Abstract
Overexpression of Bcl-XL, an antiapoptotic Bcl-2 family member, occurs in a majority of head and neck squamous cell carcinomas (HNSCCs) and correlates with chemotherapy resistance in this disease. Overexpression of Bcl-2 is also observed in HNSCC, albeit less frequently. We have previously shown that peptides derived from the BH3 domains of proapoptotic proteins can be used to target Bcl-XL and Bcl-2 in HNSCC cells, promoting apoptosis. In this report, we examined the impact of ABT-737 (for structure, see Nature 435 677-681,2005), a potent small-molecule inhibitor of Bcl-XL and Bcl-2, on HNSCC cells. As a single agent, ABT-737 was largely ineffective at promoting HNSCC cell death. By contrast, ABT-737 strongly synergized with the chemotherapy drugs cisplatin and etoposide to promote HNSCC cell death and loss of clonogenic survival. Synergism between ABT-737 and chemotherapy was associated with synergistic activation of caspase-3 and cleavage of poly(ADP-ribose) polymerase. Treatment with ABT-737 plus chemotherapy resulted in dramatic up-regulation of proapoptotic Noxa protein, and small interfering RNA (siRNA)-mediated inhibition of Noxa up-regulation partially attenuated cell death by the synergistic combination. Treatment with cisplatin or etoposide, alone or in combination with ABT-737, resulted in substantial down-regulation of Mcl-1L, a known inhibitor of ABT-737 action. Further down-regulation of Mcl-1L using siRNA failed to enhance killing by the cisplatin/ABT-737 synergistic combination, indicating that chemotherapy treatment of HNSCC cells is sufficient to remove this impediment to ABT-737. Together, our results demonstrate potent synergy between ABT-737 and chemotherapy drugs in the killing of HNSCC cells and reveal an important role for Noxa in mediating synergism by these agents.
Head and neck squamous cell carcinoma (HNSCC) is a common human cancer, and the 5-year survival rates for this disease have remained relatively unchanged for the past few decades (Forastiere et al., 2001; Gibson and Forastiere, 2006; Jemal et al., 2007). The treatment of HNSCC is hampered by frequent resistance to conventional chemotherapeutic agents. In addition, chemotherapy drugs currently used to treat HNSCC, including cisplatin, are associated with considerable toxicities (Gibson and Forastiere, 2006). Synergistic drug combinations, which would be useful for enhancing treatment efficacy and reducing adverse toxicities, have not been developed for this disease. Thus, there is a need to identify agents that can be used to circumvent chemotherapy resistance and/or synergize with conventional chemotherapy drugs in the killing of HNSCC cells.
The resistance of HNSCC cells to chemotherapy is due in part to the expression of antiapoptotic members of the Bcl-2 protein family, including Bcl-XL and Bcl-2. Bcl-XL is overexpressed in a majority of primary HNSCC specimens, whereas overexpression of Bcl-2 occurs somewhat less frequently (Drenning et al., 1998; Trask et al., 2002). It is noteworthy that Bcl-XL overexpression correlates with chemotherapy resistance in patients with HNSCC (Trask et al., 2002). Antisense-mediated down-regulation of Bcl-XL and Bcl-2 has been shown to sensitize HNSCC cell lines to chemotherapy-induced apoptosis (Sharma et al., 2005). Bcl-XL and Bcl-2 inhibit chemotherapy-induced apoptosis by binding and sequestering proapoptotic members of the Bcl-2 protein family (Danial and Korsmeyer, 2004). We have shown that cell-permeable peptides derived from the BH3 domains of proapoptotic Bax and Bad localize to mitochondria, the site of Bcl-XL/Bcl-2 expression, in HNSCC cells (Li et al., 2007). The BH3 peptides promote apoptosis signaling and HNSCC cell death, albeit at relatively high concentrations. The naturally occurring compound (-)-gossypol, an inhibitor of Bcl-XL and Bcl-2, also has been shown to promote apoptosis of HNSCC cells, both in vitro and in vivo (Bauer et al., 2005; Wolter et al., 2006). These studies have hinted at the potential therapeutic benefit of targeting Bcl-XL/Bcl-2 in head and neck cancers.
A number of small-molecule inhibitors of antiapoptotic Bcl-2 family members have been identified. Among these, the compound with the highest affinity for Bcl-XL and Bcl-2 is ABT-737 (Oltersdorf et al., 2005; Zhai et al., 2006). ABT-737 binds antiapoptotic Bcl-XL, Bcl-2, and Bcl-w but shows little affinity for antiapoptotic Mcl-1L and A1/Bfl (Oltersdorf et al., 2005). When used as a single agent, ABT-737 induces apoptosis in the low micromolar range in small-cell lung cancer (SCLC) cells (Oltersdorf et al., 2005), as well as cell lines and primary cells representing a variety of hematological malignancies, including acute myeloid leukemia, follicular lymphoma, chronic lymphocytic leukemia, and multiple myeloma (Oltersdorf et al., 2005; Konopleva et al., 2006; Kuroda et al., 2006; van Delft et al., 2006; Chauhan et al., 2007; Del Gaizo Moore et al., 2007). Monotherapeutic ABT-737 also exhibits in vivo efficacy against SCLC and leukemia xenografts (Oltersdorf et al., 2005; Konopleva et al., 2006). However, cell lines derived from other types of solid tumors have failed to demonstrate sensitivity to single agent ABT-737, and resistance to this compound is associated with aberrant overexpression of Mcl-1L (Konopleva et al., 2006; van Delft et al., 2006; Chen et al., 2007; Lin et al., 2007b; Tahir et al., 2007; Wesarg et al., 2007; Huang and Sinicrope, 2008). Despite these limitations, ABT-737 has shown the ability to sensitize cells derived from both solid tumor and hematopoietic malignancies to conventional anticancer agents (Oltersdorf et al., 2005; Tahir et al., 2007). ABT-737 sensitizes ovarian cancer cells to carboplatin (Witham et al., 2007) and enhances apoptosis induction by tumor necrosis factor-related apoptosis-inducing ligand in pancreatic, prostate, renal, and lung cancer cells (Huang and Sinicrope, 2008; Song et al., 2008). In addition, ABT-737 sensitizes cells representing hematopoietic malignancies to agents including bort-ezomib (Paoluzzi et al., 2008), N-(4-hydroxyphenyl)retinamide (Kang et al., 2008), imatinib (Kuroda et al., 2006), vincristine (Kang et al., 2007), dexamethasone (Kang et al., 2007; Trudel et al., 2007), and melphalan (Trudel et al., 2007).
