Abstract
Currently available long-acting inhaled bronchodilators (tiotropium, salmeterol, formoterol) have demonstrated beneficial effects on exacerbations in placebo-controlled trials. However, there have been no direct comparisons of these drugs with exacerbations as the primary outcome and consequently COPD treatment guidelines do not indicate a preference for either bronchodilator. Therefore, an international, randomized, double-blind, double-dummy, parallel-group clinical trial has been designed to investigate the comparative efficacy of 2 long-acting bronchodilators tiotropium 18 μg daily and salmeterol 50 μg bid on exacerbations. The trial will include at least 6800 randomized patients with diagnosis of COPD, ≥ 10 pack-year history of smoking, post-bronchodilator FEV1 ≤ 70% predicted, and a history of exacerbations in the previous year. The primary endpoint is time to first COPD exacerbation. Secondary endpoints include number of exacerbations and time to premature discontinuation of trial medication. The trial has been designed to address several of the challenges in studying exacerbations in a controlled trial by a symptom and event-based definition of exacerbations, frequent follow-up contacts, selection of time to first event as the primary endpoint and using exposure adjusted analysis when examining number of events. Other challenges in designing exacerbation trials such as differential discontinuation and follow-up of discontinued patients are discussed.
Keywords: chronic obstructive pulmonary disease, exacerbation, salmeterol, study methodology, tiotropium
Introduction
Acute COPD exacerbations contribute considerably to the morbidity associated with COPD. Exacerbations result in significant health care costs, disability, and are also responsible for premature death. Therefore, exacerbations are currently one of the most relevant outcome parameters in COPD trials. A differential effect of different drugs on exacerbations may affect the decision making of health care providers about first-line maintenance treatment.1–3
Current guidelines recommend long-acting bronchodilators as first line maintenance therapy for moderate, severe and very severe COPD, with a preference for inhaled medications over oral theophyllines.1,2 Presently, the once-daily anticholinergic tiotropium and the twice-daily beta 2-agonists salmeterol and formoterol are the most widely used maintenance medications with no specific guidance as to which agent is recommended as a first choice. Both classes of agents have been demonstrated to be effective and hold favorable safety profiles.4–6
It has been shown that tiotropium and long-acting beta 2-agonists can have positive effects on the basic physiology of airflow limitation and hyperinflation as well as on patient-reported outcomes in patients with COPD.2 The evidence level has not generally shown clear distinctions between the two classes on more clinically oriented outcomes.
Direct comparisons between tiotropium and long-acting beta 2-agonists in patients with moderate to very severe COPD have generally been short term, with one published report of 2 combined studies being of 6 months duration in 1207 patients.7 The studies of tiotropium compared with placebo and with salmeterol indicated superior bronchodilator efficacy.7–11 However, these studies were not adequately powered to detect a difference in exacerbations. Nevertheless studies of 26 weeks’ duration may be considered as limited in terms of clinical conclusions. The evaluation of exacerbations should include an extended period of observation to minimize effects of seasonal variations. Studies of at least 1 year’s duration may therefore have advantages over shorter studies. Therefore, a 1-year study was designed to compare tiotropium to salmeterol with the primary outcome of exacerbations. The methodology involved in such a study raises challenging issues in study design that are discussed here. The purpose of this report is to describe the design of a recently initiated study in order to highlight issues related to study design for active comparator trials in COPD.
Methods
Study design
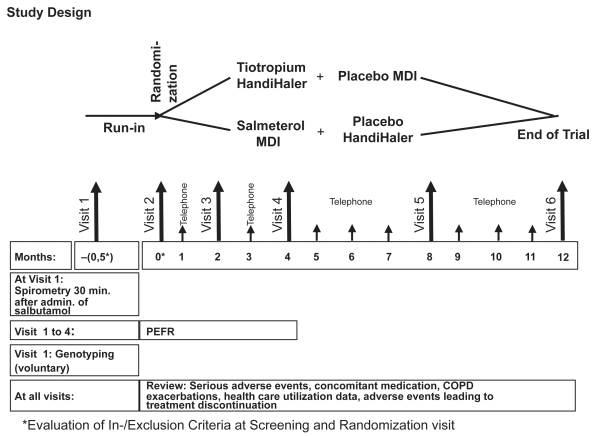
This is a 1-year international double-blind, double-dummy, randomized, parallel group trial to evaluate the effect of tiotropium 18 μg once-daily via the HandiHaler® compared to salmeterol hydroflouroalkane (HFA) 50 μg bid via metered dose inhaler on exacerbations of COPD. The study involves a 2-week run-in period followed by a 1-year treatment phase. At run-in, patients on long-acting anticholinergic drug or a combination anticholinergic/beta-adrenergics are converted to a short-acting anticholinergic, which is discontinued at randomization to study drug. Patients taking long-acting beta 2-agonists continue their use during the run-in period. The run-in period with short-acting anticholinergics is necessary due to the prolonged terminal elimination half-life of tiotropium (between 5 and 6 days). If this were not considered, prior tiotropium patients entering the salmeterol arm might contribute biased data due to a potential mixed effect of tiotropium and salmeterol. The short half-life of ipratropium used in the run-in period prevents the wash-out concern with tiotropium. The long-acting beta agonist salmeterol can be administered just before randomization as its duration of action is only up to 12 hours, is rapidly eliminated with a plasma half-life of between 2 and 8 hours and is extensively metabolized.
