Abstract
Hypothesis
Nitrotyrosine, a reaction product of peroxynitrite and proteins, could be demonstrated in the postmortem examination of brain tissue of full‐term neonates who had severe perinatal asphyxia.
Methods
The brain tissue of 22 full‐term neonates who died after severe perinatal asphyxia was examined, including cerebral cortex, basal ganglia, thalamus, hippocampus, brain stem, olives and cerebellum. Median age at death was 52 h. Routine histopathological examination and additional immunohistological staining were carried out with anti‐cysteine protease protein 32 antibodies to detect activated caspase 3, anti‐nitrotyrosine antibodies to detect nitrotyrosine and anti‐CD68 antibodies to detect activated microglia and macrophages, which might be associated with the production of nitric oxide. Staining was scored as none, weak (1–25% positive cells), moderate (26–75% positive cells) or severe (>75% positive cells).
Results
14 patients showed global injury, 4 showed injury of the basal ganglia and thalamus, and 4 showed predominantly parasagittal brain injury. One neonate without perinatal asphyxia served as a control. Nitrotyrosine staining of neurones was shown in all neonates with asphyxia, mostly in the thalamus (70%) and inferior olives (68%). Total nitrotyrosine staining tended to be less in the base of the pons and inferior olives of neonates with parasagittal brain injury. Activated caspase 3 was found mostly in the thalamus (60%) and hippocampus (53%). Positive CD68 staining was mainly present in the thalamus (70% positive).
Conclusion
Nitrotyrosine was found in brain tissue of full‐term neonates, suggesting that nitric oxide toxicity might have a role in hypoxic–ischaemic brain injury at term. This may be relevant for neuroprotective strategies in full‐term neonates with perinatal asphyxia.
Perinatal asphyxia followed by reperfusion is an important cause of permanent brain injury in full‐term neonates admitted to a neonatal intensive care unit.1,2 Recently, animal experiments have shown that excessive amounts of nitric oxide are generated during hypoxia, and even more so during reperfusion.3,4,5 Nitric oxide and superoxide combine to form peroxynitrite,6 a compound able to form nitrotyrosine in proteins, especially at lower pH values.7,8 Animal experiments in the Wilhelmina Children's Hospital, University Medical Centre Utrecht, Lundlaan, Utrecht, The Netherlands, showed that the selective inhibition of neuronal and inducible nitric oxide synthase (nNOS and iNOS, respectively) after cerebral hypoxia–ischaemia provided both short‐term and long‐term neuroprotection.9,10,11 It has been documented in animals that the expression of nitric oxide synthase (NOS) isoforms may be dependent on the developmental stage and time after the hypoxic–ischaemic insult.12 The role of nitric oxide in human neonatal brain injury has been reviewed recently.13 Nitric oxide toxicity may occur in human neonates with cystic periventricular leucomalacia, and in neonates with severe, but not mild hypoxic–ischaemic encephalopathy.14,15,16
As knowledge of these pathways is of utmost importance for the development of neuroprotective strategies, including the inhibition of NOS isoforms, we studied nitrotyrosine formation in the brain of asphyxiated full‐term human neonates who died within the first week of life. As activated microglia and macrophages can produce nitric oxide, CD68 staining was carried out to identify these cells. In addition, staining for activated caspase 3 cysteine protease protein 32 (CPP) was carried out to identify cells in the process of apoptosis, which could be related to nitrotyrosine formation.