The sensitivity of HNSCC cells to ABT-737 has not been investigated. We report that ABT-737 is ineffective as a monotherapy against HNSCC cells but potently synergizes with chemotherapy to kill these cells. Up-regulation of Noxa was found to play an important role in mediating the synergistic effects of ABT-737 and chemotherapy drugs. Moreover, because of potent down-regulation of Mcl-1L by chemotherapy drugs, the impact of ABT-737 was not limited in cells treated with synergistic combinations of ABT-737 plus chemotherapy. These findings suggest that combination of highly selective Bcl-XL/Bcl-2-targeting agents with conventional chemotherapy drugs may be an effective means for achieving synergistic antitumor effects in patients with HNSCC.
Materials and Methods
Cell Lines and Reagents. UM-22A, UM-22B, and 1483 are human HNSCC cell lines (Lin et al., 2007a) and were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics. ABT-737 and A-793844 were provided by Abbott Laboratories (Abbott Park, IL), dissolved in DMSO, and stored at -80°C as 10 mM stocks. Cisplatin was obtained from the University of Pittsburgh Cancer Institute Pharmacy, and etoposide was from Sigma (St. Louis, MO). Lipofectamine 2000 reagent was obtained from Invitrogen (Carlsbad, CA), and Annexin V-fluorescein isothiocyanate apoptosis detection kits were from BD Biosciences (San Jose, CA). Anti-Bcl-2 antibody was from Dako (Glostrup, Denmark), and antibodies against Bcl-XL, Bax, Noxa, and Mcl-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against PARP and Bak were obtained from Cell Signaling Technology (Danvers, MA), and anti-caspase-3 was from Assay Designs (Ann Arbor, MI). Anti-β-actin antibody was from Sigma. Horseradish peroxidase-conjugated secondary antibodies were purchased from Promega (Madison, WI).
Cell Viability Assays and Determination of Synergy. Cellular sensitivities to individual agents, or combinations of agents, were determined using trypan blue exclusion assays. Before treatment, UM-22A cells were seeded in triplicate at 10,000 cells/well, UM-22B at 5000 cells/well, and 1483 at 8000 cells/well in 48- or 96-well plates. After growth overnight, the cells reached approximately 50% confluence. The cells were then treated for 48 h at 37°C with varying doses of each agent or combinations of the agents. Treatment with drug diluent alone (0.1% DMSO) was used as a control in each experiment. After the addition of trypan blue, cell viabilities were determined by counting a minimum of 300 total cells per data point. Data were analyzed using GraphPad PRISM software (San Diego, CA) to determine IC50 values. The method of Chou and Talalay (1984) was used to assess synergy, and combination indexes (CIs) were calculated using CalcuSyn V2 software (BIOSOFT, Cambridge, UK). CI values lower than 1.0 were considered evidence of synergism.
In certain experiments, cell viabilities were determined by flow cytometric analysis of Annexin V staining. For these experiments, UM-22A or UM-22B cells were seeded in six-well plates and treated for 48 h with ABT-737, cisplatin, etoposide, or combinations of ABT-737 and chemotherapy. After treatment, adherent cells were detached from plates using trypsin and combined with floating cells. Cells were then washed twice with cold PBS and resuspended in 1× Annexin V binding buffer at a concentration of 1 × 106 cells/ml. Single-cell suspensions (100 μl) were then transferred to 5-ml culture tubes and stained with 5 μl of Annexin V-fluorescein isothiocyanate and 5 μl of propidium iodide for 15 min at room temperature in the dark. After staining, 1× Annexin V binding buffer (300 μl) was added to each tube, and the samples were placed on ice. Two-color flow cytometric analyses were performed using an Epics Coulter XL flow cytometer (Beckman Coulter, Fullerton, CA).
Clonogenic Assays. Cells were seeded in 100-mm dishes (3 × 106/dish), grown overnight, and then treated for 1 h with ABT-737 or cisplatin, alone or in combination. After treatment, cells were washed twice with PBS and detached from plates using trypsin. Single-cell suspensions were diluted in DMEM containing 10% FBS and an equal number of cells replated into six-well plates. The cells were grown for 12 to 15 days, and then colonies were stained for 30 min in a solution of 6% glutaraldehyde and 0.5% crystal violet in water. The plates were washed in water (5-10 times) until no more dye was detected in the rinse. After air-drying, colonies composed of 50 or more cells were counted.
Immunoblotting. For immunoblotting experiments, treated cells were scraped from plates, centrifuged at 1300 rpm for 5 min at 4°C, washed once in cold PBS, then lysed for 10 min on ice in 150 mM NaCl, 50 mM Tris pH 8.0, 0.1% SDS, 1% Nonidet P-40, 20 μg/ml aprotinin, 3 μg/ml leupeptin, and 1.5 mM phenylmethylsulfonyl fluoride. The lysates were centrifuged at 14,000 rpm and 4°C for 2 min, and the supernatants were transferred to new tubes. Bio-Rad Protein Assay dye concentrate (Bio-Rad, Hercules, CA) was then used to determine protein concentrations in the lysates. For detection of caspase-3 cleavage products and determination of protein levels of Bcl-2, Bcl-XL, Mcl-1, Bax, Bak, Noxa, Bik, and β-actin, proteins (20 μg/lane) were electrophoresed on 12.5% SDS-PAGE gels. For detection of PARP cleavage products or Mcl-1L levels, proteins were electrophoresed on 10% SDS-PAGE gels. After electrophoresis, proteins were transferred to nitrocellulose membranes for 3 h at 45 V and 4°C. The membranes were blocked at room temperature for 1 h in TBST buffer (150 mM NaCl, 50 mM Tris, pH 8.0, and 0.1% Tween 20) containing 5% nonfat milk. The blocked membranes were washed with TBST buffer, probed overnight at 4°C with primary antibodies, washed again in TBST, then probed for 1 h at room temperature with secondary antibodies. After four final washes in TBST, the membranes were developed using enhanced chemiluminescence reagent, according to the directions provided by the manufacturer (PerkinElmer Life and Analytical Science, Inc., Waltham, MA).