DNA is being extracted from blood samples according to standard molecular methods and analyzed by standard genotyping technologies to examine possible effects of beta-adrenergic single nucleotide polymorphism on lung function in patients with COPD. Clinic visits are scheduled at 2, 4, 8 and 12 months. Monthly telephone contacts are scheduled between clinic visits (Figure 1). During the active study period, patients are allowed to take any COPD concomitant medication except long-acting beta 2-agonists (alone or in a fixed combination with inhaled steroids) and anticholinergic drugs (alone or in a fixed combination with short-acting beta 2-agonists). Spacer devices are not provided for salmeterol metered dose inhaler (MDI). All patients are offered salbutamol as rescue medication.
Figure 1.
Study design.
Abbreviations: MDI, metered dose inhaler; PEFR, peak expiratory flow rate.
Patients prematurely withdrawn from study medication will be followed through telephone contact according to the clinic visit schedule for the one-year period from randomization to determine vital status. The first patient was recruited in January 2008 and study results should be available in 2010. The study is conducted in accordance with the Declaration of Helsinki (1996) and Good Clinical Practice Guidelines.12 All patients must provide written informed consent before participating in the study. Ethics committees and authorities of all participating countries have approved this protocol. The trial is registered in clinicaltrials.gov under identifier NCT00563381. The study ID number is 205.389.
Organizational committee
The study is being guided by a steering committee consisting of external clinical experts and representatives of Boehringer Ingelheim. The committee will remain blinded to data through the study period and is responsible for protocol amendments, analyses planning, data capture of health care resource use and cost data analysis, interpretation of data, and publication planning.
Study subjects
Criteria for participation include age 40 years or older, diagnosis of COPD, post-bronchodilator FEV1 ≤ 70% of predicted normal and FEV1 ≤ 70% of FVC and a smoking history of ≥10 pack years. Patients must have a history of at least 1 COPD exacerbation within the past year requiring treatment with antibiotics and/or systemic steroids and/or requiring hospitalization. Patients with significant diseases other than COPD that would preclude participation in the trial or interpretation of the results are excluded. Patients with a current diagnosis of asthma, severe cardiovascular disorders and use of systemic corticosteroid medication at unstable doses are also excluded. Patients with any respiratory infection or COPD exacerbation in the 4 weeks prior to the screening Visit (Visit 1) or during the run-in period are not permitted to participate. In the case of a respiratory infection or COPD exacerbation during the run-in period, the latter may be extended up to 4 weeks to allow for randomization after the respiratory infection or COPD exacerbation is resolved.
Efficacy, health- and economic outcomes
The primary endpoint is time to first COPD exacerbation within 1 year. Secondary endpoints include other exacerbation endpoints including hospitalizations due to COPD exacerbations (Table 1). A symptom and event based definition of exacerbation is being used in the trial. A COPD exacerbation is defined as a complex of respiratory events/symptoms (increase or new onset) of more than 1 of the following: cough, sputum, wheezing, dyspnoea or chest tightness with at least 1 symptom lasting at least 3 days requiring treatment with antibiotics and/or systemic steroids and/or hospitalization. The onset of an exacerbation is defined by the onset of the first new or increased reported symptom. The end of the exacerbation will be based on the clinical assessment of the investigator.
Table 1.
