal fluid from infected neonates were analysed for expression of surface protein, using rabbit antisera against highly purified preparations of the α and Rib proteins. According to this analysis, 14 of the 30 isolates expressed the α protein and 14 isolates expressed Rib, and two strains did not express any of these proteins. There were six different serotypes (Ia, Ib, II, III, IV, and V) and two non‐typable among the strains (table 2). It is important, however, to note that strains of serotype V express a protein that is closely related to, but not identical with, the Rib protein.32 This Rib‐like protein is identical with the R28 protein (also known as Alp3), a protein with chimeric structure expressed by both GBS and group A streptococci.18,28,29 Because the R28 protein of GBS type V strains cross reacts with Rib, and because these proteins elicit cross protective immunity,30 we have classified R28 and Rib as one antigen that is recognised by anti‐Rib serum (table 2).
Antibodies to α and Rib in sera from neonates and their mothers and female blood donors
IgG antibodies that recognise purified protein α and Rib in ELISAs were detected in sera from newborn infants, their mothers, and female blood donors (table 3). A covariation was observed between concentrations of antibodies to α and Rib in sera from the neonates, both in cases and controls. These covariations were also observed in sera from their mothers and from female blood donors (table 3). These data suggest that α and Rib may cross react in humans.
Table 3 Correlation between concentrations of antibodies to protein α and Rib in sera from 29 cases with invasive group B streptococcal infection, 60 non‐infected controls, and 100 female blood donors.
| Correlation coefficient | 95% CI | |
|---|---|---|
| Anti‐α versus anti‐Rib in neonatal sera | ||
| Cases | 0.82 | 0.65 to 0.91 |
| Controls | 0.73 | 0.58 to 0.83 |
| Anti‐α versus anti‐Rib in maternal sera | ||
| Cases | 0.88 | 0.78 to 0.95 |
| Controls | 0.80 | 0.69 to 0.88 |
| Anti‐α versus anti‐Rib in sera from female blood donors | 0.73 | 0.62 to 0.81 |
CI, Confidence interval.
Concentrations of serum antibodies to protein Rib in mothers of neonates infected with a Rib‐expressing strain and in mothers of non‐infected neonates were lower than in female blood donors (p = 0.003 and p = 0.01 respectively; table 4). For mothers of neonates infected with an α‐expressing strain, concentrations of antibodies to Rib were also lower than in blood donors. This difference, however, did not reach statistical significance. In sera from mothers of neonates infected with a Rib‐expressing strain, the concentrations of antibodies to α were lower than those of blood donors (p = 0.003; table 4).
Table 4 Mean, median, and range of concentrations of antibodies to protein α and Rib in sera from case mothers (nα = 13 and nRib = 14) and control mothers (n = 60) compared with antibody concentrations in sera from female blood donors (n = 100) using Wilcoxon two sample test.
| Mothers of neonates infected with | Mothers of non‐infected neonates | Female blood donors | ||||||
|---|---|---|---|---|---|---|---|---|
| α‐expressing strain | Rib‐expressing strain | |||||||
| Anti‐α | Anti‐Rib | Anti‐α | Anti‐Rib | Anti‐α | Anti‐Rib | Anti‐α | Anti‐Rib | |
| Mean | 0.35 | 0.34 | 0.16 | 0.21 | 0.29 | 0.34 | 0.31 | 0.45 |
| Median | 0.19 | 0.29 | 0.10 | 0.18 | 0.22 | 0.23 | 0.22 | 0.34 |
| Range | 0.05–1.22 | 0.03–1.25 | 0.01–0.46 | 0.03–0.52 | 0.02–1.21 | 0.04–1.65 | 0.01–0.99 | 0.05–1.71 |
| p Value | 0.98* | 0.16† | 0.003* | 0.003† | 0.75* | 0.01† | – | – |
Antibody concentrations are presented as absorbance values.
*Compared with anti‐α in sera from female blood donors.