When blots were reprobed with anti-β-actin, they were stripped by incubating for 45 min at 37°C in 0.1 M glycine, pH 2.9. The stripped blots were then washed in TBST buffer before incubating in blocking solution.
siRNA Transfection. Before transfection, 2 × 106 cells were seeded into 100-mm dishes. After overnight growth, the cell culture medium was replaced with DMEM/10% FBS without antibiotics. Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was then used to transfect annealed, double-stranded Noxa, Mcl-1, or nonspecific siRNAs (100 nM) into the cells, following the manufacturer's instructions. After 6 h, the cell culture medium was replaced with fresh DMEM supplemented with 10% FBS and antibiotics, and incubation continued for an additional 18 h at 37°C. The cells were then either left untreated or were treated for 24 h with ABT-737 alone, chemotherapy alone, or ABT-737 plus chemotherapy. Trypan blue exclusion assays were used to assess cell viabilities, and immunoblotting was used to verify inhibition of Noxa or Mcl-1 expression. Nonspecific siRNA was obtained from Ambion (Austin, TX), as were siRNAs targeting Noxa (5′-GUAAUUAUUGACACAUUUCTT-3′) and Mcl-1 (5′-GGACUUUUAGAUUUAGUGATT-3′).
Statistics. Statistical analyses were performed using Prism software (version 4; GraphPad Software, Inc., San Diego, CA). Comparisons between groups were carried out by one-way ANOVA followed by Tukey's multiple comparison test. P values less than 0.05 were considered significant.
Results
ABT-737 Is Ineffective as a Single Agent against HNSCC Cell Lines. With the exception of small-cell lung cancer, cells derived from solid tumor malignancies have failed to demonstrate substantial sensitivity to ABT-737 treatment alone. To determine the impact of ABT-737 on HNSCC cells we used three HNSCC cell lines: UM-22A, UM-22B, and 1483 (Lin et al., 2007a). UM-22A and UM-22B originated from the same patient but were derived from primary tumor and a cervical lymph node metastasis, respectively. Cell line 1483 originated from the primary tumor of a different patient. As shown in Table 1, cells were treated with varying concentrations of ABT-737, followed by performance of trypan blue exclusion assays and determination of IC50 values. As a control, cells were also treated with A-793844, an enantiomeric compound known to possess markedly reduced binding affinity for Bcl-XL and Bcl-2 (Oltersdorf et al., 2005). In addition, cells were treated with cisplatin, a chemotherapy drug commonly used in the clinical treatment of HNSCC, or with etoposide. Single agent ABT-737 was largely ineffective against the three HNSCC cell lines, exhibiting IC50 values ranging from 13.8 to 53.6 μM. These values were similar to those obtained with either single agent cisplatin (IC50 values from 11.1 to 23.6 μM) or single agent etoposide (IC50 values from 27.6 to 34.5 μM). Curiously, the metastatic variant UM-22B was somewhat more resistant to cisplatin than were UM-22A cells. Expression profiling of Bcl-2 family members (Supplemental Fig. 1) revealed that UM-22B cells exhibit elevated levels of antiapoptotic Bcl-2 and reduced levels of proapoptotic Bim, which may play a role in the increased drug resistance of these cells.
TABLE 1.
IC50 values of single-agent ABT-737, cisplatin, etoposide, or A-793844 against HNSCC cell lines
UM-22A, UM-22B, and 1483 cells were seeded in 48-well plates, grown overnight, and then treated for 48 h with varying doses of the indicated agents. Cell viabilities were assessed in triplicate via trypan blue exclusion assays, and IC50 values were calculated.
|
Cell Line |
IC50 |
|||
|---|---|---|---|---|
| ABT-737 | Cisplatin | Etoposide | A-793844 | |
| μM | ||||
| UM-22A | 53.6 | 14.5 | 34.5 | >100 |
| UM-22B | 36.2 | 23.6 | 34.3 | >100 |
| 1483 | 13.8 | 11.1 | 27.6 | 42.6 |
As a single agent, ABT-737 was unable to overcome the inherent drug resistance of HNSCC cells. However, it should be noted that the modest activity of single agent ABT-737 exceeded that obtained with A-793844, which exhibited IC50 values from 42.6 μM to greater than 100 μM. Because ABT-737 and A-793844 differ markedly in their abilities to bind Bcl-XL and Bcl-2, this suggests that the binding of these proteins by ABT-737 alone provides a weak apoptotic stimulus for HNSCC cells.