Methods
Subjects
Subjects were 22 full‐term neonates admitted to the neonatal intensive care unit at Wilhelmina Children's Hospital between 2000 and 2002. All were admitted because of severe perinatal asphyxia and hypoxic–ischaemic encephalopathy. Inclusion criteria were at least three of the following signs, as reported previously: abnormal fetal heart rate patterns, a 5‐min Apgar score of <7 and the need for resuscitation at birth, umbilical cord pH or first postnatal pH <7.0, and signs of hypoxic–ischaemic encephalopathy.2 Table 1 shows the clinical details of the neonates. They died at a median postnatal age of 52 (range 11–158) h. During admission routine amplitude‐integrated electroencephalography (aEEG) and brain imaging (ultrasound and, whenever possible, magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy) were carried out to assess the severity of brain injury according to our clinical protocol. The infants died after withdrawal of intensive care owing to the combination of irreversible brain injury and multiple organ failure. One neonate (gestational age 41 weeks, birth weight 3500 g) with spinal muscular atrophy, but without any sign of hypoxic–ischaemic encephalopathy, served as a control. The infant was intubated rapidly after birth because of respiratory insufficiency before severe hypoxaemia had developed. He died on the sixth day of life after discontinuation of mechanical ventilation, and was examined within 6 h after he died.
Table 1 Clinical data of the neonates with perinatal asphyxia.
| Gestational age, weeks (mean (SD)) | 40 (1) |
| Birth weight, g (mean (SD)) | 3330 (495) |
| Male:female | 13:9 |
| Small‐for‐gestational age | 3 |
| Birth | |
| Home | 5 |
| Vaginal (unassisted) in hospital | 5 |
| Vaginal (breech) | 3 |
| Vaginal (vacuum or forceps) | 4 |
| Emergency caesarean section | 5 |
| Abnormal fetal heart rate patterns | 17 |
| Cranial ultrasound scans | |
| BG, thalamus | 4 |
| Subcortical white matter | 7* |
| BG, thalamus and subcortical white matter | 8 |
| Haemorrhage | 2* |
| No abnormalities | 2 |
| Postnatal age at death, h (median (range)) | 52 (11–158) |
| Seizures, encephalopathy, n (%) | 22 (100) |
| Isoelectric aEEG, n (%) | 5 (23) |
aEEG, amplitude‐integrated electroencephalography; BG, basal ganglia.
*Including one neonate with both white matter abnormalities and blood around the tentorium.
Five neonates with asphyxia had isoelectric aEEG tracings; the remaining 17 neonates had severe suppression‐burst or continuous low voltage.17 The control patient without asphyxia had a normal aEEG until the start of sedation before extubation.
All patients died within 1 h of withdrawal of mechanical ventilation. Postmortem examinations were performed within 6 h (n = 14) and within 24 h (n = 8) of death. Brain injury was categorised on the basis of histology and according to Volpe as selective neuronal necrosis (global injury, n = 14), lesions of mainly the deep nuclear structures (basal ganglia and thalamus, n = 4) and parasagittal cerebral injury (cortex and subcortical white matter) in the watershed areas, including the cerebral cortex (n = 4).18
This study was approved by the medical ethical committee of Wilhelmina Children's Hospital, University Medical Centre, Utrecht, and informed parental consent was obtained in all cases.
Histology
Brain tissue obtained at postmortem examination was fixed using 4% buffered paraformaldehyde for 4–6 weeks. Routine samples for histology were taken from the cerebellum, inferior olive, pons, basal ganglia, thalamus, hippocampus, and occipital and temporal cortex. The samples were embedded in paraffin wax by standard histological procedures. Histological sections were cut at 5–6 μm and were mounted on coated slides. Tissue was stained with haematoxylin and eosin. For all immunohistochemical staining, the same antigen‐retrieval method was followed. Before staining with antibodies, slides were placed in boiling citrate buffer (pH 6.0) for 20 min. To study nitric oxide toxicity, sections were stained with rabbit anti‐nitrotyrosine antibodies (Upstate, Charlottesville, Virginia, USA) and counterstained with haematoxylin. Monoclonal mouse antibodies against CD68/ED1 (Novo Castra, Newcastle, UK) were used to determine the presence of activated microglia and macrophages. Apoptosis was visualised using a rabbit antibody against CPP and activated caspase 3 (Dako, Carpinteria, California, USA). Staining of anti‐nitrotyrosine and anti‐CPP was followed by goat anti‐rabbit biotin (Vector Laboratories, Burlingame, California, USA). Staining was visualised using vectastain ABC (Vector Laboratories) and diaminobenzidine. Staining of anti‐CPP was carried out by horse anti‐mouse antibodies (Vector Laboratories). The results were visualised using streptavidine and diaminobenzidine.