†Compared with anti‐Rib in sera from female blood donors.
Distribution of antibodies to α and Rib in neonatal versus maternal sera
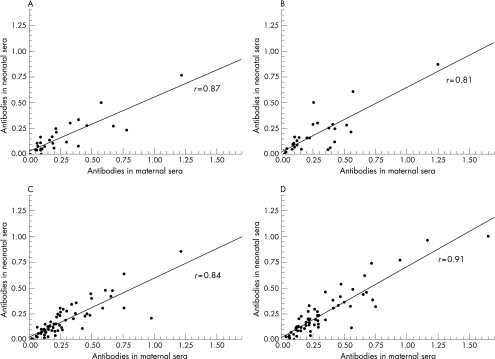
Figure 1 shows the distribution and correlation coefficients of α and Rib antibodies in neonatal versus maternal sera. A clear positive linear relation was observed for concentrations of antibodies to protein α and Rib, between neonatal sera and maternal sera, in both cases (fig 1A,B) and controls (fig 1C,D), indicating that the antibodies are transferred transplacentally.
Figure 1 Distribution and association between concentrations of antibodies to protein α and Rib in neonatal and maternal sera. (A) Anti‐α in cases with invasive group B streptococcal (GBS) infection. (B) Anti‐Rib in cases with invasive GBS infection. (C) Anti‐α in non‐infected controls. (D) Anti‐Rib in non‐infected controls. The x and y axis indicate the absorbance value at 405 nm. Pearson correlation was used.
Determinants for invasive neonatal GBS infection
The concentrations of antibodies to protein α and Rib in sera from neonates infected with GBS strains expressing Rib were associated with invasive infection (p = 0.03 and p = 0.03 respectively), in a model in which sex and gestation were adjusted for (table 5). The adjusted OR was 0.0007 (95% CI 0.00 to 0.54) for antibodies to α and 0.002 (95% CI 0.00 to 0.57) for antibodies to Rib. No statistically significant association was detected between invasive infection with strains expressing protein α and concentrations of antibodies to the α and Rib antigens (table 5). OR for the concentration of antibodies to Rib, however, showed a protective effect. In this study, gestational age was associated with invasive GBS infection, independent of protein expression on the bacterial surface of the infecting strain. These ORs varied between 1.21 and 1.30, and the p values were ⩽0.007. No association was observed between sex and invasive GBS infection.
Table 5 Associations, adjusted for gestational age and sex, between invasive group B streptococcal (GBS) infection and concentrations of antibodies to protein α and Rib in serum from neonates.
| Infecting isolate | No of sera (cases and controls) | Antibody concentration | |||||
|---|---|---|---|---|---|---|---|
| Anti‐α | Anti‐Rib | ||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| All isolates* | 89 | 0.11 | 0.003 to 3.86 | 0.22 | 0.07 | 0.004 to 1.38 | 0.08 |
| α‐expressing | 73 | 1.17 | 0.02 to 67.22 | 0.94 | 0.43 | 0.02 to 12.00 | 0.62 |
| Rib‐expressing | 74 | 0.0007 | 0.000 to 0.54 | 0.03 | 0.002 | 0.000 to 0.57 | 0.03 |
Analyses were by multiple logistic regression including all neonates with GBS infection and controls (n = 89), neonates with GBS infection caused by strains expressing protein α and Rib, and controls (nα = 73 and nRib = 74).
*All GBS isolates with protein α, Rib, or neither of these proteins expressed on the bacterial surface.
OR, Odds ratio; CI, confidence interval.
Discussion
We analysed concentrations of antibodies to the α and Rib proteins in neonates with invasive GBS infection and in their mothers. The most noteworthy finding is the strong association between neonatal concentrations of antibodies to the GBS proteins α (OR 0.0007) and Rib (OR 0.002) and invasive infection caused by strains expressing Rib (table 5). For neonatal infections caused by strains expressing the α protein, no such association could be shown. There was a tendency, however, for Rib antibodies to protect against α‐expressing strains, but we do not know if this was a spurious correlation or a real effect. It is possible that a larger study would clarify the latter observation. Sex and gestational age of the neonates were considered as possible confounders and were therefore adjusted for in the statistical analyses.