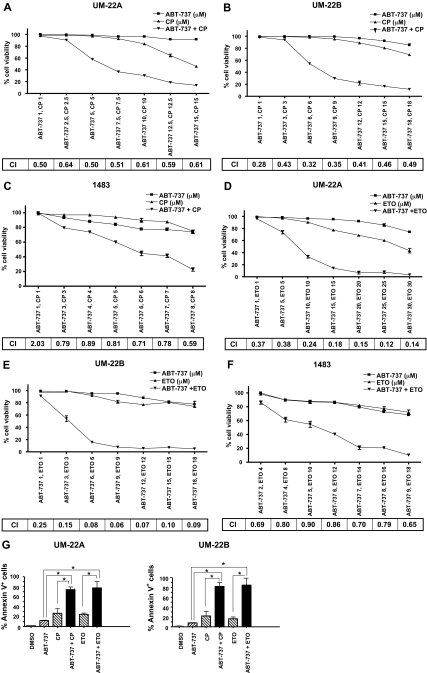
ABT-737 Synergizes with Chemotherapy to Kill HNSCC Cells. Bcl-XL and Bcl-2 are overexpressed in a high percentage of HNSCC tumors, and overexpression of Bcl-XL has been shown to correlate with chemotherapy resistance in this disease (Trask et al., 2002). Therefore, we predicted that a small-molecule inhibitor of Bcl-XL and Bcl-2 (i.e., ABT-737) might markedly enhance the sensitivity of HNSCC cells to chemotherapy drugs. To investigate this possibility, UM-22A (Fig. 1A), UM-22B (Fig. 1B), and 1483 (Fig. 1C) cells were treated for 48 h with ABT-737 alone, cisplatin alone, or varying doses of a constant ratio of ABT-737 plus cisplatin. Potential synergism was assessed by calculating combination index (CI) values according to the method of Chou and Talalay (1984), where CI values less than 1.0 are indicative of synergy. As shown, CI values well below 1.0 were observed in all three cell lines at multiple doses of the ABT-737/cisplatin combination, indicating potent synergy between these two agents. To determine whether ABT-737 would demonstrate synergy with other chemotherapy drugs in HNSCC cells, we treated cells with varying doses of a constant ratio of ABT-737 plus etoposide (Fig. 1, D-F). Again, CI values well below 1.0 were observed, pointing to potent synergy. To confirm synergy between ABT-737 and either cisplatin or etoposide, treated UM-22A and UM-22B cells were evaluated in Annexin V/propidium iodide flow cytometric assays (Fig. 1G; a representative experiment is shown in Supplemental Fig. 2). Results obtained in these experiments were quantitatively and qualitatively similar to those obtained in trypan blue exclusion assays (Fig. 1, A-F) and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt assays (data not shown).
Fig. 1.
ABT-737 synergizes with chemotherapy to kill HNSCC cells. A to C, UM-22A, UM-22B, and 1483 cells were seeded in 96-well trays, grown overnight, and then treated for 48 h with ABT-737 alone, cisplatin (CP) alone, or ABT-737 plus cisplatin. D to F, UM-22A, UM-22B, and 1483 cells were seeded as above and then treated for 48 h with ABT-737 alone, etoposide (ETO) alone, or ABT-737 plus etoposide. After treatment, trypan blue exclusion assays were performed to determine the percentage of viability. Data points represent the average of triplicate wells, and error bars represent S.D. CIs were calculated using CalcuSyn version 2 software and are shown for each combination of the drugs. G, UM-22A and UM-22B cells were seeded in six-well plates and treated for 48 h with 0.1% DMSO, 10 μM ABT-737, 10 μM cisplatin, 10 μM etoposide, or ABT-737 plus chemotherapy drug (10 μM each). After treatment, adherent cells were detached with trypsin and combined with floating cells. The percentage of Annexin V-positive cells was determined by flow cytometric analysis. *, P < 0.01.
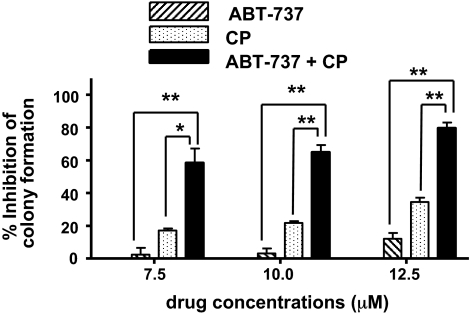
Synergy between ABT-737 and standard chemotherapy drugs in the killing of HNSCC cells was further confirmed in clonogenic survival assays (Fig. 2). UM-22A cells were treated for 1 h with 0.1% DMSO (drug diluent), ABT-737 alone, cisplatin alone, or ABT-737 plus cisplatin (1:1 fixed ratio). After treatment, cells were replated in medium lacking drugs, and colonies were counted after 12 to 15 days (a representative experiment is shown in Supplemental Fig. 3). The loss of clonogenic capacity in cells treated with ABT-737 plus cisplatin exceeded the combined losses in cells treated with ABT-737 alone and cisplatin alone, demonstrating superadditive killing by the ABT-737/cisplatin combination.
Fig. 2.
ABT-737 and cisplatin synergize in clonogenic survival assays. UM-22A cells were treated for 1 h with 0.1% DMSO, ABT-737 alone (7.5, 10, or 12.5 μM), cisplatin alone (7.5, 10, or 12.5 μM), or ABT-737 plus cisplatin (equal concentrations of each). The treated cells were washed twice in PBS, detached from plates, diluted in DMEM containing 10% FBS, then replated in six-well plates. Colonies were stained with crystal violet solution, and colonies composed of 50 cells or greater were counted. Data were plotted as the percentage of inhibition of colony formation relative to DMSO-treated cells. The graphed data represent the average of three independent experiments, and error bars represent S.D. P values were calculated using one-way ANOVA followed by Tukey's multiple comparison test. *, P < 0.01; **, P < 0.001.