Negative controls were created after omission of the specific (primary) antibody, and no staining was observed.
Positive immunohistological staining was scored using semiquantitative analysis by two observers unaware of the group assignment: no staining (score 0), weak staining (positive cells <25%; score 1), moderate staining (positive cells 26–75%; score 2) and severe staining (positive cells >75%; score 3). Only those samples with moderate2 or severe staining3 were considered to be positive in further analyses.
Statistical analysis
Staining for nitrotyrosine was compared with staining for CPP and CD68, and histological signs of hypoxic–ischaemic tissue injury. Findings in neonates with different patterns of brain injury were compared using Mann–Whitney U or χ2 tests where appropriate. Data were statistically analysed using SPSS V.10.0.
Results
Nitrotyrosine
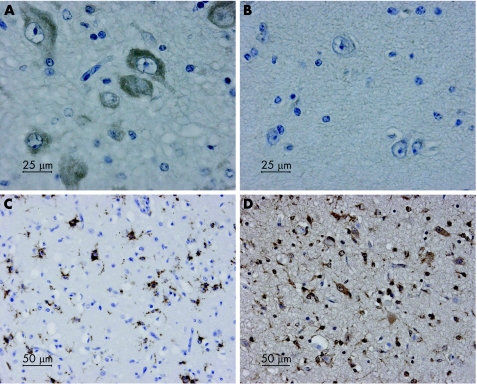
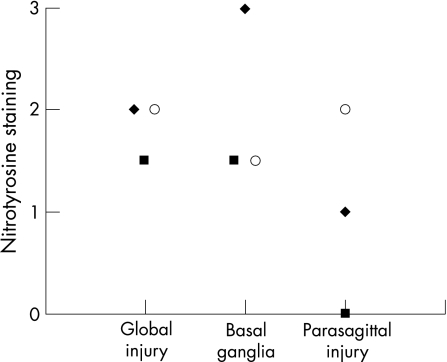
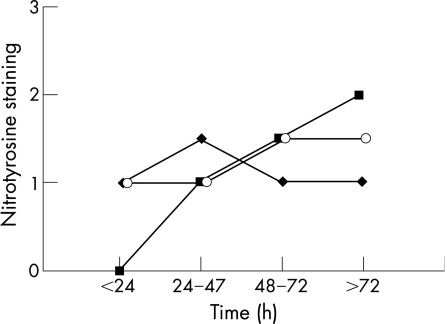
Figure 1 shows an example of positive and negative nitrotyrosine staining of neurones in the thalamus. Nitrotyrosine staining of neurones was seen in all regions of the brain after perinatal asphyxia (table 2). The thalamus, base and motor nuclei of the pons and inferior olives were often positive. In the cerebellum, nitrotyrosine staining was present mainly in Purkinje cells and in the dentate nucleus. Nitrotyrosine staining of the pons and inferior olives tended to be less prominent in neonates with parasagittal brain injury (fig 2). Figure 3 shows overall staining of nitrotyrosine versus time after perinatal asphyxia. We found no relationship between staining and postmortem interval.
Figure 1 Neurones in the thalamus with severe cytoplasmatic staining for nitrotyrosine (A, score 3) and with no nitrotyrosine staining (B, score 0). CD68 staining in basal ganglia (C, score 3) and cysteine protease protein 32 (activated caspase 3)‐positive cells in the basal ganglia (D, score 3).
Table 2 Results of histological studies.
| NT | CD68 | CPP | Cell loss, cytotoxic oedema | |
|---|---|---|---|---|
| Thalamus | 70 | 70 | 60 | 95 |
| Basal ganglia | 24 | 33 | 33 | 95 |
| Cerebral motor cortex | 29 | 27 | 36 | 86 |
| Hippocampus | 26 | 26 | 53 | 95 |
| Pons | 29 | 52 | 10 | 55 |
| Olives | 68 | 41 | 32 | 32 |
| Cerebellum | 33 | 27 | 36 | 50 |
CPP, cysteine protease protein 32; NT, nitrotyrosine.
