Abstract
The Visual Simplified Respiratory Questionnaire (VSRQ) was designed to assess health-related quality of life (HRQoL) in patients with chronic obstructive pulmonary disease (COPD). It contains eight items: dyspnea, anxiety, depressed mood, sleep, energy, daily activities, social activities and sexual life. Psychometric properties were assessed during a clinical trial that evaluated the impact of tiotropium on HRQoL of COPD patients. These included the determination of structure, internal consistency reliability, concurrent validity with the St George’s Respiratory Questionnaire (SGRQ), test – retest reliability, clinical validity and responsiveness to change over two weeks. Minimal important difference (MID) was calculated; cumulative response curves (CRC) were based on the dyspnea item. Psychometric analyses showed that VSRQ structure was unidimensional. The questionnaire demonstrated good internal consistency reliability (Cronbach’s alpha = 0.84), good concurrent validity with SGRQ (Spearman = −0.70) and clinical validity, good test-retest reproducibility (ICC = 0.77), and satisfactory responsiveness (standardized response mean = 0.57; Guyatt’s statistic = 0.63). MID was 3.4; CRC median value of the ‘minimally improved’ patients was 3.5. In conclusion, VSRQ brevity and satisfactory psychometric properties make it a good candidate for large studies to assess HRQoL in COPD patients. Further validation is needed to extend its use in clinical practice.
Keywords: chronic obstructive pulmonary disease, VSRQ, SGRQ, health-related quality of life, minimal important difference
Introduction
COPD adversely impacts emotional and physical aspects such as fatigue and muscle weakness, sleep and mood, exacerbations.1–5 Beside smoking cessation, symptom relief and improvement in Health-Related Quality of Life (HRQoL) are major goals of currently available treatments and key points in patients’ management.6,7 Nowadays, HRQoL is considered as a major endpoint in clinical trials for new COPD drugs, together with lung function parameters such as forced expiratory volume in one second (FEV1).
Generic instruments measuring HRQoL such as the Quality of Well-Being Scale (QWB) and the Sickness Impact Profile (SIP) may be used in COPD patients but showed a low sensitivity.8 Amongst the disease-specific questionnaires,9–12 the St George’s Respiratory Questionnaire (SGRQ) evaluates patient’s health status, including symptoms, activities and psychosocial impacts of COPD and asthma.13,14 It has been used extensively in clinical trials; yet, its length of completion limits its adoption as a tool for routine use. The Chronic Respiratory Questionnaire (CRQ) is used in patients with chronic respiratory diseases15,16 and has shown high sensitivity to changes, but the individualization of its dyspnea domain likely increases its complexity for both patients and interviewers.17 Shorter questionnaires such as the Airways Questionnaire 20 (AQ20) measure HRQoL in patients with COPD and asthma, but demonstrate poor discriminative power.18
In the landscape of HRQoL assessment in COPD, there is a need for questionnaires specifically designed and validated for an easy assessment in outpatient settings, real-life studies and/or routine care of the individual. The Visual Simplified Respiratory Questionnaire (VSRQ) was developed in order to offer an alternative to the already existing reference instruments to researchers and clinicians. It quickly measures and interprets HRQoL in COPD patients. In the present paper, we describe the VSRQ validation as well as its responsiveness and minimal important difference (MID) in a clinical trial setting designed to evaluate the effect of tiotropium treatment on HRQoL in COPD.19 The SGRQ was taken as a reference for comparison.
Methods
Study design and patients’ population
The VSRQ was initially developed by a team of three pulmonary physicians involved in COPD management (TP, ABT, and JMG), and a specialist of HRQoL (BA), after a thorough and extensive analysis of existing generic and COPD specific questionnaires. Selection of questions was further based on two preliminary studies of questionnaires with five to eight visual analog scales.20. Formulation of questions was further refined after ten face-to-face comprehension tests with COPD patients. Its final structure contains eight items covering dyspnea, state of anxiety, depressed mood, quality of sleep, energy, daily activities, social activities and sexual life. Each question is administered separately by the physician, through a fenestrated cardboard, and is assessed on a 10-cm long horizontal numerical rating scale that ranged from 0 to 10, with gradation lines every 1 cm; lower scores indicate higher impact on patients’ HRQoL. Labels of each extremity are specific to the item.