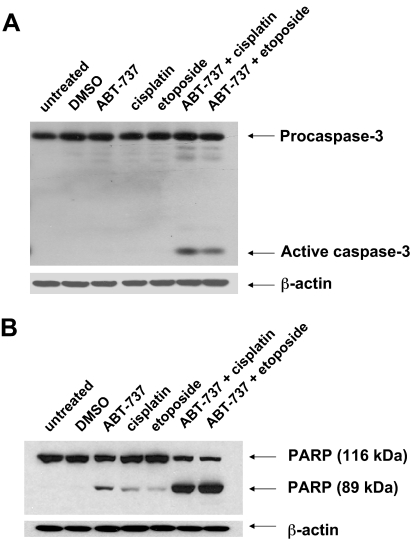
ABT-737 and Chemotherapy Induce Synergistic Activation of Apoptosis Signaling. To determine whether combinations of ABT-737 and chemotherapy drugs induce synergistic activation of apoptosis signaling in HNSCC cells, we examined processing/activation of caspase-3 and cleavage of PARP, a caspase-3/-7 substrate (Fig. 3). For these experiments, UM-22A cells were treated for a shorter length of time (24 h) with 10 μM concentrations of each agent alone, or the combination of ABT-737 plus chemotherapy drug (each at 10 μM). Relatively little, if any, processing of procaspase-3 to active caspase-3 was detected in untreated cells or in cells treated with DMSO, ABT-737 alone, cisplatin alone, or etoposide alone (Fig. 3A). Likewise, only low levels of PARP cleavage were detected after treatment with single agents (Fig. 3B). By contrast, the combination of ABT-737 plus cisplatin, or ABT-737 plus etoposide, resulted in marked appearance of active caspase-3 and cleavage of PARP protein. The degree of caspase-3 activation and PARP cleavage in response to the combination treatments greatly exceeded the sum of these events occurring in cells treated with ABT-737 alone or chemotherapy alone. Thus, ABT-737 synergized with chemotherapy drugs to induce key apoptosis signaling events in HNSCC cells.
Fig. 3.
Synergistic activation of caspase signaling by the combination of ABT-737 plus chemotherapy. UM-22A cells were left untreated or were treated for 24 h with 0.1% DMSO, 10 μM ABT-737 alone, 10 μM cisplatin alone, 10 μM etoposide alone, the combination of ABT-737 plus cisplatin (10 μM each), or the combination of ABT-737 plus etoposide (10 μM each). After treatment, whole-cell lysates were prepared, and proteins (20 μg/lane) were electrophoresed on SDS-PAGE gels, transferred to nitrocellulose, and then probed with antibodies against caspase-3 (A) or PARP (B). Blots were stripped and reprobed with anti-β-actin to demonstrate equal protein loading. Similar results were seen in three independent experiments.
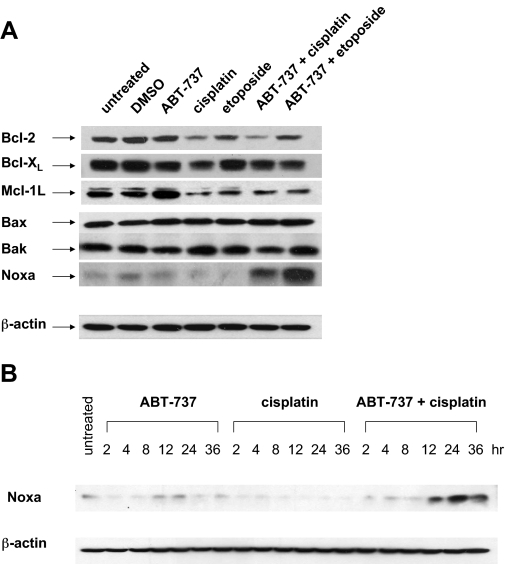
Noxa Is Potently Up-Regulated by ABT-737/Cisplatin and Mediates HNSCC Killing by This Combination. To investigate the mechanism whereby combinations of ABT-737 and chemotherapy drugs synergistically induce caspase activation and HNSCC cell death, we examined the impact of the agents, alone and in combination, on the expression levels of Bcl-2 family members (Fig. 4A). Treatment with ABT-737 alone did not substantially alter levels of the antiapoptotic proteins Bcl-2 and Bcl-XL or the proapoptotic proteins Bax, Bak, and Noxa but did cause modest induction of antiapoptotic Mcl-1L. Treatments incorporating cisplatin or etoposide, either alone or in combination with ABT-737, resulted in modest reduction in Bcl-2 and Bcl-XL and a very dramatic reduction in Mcl-1L. Overexpression of Mcl-1L in leukemia and solid tumor cell lines has been shown to correlate with resistance to ABT-737 (Konopleva et al., 2006; van Delft et al., 2006; Chen et al., 2007; Lin et al., 2007b; Tahir et al., 2007; Wesarg et al., 2007; Huang and Sinicrope, 2008). Thus, the ability of cisplatin or etoposide to promote down-regulation of Mcl-1L in HNSCC cells may serve to sensitize these cells to ABT-737.
Fig. 4.
Synergistic up-regulation of proapoptotic Noxa by the combination of ABT-737 plus chemotherapy. A, UM-22A cells were left untreated or were treated for 24 h with 0.1% DMSO, 10 μM ABT-737 alone, 10 μM cisplatin alone, 10 μM etoposide alone, ABT-737 plus cisplatin (10 μM each), or ABT-737 plus etoposide (10 μM each). After treatment, whole-cell lysates were prepared and subjected to immunoblotting for the indicated proteins. Similar results were seen in three independent experiments, and representative blots are shown. B, UM-22B cells exposed to ABT-737, cisplatin, or ABT-737 plus cisplatin for the indicated number of hours were subjected to immunoblotting for Noxa. The depicted results are representative of three independent experiments.
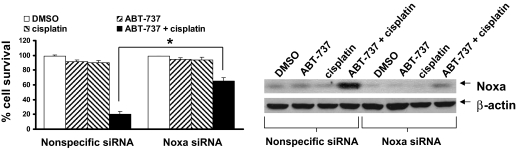
We also observed that the combination of ABT-737 plus cisplatin or ABT-737 plus etoposide resulted in striking up-regulation of Noxa relative to untreated cells or cells treated with single agents (Fig. 4A). Time-course analysis revealed that Noxa was induced as early as 12 h after cotreatment with ABT-737 and cisplatin (Fig. 4B). Noxa is a BH3 domain-only, proapoptotic, Bcl-2 family member and is known to potently bind and inhibit Mcl-1L (Chen et al., 2005). To determine the role of Noxa up-regulation in cell death mediated by this combination, we used siRNA treatment to prevent the up-regulation of Noxa. As shown in Fig. 5, Noxa was potently up-regulated in UM-22A cells transfected with a nonspecific siRNA and treated with ABT-737 plus cisplatin. By contrast, transfection with Noxa siRNA largely attenuated Noxa up-regulation by this combination. It is noteworthy that inhibition of Noxa up-regulation significantly inhibited cell death induced by ABT-737/cisplatin (p < 0.01). Likewise, Noxa siRNA also inhibited cell death resulting from the ABT-737/etoposide combination (Supplemental Fig. 4). These results indicate that up-regulation of Noxa is at least partially responsible for mediating the induction of HNSCC cell death by synergistic combinations of ABT-737 plus chemotherapy.