Our results should be valid, as all but five cases reported to us of invasive GBS infection diagnosed by positive culture from blood or cerebrospinal fluid during the three year study period were included in the study. All recruited mothers consented to participate in the study. Two neonates with GBS infection diagnosed several weeks after birth were regarded as a separate group with different characteristics and were not further considered in this study. The control neonates were recruited from the population that generated the cases. The GBS colonisation status of the control mothers was not recorded because general screening for GBS in pregnant women is not routine in the study area. In addition, the accuracy of detecting colonisation is influenced by many factors—for example, the body sites sampled, the number of cultures obtained, and the timing of culture collection in relation to delivery.8 Moreover, we do not know if high concentrations of antibodies to the α and Rib protein would actually reduce or even eliminate maternal GBS colonisation. If the latter were the case, the recruitment of the control group would have been biased, as women with high concentrations of α and Rib antibodies would have been excluded from the study.
Characterisation of the GBS strains isolated from the 30 infected infants showed that 47% expressed protein α on the bacterial surface and 47% expressed Rib or a protein that cross reacts with Rib. This result corroborates previous reports that either α or Rib are expressed by most human invasive GBS strains.12,13,33
What is already known on this topic
Animal studies have shown that vaccine induced antibodies to proteins exposed on the bacterial cell surface of GBS confer protective immunity
Antibodies to GBS cell surface proteins seem to occur naturally in human sera; however, studies comparing serum concentrations of antibodies to GBS proteins from infected neonates and their mothers with concentrations from non‐infected neonates are scarce
The clear relation between the concentrations of antibodies to the GBS cell surface proteins α and Rib in individual sera (table 3) together with the finding that low concentrations of neonatal antibodies to α and Rib were associated with invasive infection caused by strains expressing Rib (table 5) may indicate that human antibodies to α and Rib show cross reactivity. In a previous experiment with mouse antibodies,13 a very weak immunological cross reactivity between the α and Rib proteins was observed. Moreover, immunisation of mice with native protein Rib conferred partial protection against experimental infections with α‐expressing strains. The cross reactivity is possibly more extensive for human antibodies. The present study measured naturally occurring human antibodies which were probably produced as an immunological response to native streptococcal proteins exposed on bacteria that colonise the mucosal surfaces in the intestinal and urogenital tracts. It is possible that these human antibodies interact with conformational epitopes shared by the native forms of α and Rib.
The concentrations of antibodies to α and Rib seemed to be generally lower in sera from mothers of infected and non‐infected neonates than from non‐pregnant women represented by female blood donors (table 4). The observed differences in antibody concentrations between pregnant and non‐pregnant women may be, at least in part, explained by the decrease in IgG caused by haemodilution during pregnancy.34
In our study, we found an association between GBS infection and gestational age. This association may reflect the increasing number of neonates born (and exposed to GBS) during late pregnancy. On the other hand, among the GBS infected neonates in this study, there were no differences between gestational age and time of first symptoms of infection in preterm and term neonates. Most of the neonates showed signs of illness during the first few hours after birth, indicating that they were probably infected in utero. These observations suggest that GBS plays an active role in onset of labour and delivery in many cases, both in early and late pregnancy. However, the association between GBS infection and gestational age needs to be studied in a separate study.
In summary, this study shows a clear association between concentrations of antibodies to GBS proteins in sera from neonates and their mothers, which indicates that these antibodies are transferred to the fetus during pregnancy. More importantly, low concentrations of neonatal antibodies to the GBS proteins α and Rib were shown to be associated with invasive GBS disease caused by strains expressing Rib. These findings indicate that naturally occurring antibodies to GBS proteins may be involved in the defence against GBS infection.