The VSRQ was included in a clinical trial that aimed to determine the impact of tiotropium on HRQoL in patients with mild to severe COPD, as defined by the 1995 American Thoracic Society criteria.19 Patients with very severe COPD requiring long term oxygen therapy were excluded. Complete description of the criteria patients had to meet to be eligible is found in the treatment-effect article.19 The study was a French multicenter, nine-month, double blind, placebo controlled-trial. Physicians administered the VSRQ and the SGRQ at screening visit (V1), baseline-visit (14 days, V2), three-month visit (V3), six- and nine-month visits. At V1, physicians also completed a case report form and established a global health assessment for each patient. Spirometric measures were performed at each visit, and included FEV1, forced vital capacity (FVC), FEV1/FVC and inspiratory capacity (IC).19 Trial medication (tiotropium or placebo) was introduced at V2. Design and rules regarding treatment protocol during the study are fully described in the treatment-effect paper.19
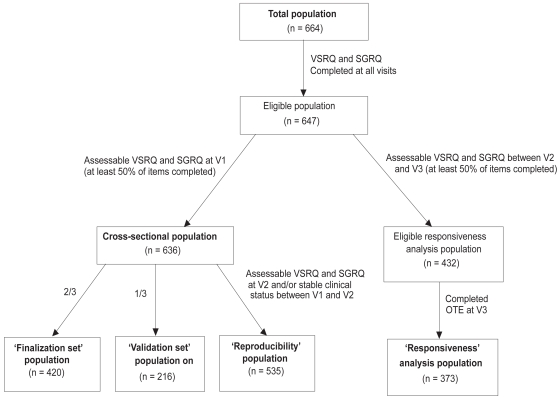
Reliability and validity of the questionnaire were determined at V2, on the cross-sectional population, ie, all subjects for whom VSRQ and SGRQ questionnaires were completed at all visits, and for which at least 50% of all VSRQ and SGRQ items were filled out at V1 (Figure 1). In order to prevent a learning effect bias, the cross-sectional population was randomly split into a 2:1 ratio: 2/3 of the patients were included in the finalization step of the VSRQ (‘finalization set’ population), and 1/3 in the validation step (‘validation set’ population).21 The responsiveness of the questionnaire was assessed on the longitudinal population (patients for whom VSRQ and SGRQ were completed at V2 and V3 and were assessable, ie, at least 50% of the items completed) (Figure 1). The reproducibility was measured on patients with assessable VSRQ and SGRQ at V2 and a clinically stable status (ie, no COPD exacerbation) between V1 and V2 (Figure 1).
Figure 1.
Composition and definition of the populations included in the analyses.
Statistical and psychometric validation methodology
Validity is the degree to which the instrument measures what it is supposed to measure.22,23 Several types of validity are distinguished. Principal Component Analysis (PCA) with Varimax Rotation was used to test the unidimensional structure of the questionnaire.24 The final structure of the VSRQ was evaluated by performing a multitrait analysis describing item convergent validity. Correlation between each item and the dimension was satisfactory if it achieved ≥0.40 for item convergent validity criterion.25 Floor and ceiling effects were determined in order to assess the ability of the scores to evaluate all severity levels in the study population. Concurrent validity consists in analyzing correlation levels between dimensions of the studied questionnaire and those of a questionnaire measuring similar concepts. A newly developed questionnaire should display a moderate correlation (0.40 to 0.70) with a well-established tool to conclude to good concurrent validity but no redundancy;22 Spearman correlation coefficients were calculated between VSRQ and SGRQ. Clinical validity evaluates the extent to which a questionnaire is able to detect variability among patients with different clinical severity levels. Patients’ clinical health status was defined based on the physician global assessment at V1 prior to the pulmonary function testing. This resulted in their allocation into four groups: “poor”, “fair”, “good” and “excellent”. Patients’ health status evaluation was based on the need for concomitant therapy, number and severity of exacerbations, severity of cough, exercise limitation, and physical findings (eg, wheezing).
In addition to descriptive analyses, nonparametric Kruskal-Wallis test (when comparing three groups or more of patients) or Mann-Whitney Wilcoxon test (when comparing two groups of patients) were used for group comparisons.
Reliability is the degree to which an instrument is free from random error; it is evaluated by measuring internal consistency reliability and reproducibility.26 Internal consistency reliability refers to the homogeneity of the items of the scale and was assessed by Cronbach’s alpha coefficient determination.27 A coefficient ≥0.70 was considered satisfactory.24 Reproducibility establishes the stability of an instrument over time in a stable population.23 Intraclass correlation coefficients (ICC) and concordance correlation coefficients (CCC) were calculated to measure the reproducibility of VSRQ and SGRQ between V1 and V2.28,29 Patients were considered stable if they did not experience an exacerbation episode over the 14 days. For group comparison, coefficient ≥0.70 was considered satisfactory.24
Responsiveness is the instrument’s ability to detect clinically important change over time,30 and is usually described by the effect-size (ES), standardized response mean (SRM) and Guyatt’s statistic.30,31 Changes are interpreted as “low change” (values close to 0.20), “moderate change” (values close to 0.50), and “important change” (values close to 0.80).32 The assessment of a significant change from zero was made by performing the Wilcoxon signed-rank test. The ability of the questionnaire to reflect underlying change was defined over 3 months (between V2 and V3). At V3, patients rated changes for each of the eight concepts covered by the VSRQ using the Overall Treatment Effect (OTE) questionnaire together with the VSRQ completion. When patients stated a health improvement or deterioration, they further reported the level of change on a 15-point rating scale, from −7 (“a great deal worse”) through 0 (“no change”) to +7 (“a great deal better”).33 According to their responses to the OTE, patients were allocated into three subgroups: “worsened”, “stable” and “improved”.
The MID, for the clinical interpretation of change in HRQoL scores, was calculated according to the methodology developed by Juniper and colleagues.33,34 Briefly, from their responses to the OTE, patients were classified based on the 15-point scale as follows: patients with scores of −7 to −2 were considered as having a “worsened” health status, patients with scores of −1 to 1 as “stable”, patients with scores of 2 to 3 as “minimally improved”, patients with scores of 4 to 5 as “quite improved”, and patients with scores of 6 to 7 as “highly improved”. Based on the dyspnea OTE scores, cumulative response curves were drawn for each of the groups described above. In parallel, a regression was performed between the changes in VSRQ and SGRQ global score used as an anchor, in order to estimate the change in VSRQ score corresponding to the MID of the SGRQ of 4 that was previously determined by Jones.35 expressed as a percentage of
Correlations between FEV1 % pred) and VSRQ or SGRQ were predicted value (FEV1 assessed at V1 by calculating Spearman coefficients.