Efficacy and safety endpoints
| Primary efficacy endpoint |
|---|
| Time to first COPD exacerbation |
| Secondary efficacy endpoints |
| Occurrence of at least one exacerbation |
| Number of COPD exacerbations |
| Time to first hospitalization due to COPD exacerbation |
| Occurrence of at least one hospitalization due to COPD exacerbations |
| Number of hospitalizations due to COPD exacerbations |
| Time to premature discontinuation of trial medication |
| Occurrence of premature discontinuation of trial medication |
| Pre-dose morning PEFR measured by patients at home during the first 4 months of randomized treatment (weekly means will be calculated) |
| Time to first COPD exacerbation or time to discontinuation of study medication because of worsening of underlying disease, whichever comes first |
| Safety endpoints |
| Serious adverse events |
| Adverse events leading to treatment discontinuation |
| All-cause mortality during treatment with study medication |
| All-cause mortality including follow-up of vital status from patients who prematurely discontinue treatment |
| Physical examination |
| Other secondary endpoints |
| Health care utilization and absence from paid work |
Abbreviation: PEFR, peak expiratory flow rate.
Only moderate and severe exacerbations will be collected as they are considered clinically relevant. Moderate exacerbations are defined as those requiring treatment with antibiotics and/or systemic steroids; those requiring hospitalizations are categorized as severe. Exacerbations based on the trial definition as well as healthcare resources used to treat these exacerbations will be collected via a questionnaire on regular clinic visits and telephone contacts. All patients are provided with a paper diary to serve as a reminder to support the structured questionnaire clinic and telephone interview. Use of medications and resource use associated with COPD exacerbations will be recorded within the trial in order to calculate direct health care costs and productivity losses by multiplying units with respective unit cost.
Vital status and exacerbations
For patients who prematurely discontinue study medication, vital status along with the primary cause of death will be recorded. The information will be gathered via phone at the scheduled clinic visits starting 4 months after randomization until the end of the trial. In case of a patient’s death, information will be collected based on information of the treating physicians, death certificates, autopsy or other medical documentation. Exacerbations following treatment discontinuation will not be collected unless the exacerbation was fatal.
Statistical analysis
The primary and all secondary time-to-event endpoints will be analyzed using Cox proportional hazards regression stratified by center. Cochrane Mantel Haenszel (CMH) test will be used for risk ratios, Poisson regression with correction for exposure and over dispersion will be used to analyse event rates. For safety, serious adverse events and mortality will be analyzed.
A review of tiotropium studies (both placebo and active controlled studies)7–11,13–17 in patients with COPD was performed to estimate the risk of at least 1 exacerbation within 52 weeks of treatment with tiotropium and the risk reduction compared with salmeterol. Studies applied similar inclusion and exclusion criteria and collected similar information on COPD exacerbations. The planned sample size has been calculated to be 6800 patients (3400 per treatment group), assuming a 40% risk to experiencing at least one COPD exacerbation within one year for tiotropium and a constant hazard ratio of 0.90. The study has 80% power to reject the null hypothesis of equal hazards (no difference between tiotropium and salmeterol) at the 5% level of significance. A protocol defined blinded interim analysis to assess event rates will be used to determine whether the sample size may need to be adjusted.
Discussion
COPD exacerbations have a negative impact on health-related quality of life,18 may promote disease progression19,20 and are a common cause of hospitalization and mortality.21 Therefore, reducing the occurrence of exacerbations continues to be an important goal of COPD treatment. Different classes of respiratory medications have been shown to accomplish this goal by either reducing the absolute frequency or the severity of exacerbations.10,22
While lung function differences can be observed in clinical trials of active compounds,7,8 trials comparing active compounds designed to evaluate COPD exacerbations as a primary outcome are uncommon. A study by Kardos et al23 demonstrated that combination therapy with salmeterol/ fluticasone compared with salmeterol monotherapy significantly reduced the frequency of moderate/severe exacerbations in patients with severe COPD and a history of repeated exacerbations. More recently, the INSPIRE study compared the long-term effect of a fixed salmeterol/fluticasone combination versus tiotropium on COPD exacerbations, demonstrating a similar reduction in exacerbations for both active drugs.24 Nevertheless, there is sparse evidence on the comparative effect of different long-acting bronchodilators on COPD exacerbations. Consequently, contemporary guidelines do not give preference to either bronchodilator.
The study is designed to investigate the comparative efficacy of 2 long-acting bronchodilators with different modes and durations of action, ie, the once-daily long-acting anticholinergic tiotropium versus the twice-daily long-acting beta 2-agonist salmeterol. The study will be sufficiently powered to demonstrate the superiority of one of the drugs in respect of the primary endpoint, time to first COPD exacerbation. The study will involve at least 6800 randomized patients from approximately 900 study centers in 26 countries. COPD patients in the study will be treated for 1 year in order to reduce potential biases that may result from seasonal variation.