What this study adds
Low concentrations of neonatal antibodies to the GBS proteins α and Rib are associated with invasive disease caused by strains expressing Rib on the cell surface
The findings indicate that naturally occurring antibodies to GBS proteins are involved in defence against GBS infection and this may have implications for the development of a GBS vaccine based on protein antigens
Acknowledgements
We thank Kristina Håkansson for valuable help with subject recruitment and collection of sera, Arne Svensson for excellent technical advice on ELISA, and all collaborators at the participating paediatric departments for support and for reporting cases.
Abbreviations
CI - confidence interval
ELISA - enzyme linked immunosorbent assay
GBS - group B streptococcus
OR - odds ratio
PBS - phosphate buffered saline
Footnotes
Financial support: the Swedish Research Council, the Meningitis Research Foundation (UK), the “Förenade Liv” Mutual Group Life Insurance Company, the Swedish Society for Medical Research, the Royal Physiographic Society in Lund, and the trusts of Golje, Jerring, Kock, Samariten and Österlund.
Competing interests: None declared.
References
- 1.Källman J, Kihlström E, Sjöberg L.et al Increase of staphylococci in neonatal septicaemia: a fourteen‐year study. Acta Paediatr 199786533–538. [DOI] [PubMed] [Google Scholar]
- 2.Aavitsland P, Hoiby E A, Lystad A. Systemic group B streptococcal disease in neonates and young infants in Norway 1985–94. Acta Paediatr 199685104–105. [DOI] [PubMed] [Google Scholar]
- 3.Schrag S J, Zywicki S, Farley M M.et al Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 200034215–20. [DOI] [PubMed] [Google Scholar]
- 4.Persson E, Trollfors B, Brandberg L L.et al Septicaemia and meningitis in neonates and during early infancy in the Göteborg area of Sweden. Acta Paediatr 2002911087–1092. [DOI] [PubMed] [Google Scholar]
- 5.Trijbels‐Smeulders M, Gerards L J, M P C.et al Epidemiology of neonatal group B streptococcal disease in The Netherlands 1997–98. Paediatr Perinat Epidemiol 200216334–341. [DOI] [PubMed] [Google Scholar]
- 6.Persson K, Bjerre B, Elfström L.et al Longitudinal study of group B streptococcal carriage during late pregnancy. Scand J Infect Dis 198719325–329. [DOI] [PubMed] [Google Scholar]
- 7.Yancey M K, Schuchat A, Brown L K.et al The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol 199688811–815. [DOI] [PubMed] [Google Scholar]
- 8.Edwards M S, Baker C J. Group B streptococcal infections. In: Remington JS, Klein JO, eds. Infectious diseases of the fetus and newborn infant. Philadelphia: WB Saunders Company, 20011091–1156.