Main analyses were performed using SAS software (Statistical Analysis System, version 8.02; SAS Inc., Cary, NC, USA). The threshold for statistical significance was set up at 5%.
Results
Patients’ population
Of the 664 patients recruited, 636 had completed assessable questionnaires at V1 and constituted the cross-sectional population. The overall mean age of patients at V2 was 64.3 years (± 10.0); the majority were male (84.9%); 72.2% were ex-smokers, 27.4% were current smokers and 0.3% never smoked (0.1% were missing data [MD]), with a mean of 43.1 ± 21.5 pack-years. COPD was diagnosed for eight years in average. According to physicians’ global assessment, the majority of patients had “fair” (43.1%) to “good” (42.6%) % pred was 46.81; FVC (L), 2.49; health status. Mean FEV1 FEV1/FVC %, 54.95 and IC, 2.11, for patients randomized at baseline and receiving a treatment. Additional spirometric measures are described in a treatment-effect article.19
Quality of completion of the VSRQ
The return rates were very high at each visit, with 69% (V3) to 96% (V1) VSRQ received (percentages based on overall recruited population, n = 664). Similar rates were found for SGRQ (74% received at V3; 97% at V1). Percentages of MD were low for VSRQ, ranging from 1.4% to 9.9% (item 8, “embarrassment in your sexual life”) MD within V1 to V3 visits. Higher percentages were reported for the SGRQ, with 4.5% to 6.5% MD.
VSRQ finalization
Due to MD in the retrieved questionnaires, 382 patients out of the 420 were considered for the finalization study of the questionnaire (‘finalization set’). Following PCA, one factor was retained by the Mineigen criterion (Eigenvalue > 1), which accounted for 51% of the total variance, indicating that a global score could therefore be calculated (Table 1). A multitrait analysis was performed. Item-scale correlation coefficients were greater than 0.40, ranging from 0.47 to 0.73. No floor effect and no ceiling effect were reported (0.0% and 0.3%, respectively). Cronbach’s alpha coefficient for the global score was 0.85.
Table 1.
Factor pattern of the VSRQ resulting from the Principal Component Analysis (PCA)
| Itemsa (Anchors of numerical rating scale) | Correlations resulting from PCA |
|---|---|
| Have you been hindered by your shortness of breath? (0: Extremely – 10: Not at all) | 0.75779 |
| Because of your respiratory problem, have you had difficulties in performing your usual daily activities (eg house cleaning, gardening)? (0: Extremely – 10: Not at all) | 0.77796 |
| Have your respiratory problems hindered your social life and your relations with others, your family, your friend or acquaintances? (0: All the time – 10: Never) | 0.70807 |
| The quality of your sleep was (0: Very poor – 10: Excellent) | 0.61581 |
| Have you had pleasure with the same things at other times? (0: No, much less – 10: Yes) | 0.81900 |
| Have you felt energetic? (0: Never – 10: All the time) | 0.79833 |
| Were you worried about your health? (0: All the time – 10: Almost never) | 0.57470 |
| Have your respiratory problems hindered your sexual life? (0: Extremely – 10: Not at all) | 0.60476 |
Notes: Items were translated into English for the purpose of publishing, but did not follow a linguistic validation process (ie there has been no process of iterative forward and backward translation; this English version is therefore not suitable for use); Recall period is one month.
Abbreviation: VSRQ, Visual Simplified Respiratory Questionnaire.
Copyright © 2008, Boehringer Ingelheim International. All rights reserved. Any use or reproduction of this questionnaire without written authorization prohibited.
VSRQ validation and scoring
PCA with the ‘validation set’ population (n = 216 patients) confirmed the VSRQ unidimensionality, with the single factor accounting for 46% of the total variance. Item convergent validity criterion of the global score was confirmed. No floor effect and no ceiling effect were reported (0.0% and 0.5%, respectively); Cronbach’s alpha for the global score was good (0.82).
The global score of the VSRQ was calculated as the sum of the eight item scores, when all items were completed and ranged from 0 to 80, with higher scores indicating better HRQoL condition. Scores were respectively 44.58 ± 15.96 (n = 578; 58 MD) and 49.72 ± 16.44 (n = 373; 59 MD) at screening and V3 visits. Scores of SGRQ were calculated as recommended by the authors13,14 with higher scores indicating lower HRQoL. Scores were 47.18 ± 17.44 (n = 623; 13 MD) and 41.25 ± 18.64 (n = 421; 11 MD) at V1 and V3, respectively.
Psychometric properties of the VSRQ
The following analyses were performed on the total cross-sectional population (n = 636), except wherever specified.
Reliability
Cronbach’s alpha coefficient of the VSRQ global score was 0.84, showing good internal consistency reliability of the questionnaire. Cronbach’s alpha of the SGRQ total score was low (0.46), but values were good for each of the three SGRQ sub-scores (0.69 to 0.83).