The percentage of patients with a moderately or severely positive result on examination (score 2 or 3) is presented.
Figure 2 Nitrotyrosine staining (median) in the inferior olive (♦), pons (▪) and thalamus (o) plotted versus type of brain injury according to Volpe18 (as global injury, lesions mainly of the basal ganglia and thalamus, or parasagittal, watershed injury).
Figure 3 Staining (median) for nitrotyrosine (♦), CD68 (▪) and cysteine protease protein 32 (o) versus time after birth (h); all brain areas are combined.
CD68
Figure 1 is an example of positive CD68 staining. Highest percentages of CD68 staining were found in the thalamus (70%) and pons (52%). In the cerebellum, CD68‐positive cells were found in the white matter and the granular layer of the cerebellar cortex, and occasionally around remnants of Purkinje cells. Figure 3 shows the increase in CD68 during the first days after perinatal asphyxia. CD68‐positive cells were seen not earlier than at least 24 h after the hypoxic–ischaemic insult.
Activated caspase 3 cysteine protease protein 32
Figure 1 gives an example of positive CPP staining. Staining for CPP was highest in the thalamus (60%) and hippocampus (53%; table 2). In the cerebellum, both Purkinje cells and cells in the granular layer of the cerebellar cortex were CPP positive. The levels of nitrotyrosine staining correlated with the levels of CPP (p<0.05).
Discussion
We have shown the presence of nitrotyrosine in the brain tissue of full‐term neonates who died within the first week of life after severe perinatal asphyxia. Nitrotyrosine was found mostly in the thalamus, base and motor nuclei of the pons, and inferior olives. In the human neonatal brain, it is unknown how often neurones are positive for nitrotyrosine under baseline conditions—that is, without hypoxia–ischaemia. In the control neonate with spinal muscular atrophy but without perinatal asphyxia, only mild nitrotyrosine staining could be seen in neurones of the thalamus and inferior olives. If the presence of nitrotyrosine had been a normal phenomenon in human neonates, we expected that it would be present in most patients. However, many of the brain tissue specimens showed only mild or no nitrotyrosine staining (table 2). Nitrotyrosine has been found in normal animals, particularly in the neuronal nuclei.19,20 In contrast, nitrotyrosine staining of the cytoplasm has been reported after cerebral and cerebellar hypoxia–ischaemia.19 In our study, all brain specimens that scored positive for nitrotyrosine showed marked staining of the cytoplasm (fig 1), which is pathological in animal experiments. Therefore, we consider positive nitrotyrosine staining in the present patients to be a sign of nitric oxide toxicity.
The patients in our study died after intensive care treatment had been discontinued after appropriate, and repetitive, information was given by the attending medical team, mostly over some days. As described in the section on subjects, this withdrawal of care was based on a poor prognosis of neurodevelopment, given the aEEG and imaging findings. The procedure was described almost a decade ago by Dutch neonatologists.21,22 Almost all neonates received opiates before the interruption of mechanical ventilation to alleviate suffering as much as possible. Neonates died within a maximum of 1 h after discontinuation of mechanical ventilation. During this period, the neonates were hypoxaemic and nitric oxide might have been produced in the brain tissue.5 On the other hand, most neonates did not show respiratory movements after extubation, and arterial oxygen values are expected to be extremely low under these circumstances.23 As superoxide (and thus oxygen) is needed to produce peroxynitrite, we do not expect major peroxynitrite production during this agonal period, or in the postmortem phase, as hardly any oxygen is present at that time.