Long-term clinical trials in COPD are faced with several methodological challenges. Critical to the outcome is a precise and feasible definition of an exacerbation. The trial employs a symptom- and event-based definition of exacerbations, combining the COPD exacerbation definitions as proposed by Rodriguez-Roisin25 and Casaburi13 and recently endorsed by an ATS/ERS (American Thoracic Society/European Respiratory Society) Task Force.3 The trial focuses on moderate (requiring treatment with antibiotics and/or systemic steroids) and severe (requiring hospitalization) exacerbations, because the clinical relevance of mild exacerbations is unclear. Thus, it may be argued that the definition in the present trial bears the potential for underestimating exacerbations; however, distinguishing variations in day to day symptoms from a “mild” exacerbation is difficult. In addition, mild exacerbations may be dealt without any contact with the physician. Thus, the data collected would primarily rely on the patients′ interpretation of their well-being. Therefore, it is preferable to use an event-based definition that requires a decision by the physician to prescribe treatment with systemic steroids and/or antibiotics. The disadvantage of using an event-based definition of exacerbation severity is that definitions are sensitive to variations between countries and settings in medical practice with respect to prescribing antibiotics/systemic steroids and hospitalizations.
One consideration in developing definitions is using a blinded adjudication process (ie, a clinical endpoints committee) in order to achieve a standardized and objective classification of an endpoint.26 This approach is considered appropriate in determining the primary cause of death in a clinical trial.27 However, in this study there is no need to adjudicate the primary endpoint given the exacerbation criteria. Exacerbation information is collected via a pre-defined questionnaire and will have to meet the trial definition of an exacerbation.
Other issues in outcome trials include withdrawal of baseline medications and how to address non-random premature discontinuations.28 Recently, concerns have been raised as to whether withdrawal of therapies at entry to study may lead to a bias.26,29,30 This likely will have a minimal impact on the current study as patients will be treated with effective accepted maintenance treatment in both intervention groups and can continue other maintenance therapies including inhaled corticosteroids and theophyllines. However, sensitivity analyses will be performed to investigate whether maintenance therapy prior to study start has an impact on the results. Non-random premature discontinuation of patients may also introduce a bias, which argues in favor of collecting follow-up data. However, other factors should be considered. The majority of patients stopping blinded treatment with tiotropium or salmeterol are likely to be treated subsequently with tiotropium or salmeterol or both. Therefore, inclusion of data from discontinued patients will lead to a dilution of the effect or significant difficulties in attributing an effect to one drug when the patient actually may be receiving the other drug (as prescribed openly by their physician). Furthermore, there is also a high risk of lower quality data (ie, it is unlikely that reporting of exacerbations will be as reliable as prior to discontinuation). Due to these reasons, data on exacerbations following drug discontinuation will not be collected in this trial.
The time to first COPD exacerbation was selected as the primary endpoint. This choice was made as time to first exacerbation is considered to be the most robust parameter for assessing COPD exacerbations in clinical trials. It is readily understood, the statistical theory for analysis is well established and resulting estimators are generally unbiased. A recent publication outlined the complexities and challenges in examining other exacerbation endpoints such as number of events, which further supports the decision for the primary endpoint in the present trial.26 Time to first COPD exacerbation avoids major disadvantages of other exacerbation endpoints (ie, the number of exacerbations) such as: (a) separation of exacerbation events from events that are close in time, (b) change in maintenance therapy subsequent to events which may result in underestimation of exacerbation rates and uneven distribution across treatment groups, and (c) the effects of early discontinuation due to an event. Withdrawal prior to the first event that is not completely at random also affects estimates of time to first exacerbation. With regard to discontinuation, statistical methods such as Poisson regression adjust for exposure time. However, this method presumes that exacerbations observed in a patient are independent events (ie, no change in maintenance therapy due to a previous event). A disadvantage of the endpoint “time to first exacerbation” may be that the data are more difficult to interpret by clinicians.
The investigation of the comparative efficacy of two long-acting bronchodilators is the main focus of this study, but the lack of resources available to satisfy the continuously increasing demand for health care has led to an increased interest in costs and health-economic outcomes. Therefore a cost-effectiveness study will be conducted that includes all COPD-related medication costs as well as all exacerbation-related costs of healthcare utilization and absence from paid work. The direct COPD-related health care costs also include the downstream costs of the pharmacotherapies that are evaluated, ie, hospitalizations, emergency room visits, unscheduled care-giver visits, ambulance transportation and medications such as the increased use of bronchodilators, antibiotics and oral corticosteroids. Only COPD-related costs will be included and not total costs because this allows greater precision in detecting the effects of the treatments by minimizing the “noise” that occurs when all costs are included.