Fig. 5.
Inhibition of Noxa up-regulation attenuates killing by the ABT-737/cisplatin combination. UM-22A cells were seeded in 100-mm dishes, grown overnight, and then transfected for 24 h with nonspecific siRNA or Noxa siRNA, as described under Materials and Methods. The transfected cells were either then left untreated or treated for an additional 24 h with 0.1% DMSO, 10 μM ABT-737 alone, 10 μM cisplatin alone, or ABT-737 plus cisplatin (10 μM each). After treatment, cells were harvested and analyzed by immunoblotting to determine Noxa protein levels. In addition, trypan blue exclusion assays were used to determine the percentage of cell survival. The plotted data represent the average of means from three independent experiments, and error bars represent the S.E.M. P values were determined using one-way ANOVA followed by Tukey's multiple comparison test. *, P < 0.01 comparing ABT-737/cisplatin-treated cells transfected with Noxa siRNA versus nonspecific siRNA.
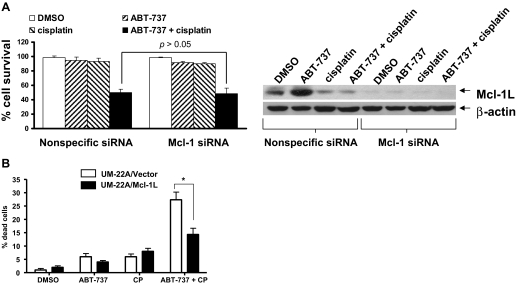
We next sought to determine whether siRNA-mediated down-regulation of Mcl-1L might serve to enhance ABT-737/cisplatin induction of cell death, because overexpression of this antiapoptotic protein is known to contribute to the resistance to ABT-737. As noted in Fig. 4A, treatments incorporating cisplatin already markedly reduced Mcl-1L levels. Transfection of cells with Mcl-1 siRNA served to further reduce the already low levels of Mcl-1L in cells treated with ABT-737 plus cisplatin (Fig. 6A). However, the further reduction of Mcl-1L levels using siRNA failed to enhance cell killing by the ABT-737/cisplatin combination (p > 0.05). This suggests that the down-regulation of Mcl-1L after treatment of HNSCC cells with chemotherapy may be sufficient to override any inhibitory effects of this protein on ABT-737 action. Alternatively, it is possible that Mcl-1L may not be a significant factor in regulating apoptosis induction by ABT-737 in HNSCC cells. To address this possibility, UM-22A cells stably transfected with an expression construct encoding Mcl-1L were treated for 48 h with the ABT-737/cisplatin combination followed by assessment of cell viability (Fig. 6B). The UM-22A/Mcl-1L cells exhibited enhanced resistance to ABT-737/cisplatin relative to vector-transfected control UM-22A cells (p < 0.01). These results supported our contention that endogenous Mcl-1L probably inhibits the action of monotherapeutic ABT-737 in HNSCC cells, but the endogenous protein is effectively removed in treatments incorporating chemotherapy.
Fig. 6.
Impact of Mcl-1 down-regulation or overexpression on killing by the ABT-737/cisplatin combination. A, UM-22A cells were seeded in 100-mm plates, grown overnight, and then transfected for 24 h with Mcl-1 siRNA or nonspecific siRNA. The cells were then left untreated or were treated for an additional 24 h with 0.1% DMSO, 5 μM ABT-737 alone, 5 μM cisplatin alone, or ABT-737 plus cisplatin (5 μM each). Immunoblotting was used to demonstrate the inhibition of Mcl-1L expression, and trypan blue exclusion assays were used to determine the percentage of cell survival. Columns represent the average of means from three independent experiments, and error bars represent S.E.M. P > 0.05 comparing ABT-737/cisplatin-treated cells transfected with Mcl-1 siRNA versus nonspecific siRNA. B, UM-22A cells stably transfected with Mcl-1L expression construct, or empty vector, were seeded in 48-well plates and treated for 48 h with DMSO, ABT-737, cisplatin, or ABT-737 plus cisplatin. After treatment, cells were detached with trypsin and combined with floating cells. Trypan blue exclusion assays were used to determine cell viabilities. *, P < 0.01. The experiment was performed three times with similar results each time.
Discussion
HNSCC is the sixth most common cancer in the United States (Jemal et al., 2007). An overall 5-year survival rate of roughly 50% places HNSCC among the most deadly of the major types of cancer (Forastiere et al., 2001; Gibson and Forastiere, 2006). Current chemotherapeutic options for HNSCC, including cisplatin, cause considerable adverse toxicities, and recurrent forms of the disease are typically highly resistant to conventional chemotherapy drugs (Gibson and Forastiere, 2006). Cetuximab, an epidermal growth factor receptor-blocking antibody, has been approved by the U.S. Food and Drug Administration for use in the treatment of HNSCC. This followed the demonstration that the addition of cetuximab to radiation therapy improved patient survival relative radiation treatment alone (Bonner et al., 2006). Although significant, the impact of cetuximab inclusion was modest in scope. Substantial improvements in therapeutic efficacies in HNSCC and reductions in the toxicities of conventional drug regimens are likely to be achieved with the identification of synergistic drug combinations. In this report, we demonstrate that ABT-737, an inhibitor of Bcl-XL and Bcl-2, potently synergizes with conventional chemotherapy drugs in the killing of HNSCC cells.