The test-retest reliability of the VSRQ and the SGRQ was measured between V1 and V2, with stable patients. ICC and CCC values ranged from 0.50 (impact on social life item) to 0.74 (impact on sexual life item) for the VSRQ. Both ICC and CCC values exceeded the 0.70 threshold for the global score of VSRQ (ie, 0.77) (Table 2). The Wilcoxon signed-rank test was not significant (p = 0.45), which demonstrated that scores remained stable over the two weeks. Similarly, ICC and CCC values were higher than 0.70 for the total score of SGRQ (0.86), with values for sub-scores ranging from 0.71 (symptoms sub-score) to 0.83 (impacts sub-scores). Changes to zero were not significant (p = 0.27).
Table 2.
Reproducibility by test-retest of VSRQ and SGRQ between screening (V1) and baseline visits (V2), ie, two weeks, in stable patients (N = 535)
| Questionnaires | N | Mean change in scores (SD) | ICC | CCC | p-value sign rank test |
|---|---|---|---|---|---|
| VSRQ global scorea | 455 | 0.32 (11.1) | 0.77 | 0.77 | 0.45 |
| SGRQ total scorea | 515 | 0.37 (9.30) | 0.86 | 0.86 | 0.27 |
Notes: 80 missing data for VSRQ; 20 missing data for SGRQ.
Abbreviations: CCC, concordance correlation coefficient; ICC, intraclass correlation coefficient; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire; VSRQ, Visual Simplified Respiratory Questionnaire.
Validity
Based on physician’s global assessment of patients’ health at V1, patients were ascribed into the group “poor”, “fair”, “good” or “excellent”. Distribution of the VSRQ global score according to each of these groups is reported in Table 3. Differences in VSRQ scores across clinical severity groups were highly significant (p < 0.0001). As patients’ global health improved, VSRQ scores increased. As patients’ global health improved, SGRQ scores decreased, with score differences between severity groups reaching statistical significance (p < 0.0001).
Table 3.
Score distribution for VSRQ and SGRQ according to global health patient groups as assessed by physicians at V1 (N = 636)
| Physician’s global assessment
|
p-valueb | ||||
|---|---|---|---|---|---|
| Poor | Fair | Good | Excellent | ||
| VSRQ | |||||
| Na | 48 | 248 | 248 | 33 | |
| Global score (mean ± SD) | 31.9 ± 14.3 | 41.7 ± 14.5 | 48.1 ± 15.7 | 57.6 ± 14.0 | <0.0001 |
| SGRQ | |||||
| Na | 54 | 271 | 263 | 34 | |
| Total score (mean ± SD) | 63.8 ± 15.2 | 49.7 ± 15.7 | 43.2 ± 16.9 | 31.8 ± 14.3 | <0.0001 |
Notes: 59 missing data for VSRQ; 14 missing data for SGRQ;
Kruskal-Wallis test.
Abbreviations: SGRQ, St George’s Respiratory Questionnaire; VSRQ, Visual Simplified Respiratory Questionnaire.
Spearman correlation coefficient value between VSRQ total score and FEV1 % pred at V1 was 0.16; coefficient was the highest for dyspnea item of VSRQ, with a value of 0.22. Correlation between SGRQ global score and FEV1 % pred was also weak (−0.26).
As presented in Table 4, overall, correlations between VSRQ items and SGRQ sub-scores were moderate (Spearman coefficients ranging from −0.26 to −0.59) and were higher between VSRQ global score and SGRQ total score (Spearman coefficient correlation = −0.70). Correlations between VSRQ global score and the three SGRQ sub-scores ranged from −0.50 to −0.68. Correlation between VSRQ and SGRQ changes between V2 and V3 was satisfactory (rho: −0.47).
Table 4.
Spearman correlation coefficients between VSRQ and SGRQ at screening visit (n = 636) (p < 0.0001)
| Shortness of breath | Daily activities impact | Social life impact | Sexual life impact | Pleasure impact | Energy impact | Worry impact | Sleep impact | VSRQ total score | |
|---|---|---|---|---|---|---|---|---|---|
| Symptoms sub-score | −0.43 | −0.40 | −0.31 | −0.26 | −0.40 | −0.39 | −0.30 | −0.32 | −0.50 |
| Impacts sub-score | −0.48 | −0.55 | −0.51 | −0.38 | −0.56 | −0.51 | −0.39 | −0.42 | −0.68 |
| Activities sub-score | −0.43 | −0.56 | −0.40 | −0.35 | −0.52 | −0.47 | −0.26 | −0.28 | −0.58 |
| SGRQ total score | −0.52 | −0.59 | −0.50 | −0.38 | −0.59 | −0.54 | −0.38 | −0.41 | −0.70 |
Abbreviations: SGRQ, St George’s Respiratory Questionnaire; VSRQ, Visual Simplified Respiratory Questionnaire.