The production of nitric oxide in children with perinatal asphyxia may have been biphasic. A primary peak of nitric oxide may have occurred during asphyxia and resuscitation, followed by a second peak during the activation of microglia and macrophages.5 The production of nitric oxide by activated microglia and macrophages has been reported previously.24 In addition, nitric oxide toxicity has been shown in neonates with severe forms of periventricular leucomalacia.15 Raised levels of nitric oxide have also been shown in the cerebrospinal fluid of neonates with severe hypoxic–ischaemic encephalopathy.16
As all neonates in our study had encephalopathy and seizures, we could not assess the influence of aEEG patterns or seizures on nitrotyrosine staining; nor could we find any relationship between the postmortem interval and nitrotyrosine staining. This suggests that neither the agonal anoxic process nor postmortem changes influenced nitrotyrosine staining.
Raised nitrotyrosine levels have been observed in the brain tissue of 12‐day‐old rats as early as 30 min after hypoxia–ischaemia.25 Recently, a biphasic pattern of nitrotyrosine production has been reported in the cortex of neonatal rats with hypoxia–ischaemia.26 However, toxicity of nitric oxide may be caused not only by the formation of peroxynitrite27,28 and nitrotyrosine but also by the direct effects of nitric oxide on mitochondria, inhibiting mitochondrial respiration.11,29 In our postmortem examination of human brain tissue, these in vivo mitochondrial changes could not be tested. It is likely, however, that the toxic effects of nitric oxide are far greater than can be shown with nitrotyrosine.
CD68 (or ED1) is a marker of activated microglia and macrophages. Activated microglia and macrophages may produce nitric oxide. In this study, nitrotyrosine staining did not correlate with CD68 staining. CD68 gradually increased during the first days after perinatal asphyxia, whereas nitrotyrosine remained raised during the first week of life. This suggests that the contribution of activated microglia and macrophages to nitrotyrosine formation may not be as important as other sources of nitric oxide.
Activated caspase 3, an important protein preceding apoptosis, was found in various brain areas.26 The CPP staining correlated positively with nitrotyrosine staining, suggesting that nitrotyrosine formation and apoptosis coincide in the asphyxiated newborn brain. Further studies in human neonates will be needed to clarify the relationship between nitric oxide production and apoptosis.
Although antenatal injury has been shown in many newborns with perinatal asphyxia,30 gliosis, ependymal damage with rosette formation and calcifications, well‐known markers of long‐standing brain injury, were not present in the neonates with asphyxia in our study who were examined in the first week of life, indicating acute, perinatal brain injury. This confirms the acute nature of the hypoxic–ischaemic insult, as previously suggested in a study using MRI.2
What is already known on this topic
Nitric oxide toxicity contributes to brain injury after perinatal asphyxia in newborn animals, but its role in human neonates is unknown.
Nitrotyrosine is produced in brain tissue by nitric oxide and superoxide.
What this study adds
Nitric oxide toxicity may have a role in brain injury of the human neonate after severe perinatal asphyxia.
This knowledge may be relevant for the development of neuroprotective strategies.
Different patterns of brain lesions have been described in the literature—for example, lesions of the basal ganglia and thalamus in acute asphyxia versus abnormalities in the subcortical white matter and parasagittal watershed injury in more chronic or less severe forms of asphyxia.18 These different patterns were also seen in our group of patients. Although four neonates had predominantly parasagittal cerebral injury (cortex and subcortical white matter) in the watershed areas, with minor histological abnormalities of the basal ganglia, histological abnormalities of the basal ganglia and thalamus could be identified in 20 of the 22 (95%) patients (table 2), because all neonates in our study had well‐documented severe, acute asphyxia with abnormal fetal heart rate patterns, low Apgar scores, severe acidosis of the umbilical artery when measured and the need for resuscitation. Overall nitrotyrosine staining tended to be lowest in the group with the watershed lesions, but this was not statistically significant.
Cranial ultrasound scans showed abnormalities in all but two neonates who died at 11 and 50 h after acute asphyxia, respectively. This indicates that the asphyxia occurred very recently, as it takes at least 24 h to see changes on ultrasound scans after perinatal asphyxia. This study confirms our previous findings indicating the usefulness of ultrasound scanning in full‐term neonates with perinatal asphyxia.31 MRI carried out on nine neonates showed abnormalities of the basal ganglia and thalamus in five, severe abnormalities of the subcortical white matter in three and watershed lesions in one neonate. In all infants, histological examination confirmed the abnormalities shown by ultrasound scans or MRI, but also showed additional and more subtle abnormalities.