We have shown that the proteasome inhibitor bortezomib synergizes with cisplatin in HNSCC cells (Li et al., 2008). Bortezomib treatment causes multiple changes in the cell, including inhibition of nuclear factor-κB, a transcription factor that is hyperactivated and contributes to the survival of HNSCC cells (Ondrey et al., 1999; Van Waes et al., 2005). In addition, bortezomib alters the ratio of pro- and antiapoptotic Bcl-2 family members in the cell. Treatment of HNSCC cells with bortezomib induces the expression of Bik, Bim, and Noxa, natural cellular antagonists of Bcl-XL and Bcl-2 (Fribley et al., 2006; Li et al., 2008). Thus, bortezomib treatment provides a means for indirect targeting of Bcl-XL and Bcl-2 in HNSCC cells. In the current study, we sought to determine whether direct targeting of Bcl-XL and Bcl-2 using a highly specific small-molecule inhibitor would result in synergism with conventional chemotherapy drugs. Indeed, the combination of ABT-737 with cisplatin or etoposide resulted in synergistic induction of HNSCC cell death, as measured by trypan blue exclusion, Annexin V, and clonogenic survival assays (Figs. 1 and 2). Synergism between ABT-737 and chemotherapy was also evident on a molecular level, as assessed by caspase-3 activation and PARP cleavage (Fig. 3), hallmarks of apoptosis.
Our studies reveal an important role for Noxa in the synergism of ABT-737 and chemotherapy drugs against HNSCC cells. Noxa expression was markedly up-regulated after treatment with ABT-737 plus chemotherapy, and synergism by this combination was partially dependent on Noxa induction (Figs. 4 and 5). Similar up-regulation of Noxa by ABT-737 plus chemotherapy has been observed in H196 SCLC cells that are highly resistant to ABT-737 alone (Tahir et al., 2007). Enforced overexpression of Noxa in ABT-737-resistant H196 SCLC cells (Tahir et al., 2007), NCI-H1299 NSCLC cells (Wesarg et al., 2007), or mouse embryo fibroblasts (van Delft et al., 2006) has been shown to confer sensitivity to ABT-737. Meanwhile, studies using gene knockout mouse embryo fibroblasts have revealed that Bax and Bak are essential for ABT-737 activity (van Delft et al., 2006; Chen et al., 2007). The HNSCC cell lines used in our studies are known to harbor mutant p53, as is typical with most HNSCC cell lines and primary patient specimens. This raises the possibility that p73, or an alternative mechanism, may play a role in Noxa induction in HNSCC cells treated with the ABT-737/chemotherapy combination.
It is noteworthy that Noxa is known to bind with high affinity to Mcl-1L, but not Bcl-XL or Bcl-2 (Chen et al., 2005). This suggests that Noxa up-regulation in response to treatment with ABT-737 plus chemotherapy may serve to functionally inactivate Mcl-1L, causing displacement of proapoptotic proteins bound to the Mcl-1L protein. The displaced proapoptotic proteins may then bind and directly activate Bax and Bak, as has been suggested by a model of direct Bax/Bak activation (Letai et al., 2002; Kuwana et al., 2005). Alternatively, proapoptotic proteins displaced from Mcl-1L may bind to Bcl-XL and Bcl-2, causing displacement of Bax and Bak, as has been suggested in a model of indirect Bax/Bak activation (Chen et al., 2005; Willis et al., 2007).
Resistance to ABT-737 has been correlated with overexpression of Mcl-1L, which does not bind this compound (Oltersdorf et al., 2005; Tahir et al., 2007). Down-regulation of Mcl-1L has been shown to confer sensitivity to ABT-737 (Konopleva et al., 2006; van Delft et al., 2006; Chen et al., 2007; Lin et al., 2007b; Tahir et al., 2007; Wesarg et al., 2007; Huang and Sinicrope, 2008). As noted above, the marked up-regulation of Noxa in HNSCC cells treated with ABT-737 plus chemotherapy probably serves to functionally inactivate Mcl-1L and promote synergism by this combination. However, we also discovered that chemotherapy alone caused substantial reduction in Mcl-1L levels, independent of Noxa up-regulation (Fig. 4). Thus, in HNSCC cells, dual repression of cellular Mcl-1L, via Noxa up-regulation and chemotherapy-induced down-regulation of the Mcl-1L protein, may explain the highly potent synergism between ABT-737 and conventional chemotherapy drugs in promoting cell death. This contention is supported by our findings that further down-regulation of endogenous Mcl-1L levels using siRNA failed to enhance killing by the ABT-737/cisplatin combination (Fig. 6A), whereas enforced overexpression of Mcl-1L inhibited cell death by this combination (Fig. 6B). These findings suggest that overexpression of endogenous Mcl-1L in HNSCC tumors will not prove an insurmountable impediment to therapies combining chemotherapy drugs and ABT-737 or next-generation derivatives, including ABT-263 (Tse et al., 2008).
Supplementary Material
Acknowledgments
We gratefully acknowledge Abbott Laboratories for providing the ABT-737 and A-793844 compounds.
This work was supported by the National Institutes of Health National Cancer Institute [Grant P50-CA097190-01A1].
Y.Z. and R.L. contributed equally to this work.