Responsiveness
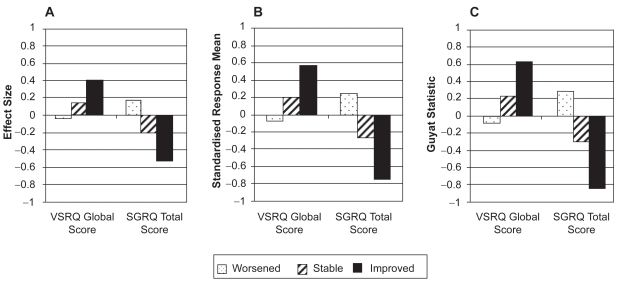
SRM and Guyatt’s statistic indicated a good responsiveness of VSRQ global score (0.57 and 0.63 for the ‘improved’ group, respectively) (Figures 2C and 2B); slightly lower ES (0.40) was observed (Figure 2A). Only change in scores in the ‘improved’ group was significantly different from zero (p < 0.0001). For SGRQ total score, Guyatt’s statistic and SRM demonstrated high responsiveness (−0.84 and −0.75, respectively; Figures 2B and 2C), while ES indicated moderate responsiveness (−0.52; Figure 2A). Change in scores was significant for all three groups, with p-values varying from <0.0001 to 0.05.
Figure 2.
Responsiveness of VSRQ global score and SGRQ total score over baseline and three- month visits according to patients’ health status groups measured by A), effect size, B) standardized response mean, and C), Guyatt’s statistic (N = 373).
In patients who have experienced a health status improvement, the overall change in VSRQ global score was 6.7; in patients who reported deterioration, the change in scores was −0.8, reflecting that VSRQ global mean score increased as patients’ health status improved (Table 5). A similar pattern was observed for SGRQ: as global rating of change improved, a decrease was observed in the SGRQ total mean score, indicating a better health condition.
Table 5.
Responsiveness to change of VSRQ and SGRQ over three months (N = 373)
| Change in score between V2 and V3 | Global rating of healtha |
||
|---|---|---|---|
| Worsened n = 53 | Stable n = 114 | Improved n = 206 | |
| VSRQ global mean score | −0.82 | 2.38 | 6.66 |
| nb (MDc) | 39 (14) | 89 (25) | 177 (29) |
| SGRQ total mean score | 3.08 | −3.33 | −9.30 |
| nb (MDc) | 48 (5) | 108 (6) | 198 (8) |
Notes: Subgroups defined from the OTE; “worsened”: all OTE scores ≤0, and at least one OTE score <−1; “stable”: all OTE scores within [−1; +1]; “improved”: all OTE scores ≥0 and at least one OTE score >1;
patients whose global rating health and complete VSRQ at V2 and V3 were available; patients whose global rating health and SGRQ with at least 41 item completed at V2 and V3 were available;
MD, ie, patients whose VSRQ was incomplete at V2 and/or V3; patients whose SGRQ contained at least 10 missing items at V2 and/or V3.
Abbreviations: MD, missing data; OTE, Overall Treatment Effect Questionnaire; SGRQ, St George’s Respiratory Questionnaire; VSRQ, Visual Simplified Respiratory Questionnaire.
Definition of the MID
Change in VSRQ score corresponding to the MID was assessed from the OTE breathlessness scale (corresponding to the dyspnea item). The MID was then determined as the mean change in the ‘improved’ group on this scale (OTE score = +2 or +3) and was 3.4. In parallel, a MID value of 3.2 for VSRQ was determined from the regression analysis between VSRQ global score and SGRQ total score.
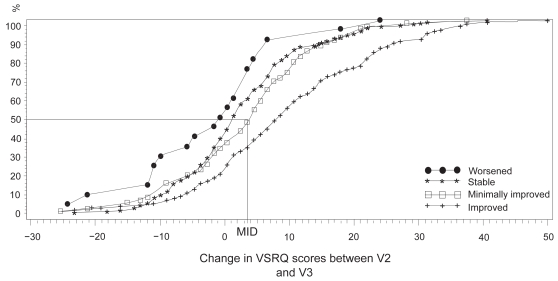
The median response of the “minimally improved” group determined from the cumulative response curves that were drawn based on the dyspnea OTE was 3.5 (Figure 3).
Figure 3.
Cumulative response curves according to the evolution of dyspnea for the four health groups defined from the dyspnea Overall Treatment Effect questionnaire. ‘Worsened patients’, n = 30; ‘stable patients’, n = 185; ‘minimally improved’, n = 83; ‘improved’, n = 116.