From this observational study, it cannot be proven that nitric oxide toxicity has a causative role in perinatal brain injury. However, these findings, as well as the findings of our recent animal experiments,9 and studies in neonates by others15,16 suggest an important role for nitric oxide in perinatal brain injury and warrant further studies.
Conclusion
Nitrotyrosine staining was shown in postmortem examination of the brain tissue of full‐term neonates with asphyxia respectively. Nitric oxide toxicity, through peroxynitrite and subsequent nitrotyrosine formation, is likely to have a role in perinatal brain injury at term.
Abbreviations
aEEG - amplitude‐integrated electroencephalography
CPP - cysteine protease protein 32
iNOS - inducible nitric oxide synthase
MRI - magnetic resonance imaging
nNOS - neuronal nitric oxide synthase
NOS - nitric oxide synthase
Footnotes
Funding: This study was funded by a grant from the “Brain Foundation of the Netherlands” (Nederlandse Hersenstichting).
Competing interests: None.
The funding source, the Brain Foundation of the Netherlands, was not involved in data collection, analysis or preparation of the manuscript, and the decision to submit the paper for publication.
References
- 1.Fellman V, Raivio K O. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res 199741599–606. [DOI] [PubMed] [Google Scholar]
- 2.Cowan F, Rutherford M, Groenendaal F.et al Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 2003361736–742. [DOI] [PubMed] [Google Scholar]
- 3.Malinski T, Bailey F, Zhang Z G.et al Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 199313355–358. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, Tominaga T, Ohnishi T.et al Electron paramagnetic resonance study on nitric oxide production during focal ischemia and reperfusion in the rat. Brain Res 199464791–96. [DOI] [PubMed] [Google Scholar]
- 5.Ioroi T, Yonetani M, Nakamura H. Effects of hypoxia and reoxygenation on nitric oxide production and cerebral blood flow in developing rat striatum. Pediatr Res 199843733–737. [DOI] [PubMed] [Google Scholar]
- 6.Siles E, Martinez‐Lara E, Canuelo A.et al Age‐related changes of the nitric oxide system in the rat brain. Brain Res 2002956385–392. [DOI] [PubMed] [Google Scholar]
- 7.Beckman J S. The double‐edged role of nitric oxide in brain function and superoxide‐mediated injury. J Dev Physiol 19911553–59. [PubMed] [Google Scholar]
- 8.Lipton S A, Choi Y B, Pan Z H.et al A redox‐based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso‐compounds. Nature 1993364626–632. [DOI] [PubMed] [Google Scholar]
- 9.Peeters‐Scholte C, Koster J G, Veldhuis W.et al Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia–ischemia. Stroke 2002332304–2310. [DOI] [PubMed] [Google Scholar]
- 10.van den Tweel E R, Peeters‐Scholte C M, Van Bel F.et al Inhibition of nNOS and iNOS following hypoxia–ischemia improves long‐term outcome but does not influence the inflammatory response in the neonatal rat brain. Dev Neurosci 200224389–395. [DOI] [PubMed] [Google Scholar]
- 11.van den Tweel E R, Van Bel F, Kavelaars A.et al Long‐term neuroprotection with 2‐iminiobiotin, an inhibitor of neuronal and inducible nitric oxide synthase, after cerebral hypoxia–ischemia in neonatal rats. J Cereb Blood Flow Metab 20052567–74. [DOI] [PubMed] [Google Scholar]
- 12.Northington F J, Tobin J R, Harris A P.et al Developmental and regional differences in nitric oxide synthase activity and blood flow in the sheep brain. J Cereb Blood Flow Metab 199717109–115. [DOI] [PubMed] [Google Scholar]
- 13.Ferriero D M. Neonatal brain injury. N Engl J Med 20043511985–1995. [DOI] [PubMed] [Google Scholar]
- 14.Beckman J S, Viera L, Estevez A G.et al Nitric oxide and peroxynitrite in the perinatal period. Semin Perinatol 20002437–41. [DOI] [PubMed] [Google Scholar]
- 15.Haynes R L, Folkerth R D, Keefe R J.et al Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol 200362441–450. [DOI] [PubMed] [Google Scholar]
- 16.Ergenekon E, Gucuyener K, Erbas D.et al Cerebrospinal fluid and serum vascular endothelial growth factor and nitric oxide levels in newborns with hypoxic ischemic encephalopathy. Brain Dev 200426283–286. [DOI] [PubMed] [Google Scholar]
- 17.Toet M C, Hellström‐Westas L, Groenendaal F.et al Amplitude integrated aEEG at 3 and 6 hours after birth in fullterm neonates with hypoxic–ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 199981F19–F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpe J J. Hypoxic‐ischemic encephalopathy: neuropathology and pathogenesis. In: Volpe JJ, eds. Neurology of the newborn. 4th edn. Philadelphia: Saunders, 2001296–330.
