Abstract
Activation of G protein-coupled α2 adrenergic receptors (ARs) inhibits epileptiform activity in the hippocampal CA3 region. The specific mechanism underlying this action is unclear. This study investigated which subtype(s) of α2ARs and G proteins (Gαo or Gαi) are involved in this response using recordings of mouse hippocampal CA3 epileptiform bursts. Application of epinephrine (EPI) or norepinephrine (NE) reduced the frequency of bursts in a concentration-dependent manner: (-)EPI > (-)NE >>> (+)NE. To identify the α2AR subtype involved, equilibrium dissociation constants (pKb) were determined for the selective αAR antagonists atipamezole (8.79), rauwolscine (7.75), 2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride (WB-4101; 6.87), and prazosin (5.71). Calculated pKb values correlated best with affinities determined previously for the mouse α2AAR subtype (r = 0.98, slope = 1.07). Furthermore, the inhibitory effects of EPI were lost in hippocampal slices from α2AAR-but not α2CAR-knockout mice. Pretreatment with pertussis toxin also reduced the EPI-mediated inhibition of epileptiform bursts. Finally, using knock-in mice with point mutations that disrupt regulator of G protein signaling (RGS) binding to Gα subunits to enhance signaling by that G protein, the EPI-mediated inhibition of bursts was significantly more potent in slices from RGS-insensitive GαoG184S heterozygous (Gαo+/GS) mice compared with either Gαi2G184S heterozygous (Gαi2+/GS) or control mice (EC50 = 2.5 versus 19 and 23 nM, respectively). Together, these findings indicate that the inhibitory effect of EPI on hippocampal CA3 epileptiform activity uses an α2AAR/Gαo protein-mediated pathway under strong inhibitory control by RGS proteins. This suggests a possible role for RGS inhibitors or selective α2AAR agonists as a novel antiepileptic drug therapy.
The noradrenergic system modulates many physiological and pathological processes within the central nervous system (CNS). Noradrenergic neurons regulate attention and arousal, sleep, and learning and memory (Pupo and Minneman, 2001) and seem to attenuate epileptic activity (Giorgi et al., 2004). The hippocampus receives substantial noradrenergic innervation in all regions, including the cornu ammonis 3 (CA3), a region essential for many cognitive functions such as spatial pattern recognition, novelty detection, and short-term memory (Kesner et al., 2004). The CA3 region possesses a dense recurrent network of excitatory axons between the pyramidal neurons that may be crucial for performing these cognitive functions but also makes the region vulnerable to overexcitation (Schwartzkroin, 1986). This region has one of the lowest seizure thresholds and is often involved in temporal lobe epilepsy, the most common human epileptic syndrome. It is clear that thoroughly delineating the inhibitory and excitatory aspects of this region is critical to understanding CNS function and dysfunction and to designing targeted therapeutic approaches.
Norepinephrine (NE) is the major neurotransmitter released by noradrenergic neurons and modulates several CA3 processes. NE has been shown to facilitate long-term potentiation, which is involved in memory formation, and antiepileptic activity (Giorgi et al., 2004) in the hippocampal CA3 region. Increased NE release in the brain has been shown to inhibit epileptiform activity, whereas reduced NE levels seem to increase seizure susceptibility (Weinshenker and Szot, 2002). Although the mechanism by which NE mediates these effects is still unclear, NE may both potentiate memory and inhibit the overexcitation associated with seizures (Jurgens et al., 2005) through the distinct and diverse expression of postsynaptic receptor subtypes (Hillman et al., 2005).
Adrenergic receptors (ARs) are divided into three major classes, each of which has a unique G protein pairing resulting in diverse physiological actions (Pupo and Minneman, 2001). Studies have suggested that βARs mediate the enhancement of long-term potentiation (Hopkins and Johnston, 1988) and memory (Devauges and Sara, 1991), whereas the antiepileptogenic actions of NE may involve α2AR activation (Giorgi et al., 2004). Pharmacological and molecular cloning studies have revealed the existence of three α2AR subtypes denoted α2A, α2B, and α2C (Bylund et al., 1994). We recently showed that NE inhibits rat hippocampal CA3 epileptiform bursts through α2AAR activation (Jurgens et al., 2007). Furthermore, specific activation of α2AARs attenuates seizures in mice elicited by chemoconvulsants (Szot et al., 2004).
ARs are part of a large and diverse family of GTP-binding (G) protein-coupled receptors (GPCRs). The extracellular signals received by GPCRs are relayed by heterotrimeric G proteins (Gαβγ) to effector enzymes and channels within the cell (Gilman, 1987). The conversion of GDP-bound inactive Gαβγ heterotrimer into activated Gα-GTP and G-βγ subunits is achieved by catalyzing nucleotide exchange on Gα subunits via GPCR activation. Once released, the subunits interact with a variety of downstream effectors in an intracellular signaling cascade (Offermanns, 2003). Deactivation of the G protein is achieved by hydrolysis of the Gα-bound GTP, a step that controls the duration of the signal. The GDP-bound Gα subunit will then reform with the G-βγ heterodimer, forming an inactive trimer once again.