Abbreviations: MID, minimal important difference = 3.5. and VSRQ is not
Discussion
Although HRQoL instruments have been widely used for studying COPD impacts on patients’ HRQoL, none of them combine brevity, comprehensive coverage of all dimensions of HRQoL (ie, physical function, psychological state, social interaction, and somatic sensation, as defined by Schipper and colleagues) and COPD specificity altogether.9,11,15,18,36 VSRQ is a new disease-specific tool assessing the impact of COPD on patients’ HRQoL in routine practice and large real-life studies. It comprises only eight items covering dyspnea, state of anxiety, depressed mood, quality of sleep, energy, daily activities, social activities and sexual life HRQoL domains. Its average length of completion is 3 min, 20 sec, much lower than the time required for the two widely used instruments SGRQ or CRQ (10 to 25 minutes).13–15 VSRQ recall period is one month, similar to that reported for SGRQ.13,14
The VSRQ showed fair psychometric properties, comparable to the SGRQ regarding most validation criteria. The correlations between the questionnaires’ scores indicated good level of consistency between the concepts measured by VSRQ and SGRQ, but no redundancy (correlations of −0.70 between VSRQ global score and SGRQ total score). The VRSQ global score was found to be more strongly correlated with the SGRQ activities and impacts sub-scores than with the symptoms sub-score, which agrees with the fact that VSRQ was developed as a HRQoL tool rather than a symptom assessment tool. In the same way, VSRQ items about sexual life, emotional and sleep impacts were the most weakly correlated with the SGRQ total score, which was expected as these former VSRQ items measure concepts that are not explicitly covered by the SGRQ. VSRQ demonstrated good reliability, with good internal consistency of each individual item between one to each of the others. Reproducibility analyses concluded to the stability of the VSRQ over 2 weeks, although somewhat slightly lower than the one observed for the SGRQ; yet it remained satisfactory and comparable to that of the recently self-administered modified version of the CRQ.17 VSRQ also showed as good ability as the SGRQ in discriminating between groups of patients presenting different levels of COPD severity, thus demonstrating that in spite of its brevity, the VSRQ was clinically valid. One could question the possible interference between VSRQ administration and health status rating by the physician. However, it has been recently reported that physician’s rating of patient’s health status was only marginally influenced by patient’s own self-rated health.37 In order to consolidate these clinical findings, it should be interesting to validate each individual item of the VSRQ by comparing it with its corresponding physiological measures, eg, dyspnea item with lung hyperinflation, daily activities item with the 6-min walking distance.
The responsiveness of the VSRQ over 3 months, though slightly lower than the SGRQ especially in detecting deterioration, was satisfactory, indicating that the VSRQ enabled to report modifications in patients’ COPD medical condition that may have occurred during this time. Comparable data were recently reported for both CRQ and SGRQ in different study settings,38,39 and one should point out that the responsiveness property of disease-specific questionnaires widely differ between clinical studies according to patients’ clinical characteristics.40 The number of worsened patients with complete VSRQ was low (n = 39), which might have compromised the sensitivity analysis in this subgroup. The change in SGRQ score was also of borderline significance in these patients.
The low correlation between FEV1 surprising. Indeed, numerous studies have shown weak relationship between lung function parameter measurements and HRQoL outcomes in COPD; such observation has also been reported recently for SGRQ.5,14,41 It is important to note that the strongest correlation was observed with the VSRQ dyspnea item, the most prominent symptom limiting daily life activities and the most frequently reported complaint of COPD patients. In other words, spirometry and the VSRQ as a HRQoL tool complement each other well to evaluate disease severity and the impact of treatment, eventually giving a more comprehensive image of the patient’s clinical condition.
Lastly, the determined MID for VSRQ was 3.4 when using a similar approach than Juniper and colleagues.33 When performing a regression analysis between changes in VSRQ and SGRQ scores, a MID value of 3.2 for VSRQ was found to be corresponding to the MID value of 4 previously determined by Jones for the SGRQ.35 The close range of these two values is remarkable enough to be highlighted. The MID of VSRQ was set at 3.4. In other terms, scores of VSRQ needed to increase by 3.4 for a patient to consider their clinical status improved. In order to support the interpretation of VSRQ, we represented the cumulative response curves of changes. For the VSRQ, the determined median value was found to be of 3.5, again very close to the two previously MID values defined above (3.2 and 3.4). It would be interesting in the future to see if these MID do indeed predict serious clinical events such as hospitalizations or deaths.
As the VSRQ is a newly developed instrument, our first aim was to consolidate the use and validation of the VSRQ in its whole. In a next step, it would be interesting to validate each of the items of the VSRQ by assessing their ability and validity to measure HRQoL when taken individually. The VSRQ brevity, simple scoring and good psychometric properties make it a good candidate for large epidemiological studies or clinical trials, where length of completion is often an obstacle to the use of HRQoL questionnaires in the protocol. Short questionnaires such as the disease-specific Clinical COPD Questionnaire (CCQ) have not been validated in clinical trials evaluating inhaled therapy (corticosteroids and/or bronchodilators) in COPD patients yet.12 Furthermore, CCQ was designed to measure clinical control in COPD patients and does not cover all four HRQoL domains.
Patient-reported outcomes are major target of COPD treatment. Although this approach needs validation studies, the use of a simple HRQoL tool such as the VSRQ to modulate treatment in individual COPD patients might prove helpful. However the use of HRQoL for the clinical management of individual patients remains controversial, since the repeatability of scores is often lower than MID, as emphasized by Jones.40 The focusing of VSRQ questions on aims of daily life and the immediate availability of scores might also facilitate the communication between the physician and his patient about their expectations in treatment benefits. This particular issue still needs further validation in different clinical settings (eg, severity level) and in larger series. The psychometric performances of the VSRQ should also be evaluated during pulmonary rehabilitation, which has a highly significant impact on HRQoL.42 Finally, it would also be interesting to investigate how the VSRQ performs in severely affected COPD subgroup of patients, particularly those with chronic respiratory failure, for whom new instruments are welcome.43–46
Developing and validating multi-language versions of the present VSRQ will be necessary to allow its implementation in future international clinical studies.
In conclusion, the VSRQ is now available for researchers and clinicians as an addition to the existing sets of HRQoL questionnaires. It is a promising tool for use in large real-life studies, epidemiological and phase IV studies, as well as in clinical practice.7,40,47,48 However, further validation in specific studies is needed.