ABBREVIATIONS: HNSCC, head and neck squamous cell carcinoma; SCLC, small-cell lung cancer; siRNA, small interfering RNA; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; PBS, phosphate-buffered saline; PARP, poly(ADP-ribose) polymerase; DMSO, dimethyl sulfoxide; MEF, mouse embryo fibroblast; CI, combination index; PAGE, polyacrylamide gel electrophoresis; TBST, Tris-buffered saline/Tween 20; ANOVA, analysis of variance.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee JS, Chen J, Wang S, Bradford CR, and Carey TE (2005) Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther 41096 -1104. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. New Engl J Med 354567 -578. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, Schlossman R, Ghobrial I, Raje N, Munshi N, et al. (2007) A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene 262374 -2380. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, and Huang DC (2005) Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17393 -403. [DOI] [PubMed] [Google Scholar]
- Chen S, Dai Y, Harada H, Dent P, and Grant S (2007) Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res 67782 -791. [DOI] [PubMed] [Google Scholar]
- Chou TC and Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22 27-55. [DOI] [PubMed] [Google Scholar]
- Danial NN and Korsmeyer SJ (2004) Cell death: critical control points. Cell 116205 -219. [DOI] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, and Letai A (2007) Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest 117112 -121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenning SD, Marcovitch AJ, Johnson DE, Melhem MF, Tweardy DJ, and Grandis JR (1998) Bcl-2 but not Bax expression is associated with apoptosis in normal and transformed squamous epithelium. Clin Cancer Res 42913 -2921. [PubMed] [Google Scholar]
- Forastiere A, Koch W, Trotti A, and Sidransky D (2001) Head and neck cancer. New Engl J Med 3451890 -1900. [DOI] [PubMed] [Google Scholar]
- Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, Hale TJ, Soengas MS, Kaufman RJ, and Wang CY (2006) Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem 28131440 -31447. [DOI] [PubMed] [Google Scholar]
- Gibson MK and Forastiere AA (2006) Reassessment of the role of induction chemotherapy for head and neck cancer. Lancet Oncol 7565 -574. [DOI] [PubMed] [Google Scholar]
- Huang S and Sinicrope FA (2008) BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res 682944 -2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, and Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 5743 -66. [DOI] [PubMed] [Google Scholar]
- Kang MH, Kang YH, Szymanska B, Wilczynska-Kalak U, Sheard MA, Harned TM, Lock RB, and Reynolds CP (2007) Activity of vincristine, L-ASP, and dexamethasone against acute lymphoblastic leukemia is enhanced by the BH3-mimetic ABT-737 in vitro and in vivo. Blood 1102057 -2066. [DOI] [PubMed] [Google Scholar]
- Kang MH, Wan Z, Kang YH, Sposto R, and Reynolds CP (2008) Mechanism of synergy of N-(4-hydroxyphenyl)retinamide and ABT-737 in acute lymphoblastic leukemia cell lines: Mcl-1 inactivation. J Natl Cancer Inst 100580 -595. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al. (2006) Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10375 -388. [DOI] [PubMed] [Google Scholar]
- Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, Kimura S, Ottmann OG, Druker BJ, Villunger A, et al. (2006) Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci U S A 10314907 -14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, and Newmeyer DD (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17525 -535. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, and Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2183 -192. [DOI] [PubMed] [Google Scholar]
- Li C, Li R, Grandis JR, and Johnson DE (2008) Bortezomib induces apoptosis via Bim and Bik up-regulation and synergizes with cisplatin in the killing of head and neck squamous cell carcinoma cells. Mol Cancer Ther 71647 -1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Boehm AL, Miranda MB, Shangary S, Grandis JR, and Johnson DE (2007) Targeting antiapoptotic Bcl-2 family members with cell-permeable BH3 peptides induces apoptosis signaling and death in head and neck squamous cell carcinoma cells. Neoplasia 9 801-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, Ferris RL, and Lai SY (2007a) Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck 29163 -188. [DOI] [PubMed] [Google Scholar]
- Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, Fesik SW, and Shen Y (2007b) `Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-X(L) inhibitor ABT-737. Oncogene 263972 -3979. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435677 -681. [DOI] [PubMed] [Google Scholar]
- Ondrey FG, Dong G, Sunwoo J, Chen Z, Wolf JS, Crowl-Bancroft CV, Mukaida N, and Van Waes C (1999) Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinog 26119 -129. [DOI] [PubMed] [Google Scholar]
- Paoluzzi L, Gonen M, Bhagat G, Furman RR, Gardner JR, Scotto L, Gueorguiev VD, Heaney ML, Manova K, and O'Connor OA (2008) The BH3-only mimetic ABT-737 synergizes the anti-neoplastic activity of proteasome inhibitors in lymphoid malignancies. Blood 1122906 -2916. [DOI] [PubMed] [Google Scholar]
- Sharma H, Sen S, Lo Muzio L, Mariggiò A, and Singh N (2005) Antisense-mediated down-regulation of anti-apoptotic proteins induces apoptosis and sensitizes head and neck squamous cell carcinoma cells to chemotherapy. Cancer Biol Ther 4 720-727. [DOI] [PubMed] [Google Scholar]
- Song JH, Kandasamy K, and Kraft AS (2008) ABT-737 induces expression of the death receptor 5 and sensitizes human cancer cells to TRAIL-induced apoptosis. J Biol Chem 28325003 -25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, Warner RB, Ng SC, Fesik SW, Elmore SW, et al. (2007) Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res 671176 -1183. [DOI] [PubMed] [Google Scholar]
- Trask DK, Wolf GT, Bradford CR, Fisher SG, Devaney K, Johnson M, Singleton T, and Wicha M (2002) Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope 112638 -644. [DOI] [PubMed] [Google Scholar]
- Trudel S, Stewart AK, Li Z, Shu Y, Liang SB, Trieu Y, Reece D, Paterson J, Wang D, and Wen XY (2007) The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res 13621 -629. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 683421 -3428. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10389 -399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes C, Chang AA, Lebowitz PF, Druzgal CH, Chen Z, Elsayed YA, Sunwoo JB, Rudy SF, Morris JC, Mitchell JB, et al. (2005) Inhibition of nuclear factor-kappaB and target genes during combined therapy with proteasome inhibitor bortezomib and reirradiation in patients with recurrent head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 631400 -1412. [DOI] [PubMed] [Google Scholar]
- Wesarg E, Hoffarth S, Wiewrodt R, Kröll M, Biesterfeld S, Huber C, and Schuler M (2007) Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer 1212387 -2394. [DOI] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315856 -859. [DOI] [PubMed] [Google Scholar]
- Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB, and Richardson A (2007) The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res 137191 -7198. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Wang SJ, Henson BS, Wang S, Griffith KA, Kumar B, Chen J, Carey TE, Bradford CR, and D'Silva NJ (2006) (-)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia 8 163-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai D, Jin C, Satterthwait AC, and Reed JC (2006) Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ 131419 -1421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.