- 9.Bevanger L. The Ibc proteins of group B streptococci: isolation of the alpha and beta antigens by immunosorbent chromatography and test for human serum antibodies against the two antigens. Acta Pathol Microbiol Immunol Scand B 198593113–119. [DOI] [PubMed] [Google Scholar]
- 10.Lancefield R C, McCarty M, Everly W N. Multiple mouse‐protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med 1975142165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michel J L, Madoff L C, Kling D E.et al Cloned alpha and beta C‐protein antigens of group B streptococci elicit protective immunity. Infect Immun 1991592023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stålhammar‐Carlemalm M, Stenberg L, Lindahl G. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med 19931771593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson C, Stålhammar‐Carlemalm M, Lindahl G. Experimental vaccination against group B streptococcus, an encapsulated bacterium, with highly purified preparations of cell surface proteins Rib and α. Infect Immun 1996643518–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson D R, Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild‐type strains of common serotypes. J Clin Microbiol 198419506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson C, Stålhammar‐Carlemalm M, Lindahl G. Protection against experimental infection with group B streptococcus by immunization with a bivalent protein vaccine. Vaccine 199917454–458. [DOI] [PubMed] [Google Scholar]
- 16.Michel J L, Madoff L C, Olson K.et al Large, identical, tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc Natl Acad Sci USA 19928910060–10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wästfelt M, Stålhammar‐Carlemalm M, Delisse A M.et al Identification of a family of streptococcal surface proteins with extremely repetitive structure. J Biol Chem 199627118892–18897. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl G, Stålhammar‐Carlemalm M, Areschoug T. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 200518102–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker C J, Kasper D L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 1976294753–756. [DOI] [PubMed] [Google Scholar]
- 20.Baker C J, Edwards M S, Kasper D L. Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection. Pediatrics 198168544–549. [PubMed] [Google Scholar]
- 21.Christensen K K, Christensen P, Lindberg A.et al Mothers of infants with neonatal group B streptococcal septicemia are poor responders to bacterial carbohydrate antigens. Int Arch Allergy Appl Immunol 1982677–12. [DOI] [PubMed] [Google Scholar]
- 22.Lin F Y, Weisman L E, Azimi P H.et al Level of maternal IgG anti‐group B streptococcus type III antibody correlated with protection of neonates against early‐onset disease caused by this pathogen. J Infect Dis 2004190928–934. [DOI] [PubMed] [Google Scholar]
- 23.Flores A E, Nelson J A, Wu X Y.et al Antibody profiles to the group B streptococcal beta antigen in maternal and infant paired sera. Acta Pathol Microbiol Immunol Scand B 199310141–49. [DOI] [PubMed] [Google Scholar]
- 24.Christensen K K, Christensen P, Duc G.et al Human IgG antibodies to carbohydrate and protein antigens in mouse protection tests with group B streptococci. Pediatr Res 198418478–482. [DOI] [PubMed] [Google Scholar]
- 25.Kim K S, Wass C A, Hong J K.et al Demonstration of opsonic and protective activity of human cord sera against type III group B streptococcus that are independent of type‐specific antibody. Pediatr Res 198824628–632. [DOI] [PubMed] [Google Scholar]
- 26.Linden V, Christensen K K, Christensen P. Correlation between low levels of maternal IgG antibodies to R protein and neonatal septicemia with group B streptococci carrying R protein. Int Arch Allergy Appl Immunol 198371168–172. [DOI] [PubMed] [Google Scholar]
- 27.Lachenauer C S, Baker C J, Baron M J.et al Quantitative determination of immunoglobulin G specific for group B streptococcal beta C protein in human maternal serum. J Infect Dis 2002185368–374. [DOI] [PubMed] [Google Scholar]
- 28.Stålhammar‐Carlemalm M, Areschoug T, Larsson C.et al The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol Microbiol 199933208–219. [DOI] [PubMed] [Google Scholar]
- 29.Lachenauer C S, Creti R, Michel J L.et al Mosaicism in the alpha‐like protein genes of group B streptococci. Proc Natl Acad Sci USA 2000979630–9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stålhammar‐Carlemalm M, Areschoug T, Larsson C.et al Cross‐protection between group A and group B streptococci due to cross‐reacting surface proteins. J Infect Dis 2000182142–149. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter A. Enzyme‐linked immunoassays. In: NR Rose HF, Fahey JL, eds. Manual of clinical laboratory immunology. 4th ed. Washington DC: ASM Press, 1992
- 32.Areschoug T, Stålhammar‐Carlemalm M, Larsson C.et al Group B streptococcal surface proteins as targets for protective antibodies: identification of two novel proteins in strains of serotype V. Infect Immun 1999676350–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevanger L. Ibc proteins as serotype markers of group B streptococci. Acta Pathol Microbiol Immunol Scand B 198391231–234. [DOI] [PubMed] [Google Scholar]
- 34.Ailus K T. A follow‐up study of immunoglobulin levels and autoantibodies in an unselected pregnant population. Am J Reprod Immunol 199431189–196. [DOI] [PubMed] [Google Scholar]