In conclusion, the trial represents a unique opportunity to study the differences between two well established long-acting bronchodilators in COPD patients with a history of moderate and severe exacerbations. The knowledge gained from the study may help physicians to guide their treatment decision and is also going to influence future national and international COPD guidelines.
Footnotes
Disclosures
Kai-Michael Beeh, Maureen Rutten-van Moelken and Claus Vogelmeier receive remuneration as consultants of Boehringer Ingelheim. Maureen Rutten-van Moelken also received funding of Boehringer Ingelheim to conduct research on the pharmacoeconomic aspects of tiotropium. Dr Beeh’s institution receives compensation for the design and performance of clinical trials from various pharmaceutical companies, including Boehringer Ingelheim.
Thomas Glaab, Bettina Hederer, Steven Kesten and Achim Müller are employees of Boehringer Ingelheim.
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.Cazzola M, MacNee W, Martinez FJ, et al. American Thoracic Society; European Respiratory Society Task Force on outcomes of COPD. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31:416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 4.Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest. 2006;130:1695–1703. doi: 10.1378/chest.130.6.1695. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson GT, Funck-Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular Safety of Salmeterol in COPD. Chest. 2003;123:1817–1824. doi: 10.1378/chest.123.6.1817. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo GJ, Nannini LJ, Rodriguez-Roisin R. Safety of long-acting beta-agonists in stable COPD: a systematic review. Chest. 2008;133:1079–1087. doi: 10.1378/chest.07-1167. [DOI] [PubMed] [Google Scholar]
- 7.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once-daily tiotropium compared with twice-daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briggs DD, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulm Pharmacol Ther. 2005;18:397–404. doi: 10.1016/j.pupt.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 10.Dusser D, Bravo ML, Iacono P MISTRAL study group. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006;27:547–555. doi: 10.1183/09031936.06.00062705. [DOI] [PubMed] [Google Scholar]
- 11.Beeh KM, Beier J, Buhl R, Stark-Lorenzen P, Gerken F, Metzdorf N ATEM study group. Efficacy of tiotropium bromide (Spiriva®) in patients with chronic obstructive pulmonary disease (COPD) of different severities. Pneumologie. 2006;60:341–346. doi: 10.1055/s-2005-919145. [DOI] [PubMed] [Google Scholar]
- 12.Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Off J Eur Commun. 2001;L121:34–44. [PubMed] [Google Scholar]
- 13.Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 14.Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. 2002;122:47–55. doi: 10.1378/chest.122.1.47. [DOI] [PubMed] [Google Scholar]
- 15.Tonnel AB, Perez T, Grosbois JM, Verkindre C, Bravo ML, Brun M TIPHON Study Group. Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD. Int J COPD. 2008;3:1–10. doi: 10.2147/copd.s2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powrie DJ, Wilkinson TMA, Donaldson GC, et al. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J. 2007;30:472–478. doi: 10.1183/09031936.00023907. [DOI] [PubMed] [Google Scholar]
- 17.Chan CKN, Maltais F, Sigouin C, Haddon JM, Ford GT SAFE Study Group. A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Can Respir J. 2007;14:465–472. doi: 10.1155/2007/192961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the Lung Health Study. Am J Respir Crit Care Med. 2001;164:358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 21.Soler-Cataluna JJ, Martinez-Garcia MA, Sanchez PR, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2007 update. Can Respir J. 2007;14:5B–32B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/ fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:144–149. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 24.Wedzicha JA, Calverley PMA, Seemungal TA, Hagan G, Ansari Z, Stockley RA INSPIRE Investigators. The prevention of COPD exacerbations by salmeterol/ fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Roisin R. Toward a consus definition for COPD exacerbations. Chest. 2000;117:398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 26.Aaron SD, Fergusson D, Marks GB, et al. Canadian Thoracic Society/ Canadian Respiratory Clinical Research Consortium. Counting, analysing and reporting exacerbations of COPD in randomized controlled trials. Thorax. 2008;63:122–128. doi: 10.1136/thx.2007.082636. [DOI] [PubMed] [Google Scholar]
- 27.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: Operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decramer M, Celli B, Tashkin DP, et al. Clinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the Uplift Trial. COPD. 2004;1:303–312. doi: 10.1081/copd-200026934. [DOI] [PubMed] [Google Scholar]
- 29.Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:842–846. doi: 10.1164/rccm.200508-1338PP. [DOI] [PubMed] [Google Scholar]
- 30.Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. Eur Respir J. 2008;31:927–933. doi: 10.1183/09031936.00098307. [DOI] [PubMed] [Google Scholar]