- 19.Rodrigo J, Alonso D, Fernandez A P.et al Neuronal and inducible nitric oxide synthase expression and protein nitration in rat cerebellum after oxygen and glucose deprivation. Brain Res 200190920–45. [DOI] [PubMed] [Google Scholar]
- 20.Golden W C, Brambrink A M, Traystman R J.et al Nitration of the striatal Na,K‐ATPase alpha3 isoform occurs in normal brain development but is not increased during hypoxia–ischemia in newborn piglets. Neurochem Res 2003281883–1889. [DOI] [PubMed] [Google Scholar]
- 21.Sauer P J. Ethical decisions in neonatal intensive care units: the Dutch experience. Pediatrics 199290729–732. [PubMed] [Google Scholar]
- 22.Cuttini M, Nadai M, Kaminski M.et al End‐of‐life decisions in neonatal intensive care: physicians' self‐reported practices in seven European countries. EURONIC Study Group. Lancet 20003552112–2118. [DOI] [PubMed] [Google Scholar]
- 23.Myers R E. Experimental models of perinatal brain damage: relevance to human pathology. In: Gluck L, eds. Intrauterine asphyxia and the developing fetal brain. Chicago: Year Book Medical Publishers, 197737–97.
- 24.Iadecola C, Zhang F, Xu S.et al Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab 199515378–384. [DOI] [PubMed] [Google Scholar]
- 25.van den Tweel E R, Nijboer C, Kavelaars A.et al Expression of nitric oxide synthase isoforms and nitrotyrosine formation after hypoxia–ischemia in the neonatal rat brain. J Neuroimmunol 200516764–71. [DOI] [PubMed] [Google Scholar]
- 26.Zhu C, Wang X, Qiu L.et al Nitrosylation precedes caspase 3 activation and translocation of apoptosis‐inducing factor in neonatal rat cerebral hypoxia–ischaemia. J Neurochem 200490462–471. [DOI] [PubMed] [Google Scholar]
- 27.Numagami Y, Zubrow A B, Mishra O P.et al Lipid free radical generation and brain cell membrane alteration following nitric oxide synthase inhibition during cerebral hypoxia in the newborn piglet. J Neurochem 1997691542–1547. [DOI] [PubMed] [Google Scholar]
- 28.Reiter C D, Teng R J, Beckman J S. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem 200027532460–32466. [DOI] [PubMed] [Google Scholar]
- 29.Brown G C, Bal‐Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol 200327325–355. [DOI] [PubMed] [Google Scholar]
- 30.Becher J C, Bell J E, Keeling J W.et al The Scottish perinatal neuropathology study: clinicopathological correlation in early neonatal deaths. Arch Dis Child Fetal Neonatal Ed 200489F399–F407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eken P, Jansen G H, Groenendaal F.et al Intracranial lesions in the fullterm infant with hypoxic ischaemic encephalopathy: ultrasound and autopsy correlation. Neuropediatrics 199425301–307. [DOI] [PubMed] [Google Scholar]