Acknowledgments
The authors would like to thank Muriel Viala (Mapi Values) for her contribution to the statistical analyses and manuscript reviewing, and François Denis and Maryse Mueser (Boehringer-Ingelheim) for their thorough reviewing of the manuscript. We would also like to thank Nicola Barnes (Mapi Values) for her contribution in reviewing the English language editing of the the manuscript.
Footnotes
Disclosure
The work was funded by Boehringer Ingelheim France. Professor Tonnel serves on the French Advisory Board Committees for Boehringer Ingelheim France, AstraZeneca, GlaxoSmithKline and Novartis. Dr Perez received research support from Boehringer Ingelheim. Drs Marie-Laure Bravo and Michèle Brun are employees of Boehringer Ingelheim. Benoit Arnould, Valérie Bosch and Isabelle Guillemin were paid consultants to Boehringer Ingelheim France.
Copyrights
The VSRQ is protected by copyright with all rights reserved to Boehringer Ingelheim, France. Do not use without permission. Boehringer Ingelheim kindly encourages the use of the questionnaire by clinicians, researchers and pharmaceutical industries. For information on, or permission to use the VSRQ, please contact Boehringer Ingelheim, France.
References
- 1.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gay PC. Chronic obstructive pulmonary disease and sleep. Respir Care. 2004;49:39–51. [PubMed] [Google Scholar]
- 3.Janssens JP, Rochat T, Frey JG, Dousse N, Pichard C, Tschopp JM. Health-related quality of life in patients under long-term oxygen therapy: a home-based descriptive study. Respir Med. 1997;91:592–602. doi: 10.1016/s0954-6111(97)90005-6. [DOI] [PubMed] [Google Scholar]
- 4.Killian KJ. Limitation to muscular activity in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:6–7. doi: 10.1183/09031936.04.00038104. [DOI] [PubMed] [Google Scholar]
- 5.Okubadejo AA, Jones PW, Wedzicha JA. Quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia. Thorax. 1996;51:44–47. doi: 10.1136/thx.51.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabbri LM, Hurd SS. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J. 2003;22:1–2. doi: 10.1183/09031936.03.00063703. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Executive summary. 2006 Cited Oct 6, 2008. Available from http://www.goldcopd.com 2006.
- 8.Lareau SC, Breslin EH, Meek PM. Functional status instruments: outcome measure in the evaluation of patients with chronic obstructive pulmonary disease. Heart Lung. 1996;25:212–224. doi: 10.1016/s0147-9563(96)80032-x. [DOI] [PubMed] [Google Scholar]
- 9.Hyland ME, BOTT J, Singh S, Kenyon CAP. Domains, constructs and the development of the Breathing Problems Questionnaire. Qual Life Res. 1994;3:245–256. doi: 10.1007/BF00434898. [DOI] [PubMed] [Google Scholar]
- 10.Hyland ME, Singh SJ, Sodergren SC, Morgan MPL. Development of a shortened version of the breathing problems questionnaire suitable for use in a pulmonary rehabilitation clinic: a purpose-specific, disease-specific questionnaire. Qual Life Res. 1998;7:227–233. doi: 10.1023/a:1024921610972. [DOI] [PubMed] [Google Scholar]
- 11.Maille AR, Koning CJM, Zwinderman AH, Willems LNA, Dijkman JH, Kaptein AA. The development of the ‘Quality-of-Life for Respiratory Illness Questionnaire (QOL-RIQ)’: a disease-specific quality-of-life questionnaire for patients with mild to moderate chronic non-specific lung disease. Respir Med. 1997;91:297–309. doi: 10.1016/s0954-6111(97)90034-2. [DOI] [PubMed] [Google Scholar]
- 12.Van Der Molen T, Willemse BW, Schokker S, Ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones PW, Quirk FH, Baveystock CM. The St. George’s Respiratory Questionnaire. Respir Med. 1991;85:25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 14.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–778. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijkstra PJ, Ten Vergert EM, Van Altena R, Otten V, Postma DS, Kraan J, et al. Reliability and validity of the chronic respiratory questionnaire (CRQ) Thorax. 1994;49:465–467. doi: 10.1136/thx.49.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory disease Questionnaire (CRQ) COPD. 2005;2:81–89. doi: 10.1081/copd-200050651. [DOI] [PubMed] [Google Scholar]
- 18.Hajiro T, Nishimura K, Jones PW, Tsukino M, Ikeda A, Koyama H, et al. A novel, short, and simple questionnaire to measure health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1874–1878. doi: 10.1164/ajrccm.159.6.9807097. [DOI] [PubMed] [Google Scholar]
- 19.Tonnel A-B, Perez T, Grosbois J-M, Verkindre C, Bravo M-L, Brun M. Effect of tiotropium on Health-Related Quality of Life as a primary efficacy endpoint in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:301–310. doi: 10.2147/copd.s2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosbois J-M, Mulkowski C, Clabaut M, Duquesnoy B, Wattier I, Bart F. Intérêt des échelles visuelles analogiques dans l’évaluation de la qualité de vie des BPCO avant et après réhabilitation respiratoire. Rev Mal Respir. 2006;23:408. [Google Scholar]
- 21.Vapnik VN. Statistical learning theory. New York: Wiley-Interscience; 1998. [Google Scholar]
- 22.Chassany O, Sagnier P, Marquis P, Fullerton S, Aaronson N. Patient-reported outcomes: The example of health-related quality of life: A European guidance document for the improved integration of health-related quality of life assessment in the drug regulatory process. Drug Inf J. 2002;36:209–238. [Google Scholar]
- 23.Scientific Advisory Committee of the Medical Outcomes Trust. Assessing health status and quality-of-life instruments: Attributes and review criteria. Qual Life Res. 2002;11:195–203. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 24.Nunnally JC, Bernstein IH. Psychometric theory. 3rd ed. New York: McGraw-Hill Inc; 1994. [Google Scholar]
- 25.Hays RD, Hayashi T. Beyond internal consistency reliability: rationale and user’s guide for Multitrait analysis program on the microcomputer. Behav Res Methods Instrum Comput. 1990;22:167–175. [Google Scholar]
- 26.Hays RD, Anderson R, Revicki D. Quality of Life assessment in clinical trials: methods and practice. Oxford: Oxford University Press; 1998. Assessing reliability and validity of measurement in clinical trials; pp. 169–182. [Google Scholar]
- 27.Cronbach LJ. Coefficient Alpha and the Internal Structure of Tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 28.Deyo RA, Diehr P, Patrick DL. Reproducibility and Responsiveness of Health Status Measures. Statistics and Strategies for Evaluation. Control Clin Trials. 1991:142–158. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 29.Hays RD, Revicki DA, Anderson R. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res. 1993;2:441–449. doi: 10.1007/BF00422218. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Deyo RA, Charlson M, Levine MN, Mitchell A. Responsiveness and validity in health status measurements a clarification. J Clin Epidemiol. 1989;42:403–408. doi: 10.1016/0895-4356(89)90128-5. [DOI] [PubMed] [Google Scholar]
- 31.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Medical Care. 1989;27:178–189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1977. [Google Scholar]
- 33.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 34.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 35.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 36.Schipper H. Guidelines and caveats for quality of life measurement in clinical practice and research. Oncology (Williston Park) 1990;4:51–57. [PubMed] [Google Scholar]
- 37.Kroenke K, Wyrwich KW, Tierney WM, Babu AN, Wolinsky FD. Physician-estimated disease severity in patients with chronic heart or lung disease: a cross-sectional analysis. Health Qual Life Outcomes. 2006;4:60. doi: 10.1186/1477-7525-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puhan MA, Soesilo I, Guyatt GH, Schunemann HJ. Combining scores from different patient reported outcome measures in meta-analyses: when is it justified? Health Qual Life Outcomes. 2006;4:94. doi: 10.1186/1477-7525-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puhan MA, Guyatt G, Goldstein R, et al. Relative responsiveness of the Chronic Respiratory Questionnaire, St Georges Respiratory Questionnaire and four other health-related quality of life instruments for patients with chronic lung disease. Respir Med. 2007;101:308–316. doi: 10.1016/j.rmed.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Jones PW. Health status: what does it mean for payers and patients? Proc Am Thorac Soc. 2006;3:222–226. doi: 10.1513/pats.200512-126SF. [DOI] [PubMed] [Google Scholar]
- 41.Rutten-van MM, Roos B, Van Noord JA. An empirical comparison of the St George’s Respiratory Questionnaire (SGRQ) and the Chronic Respiratory Disease Questionnaire (CRQ) in a clinical trial setting. Thorax. 1999;54:995–1003. doi: 10.1136/thx.54.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Duiverman ML, Wempe JB, Bladder G, Kerstjens HA, Wijkstra PJ. Health-related quality of life in COPD patients with chronic respiratory failure. Eur Respir J. 2008;32:379–386. doi: 10.1183/09031936.00163607. [DOI] [PubMed] [Google Scholar]
- 44.Windisch W, Budweiser S, Heinemann F, Pfeifer M, Rzehak P. The Severe Respiratory Insufficiency Questionnaire was valid for COPD patients with severe chronic respiratory failure. J Clin Epidemiol. 2008;61:848–853. doi: 10.1016/j.jclinepi.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Carone M, Bertolotti G, Anchisi F, Zotti AM, Donner CF, Jones PW. Analysis of factors that characterize health impairment in patients with chronic respiratory failure. Quality of Life in Chronic Respiratory Failure Group. Eur Respir J. 1999;13:1293–1300. doi: 10.1183/09031936.99.13613019. [DOI] [PubMed] [Google Scholar]
- 46.Windisch W, Freidel K, Schucher B, Baumann H, Wiebel M, Matthys H, et al. The Severe Respiratory Insufficiency (SRI) Questionnaire: a specific measure of health-related quality of life in patients receiving home mechanical ventilation. J Clin Epidemiol. 2003;56:752–759. doi: 10.1016/s0895-4356(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 47.Arnould B. Patient-Reported Outcomes in clinical practice. From measurement instruments to decision tools: much more than a simple change in format. PRO Newsletter. 2006;36:21–24. [Google Scholar]
- 48.Marquis P, Arnould B, Acquadro C, Roberts W. Patient-Reported Outcomes and Health-Related Quality of Life in effectiveness studies: pros and cons. Drug Dev Res. 2006;67:193–201. [Google Scholar]