For some Gα families (Gi/o and Gq), the rate of GTP hydrolysis can be enhanced by regulator of G protein signaling (RGS) proteins (Berman et al., 1996; Watson et al., 1996). Consequently, RGS proteins are negative modulators of signaling through receptors coupled to the Gi/o and Gq family of G proteins (Clark et al., 2008) and enhance intrinsic GTPase activity of the GTP-bound Gα subunits. This GTPase acceleration attenuates G protein signaling by resetting the Gα subunit to its inactive conformation (Hollinger and Hepler, 2002). Interfering with the activity of RGS proteins allows the Gα subunit to remain active for a longer time, effectively enhancing the signal (Lan et al., 1998; Clark et al., 2003). Therapeutic agents targeting RGS proteins could be used to enhance the effect of current GPCR-mediated drug therapies by reducing the required therapeutic dose while increasing the regional agonist specificity, thereby decreasing the possibility of side effects (Zhong and Neubig, 2001; Neubig and Siderovski, 2002).
This study investigated the role of α2ARs and RGS proteins in the antiepileptic actions of NE using field recordings of hippocampal CA3 epileptiform burst activity and a combination of selective blockers for the AR and G protein subtypes, transgenic α2AR knockout, and RGS-insensitive Gα subunit knock-in mice. Delineating which α2AR and G protein subtypes are involved in attenuating hippocampal epileptiform activity will help to further elucidate the mechanism by which NE inhibits epileptogenesis and may suggest potential targets for antiepileptic drug therapy.
Materials and Methods
Reagents
Atipamezole was made by Orion Corporation (Espoo, Finland). Desipramine, l-(-)-epinephrine (+)-bitartrate, l-(-)-norepinephrine (+)-bitartrate, d-(+)-norepinephrine (-)-bitartrate, oxymetazoline hydrochloride, pertussis toxin, picrotoxin, pindolol, and timolol maleate were obtained from Sigma-Aldrich (St. Louis, MO). Prazosin hydrochloride, rauwolscine hydrochloride, and WB-4101 were acquired from Tocris Cookson Inc. (Ellisville, MO). All chemical reagents used to make the artificial cerebrospinal fluid (ACSF) were of biological grade from J. T. Baker, Inc. (Phillipsburg, NJ) or Thermo Fisher Scientific (Waltham, MA). Isoflurane was purchased from Abbott Diagnostics (Chicago, IL).
Animals
C57BL/6J mice of both genders were used in the present study. Mice were housed two to four per cage (size 11.5 × 7 inches) under standard laboratory conditions on a 12-h light/dark cycle (lights on at 7:00 AM) in rooms maintained at a temperature of ∼22°C with a relative humidity of ∼55%. Water and dried laboratory food (Teklad Global 18% Protein Rodent Diet; Harlan Teklad, Madison, WI) were provided ad libitum. Mice were allowed to acclimate for at least 4 days after arrival (see below). All protocols described were approved by the Institutional Animal Care and Use Committee of Emory University (Atlanta, GA), the University of Michigan (Ann Arbor, MI), and the University of North Dakota (Grand Forks, ND) in accordance with National Institute of Health guidelines (Institute of Laboratory Animal Resources, 1996) and meet the guidelines of the American Association for Accreditation of Laboratory Animal Care.
Transgenic Mice
Generation of α2AAR- and α2CAR-Knockout Mice. α2A(-/-) [α2A/α2C; (-/-)/(+/+)] and α2C(-/-) [α2A/α2C; (+/+)/(-/-)] mice, maintained on a pure C57BL/6J background, were generated at Emory University using heterozygous α2C(+/-) and α2AC(+/-) mice obtained from Brian K. Kobilka (Stanford University, Stanford, CA). Genotypes were confirmed by polymerase chain reaction. All mice were reared in a specific pathogen-free facility at Emory University with a 12-h light/dark cycle (lights on at 7:00 AM) and were shipped to the University of North Dakota at age 2 to 5 months. Control animals used in these studies were wild-type (WT) C57BL/6J [α2A/α2C;(+/+)/(+/+)] mice purchased from The Jackson Laboratory (Bar Harbor, ME).
Generation of GαoG184S Heterozygous (Gαo+/GS) Knock-In Mice. The original GαoG184S ES cell line, described in Fu et al. (2004, 2006), was developed in a 129-D3 ES cell background, which never went germline. Consequently, the GαoG184S mouse strain was constructed from a 129-CJ7 ES line using methods similar to those previously reported for the Gαi2G184S strain (Fu et al., 2006; Huang et al., 2006). Specifically, we prepared a targeting construct by restriction digestion to obtain DNA fragments of the mouse Gnao gene from a Bac clone derived from 129-CJ7 DNA Bac library (ResGen; Invitrogen, Carlsbad, CA). Using those fragments, a targeting construct was prepared in the TKLNL vector (Mortensen et al., 1992). First the mutant Gαo exon 5 was produced by mutating the sequence AAAACAACTGGCATCGTAGAAA to AAAACAACTAGTATCGTAGAAA. The bases in boldface type indicate the changed codon (Gly184 to Ser184), and the underline portion designates the location of the resulting diagnostic SpeI restriction site. This mutated exon 5 and additional 5′ genomic sequence to form the “left” homology arm was cloned into TKLNL to introduce the loxP-flanked neo marker after exon 5, then the right arm genomic fragment from exons 6 to 8 was cloned 3′ of the loxP cassette in a manner similar to that for preparing the Gαi2G184S targeting vector (Fu et al., 2006; Huang et al., 2006). CJ7 ES cells were electroporated with the targeting vector, and homologous recombinants were isolated. Targeted CJ7 ES cells were microinjected into C57BL/6NCrl × (C57BL/6J × DBA/2J)F1 mouse blastocysts to generate ES cell-mouse chimeras. After identification of chimeric offspring, the mice were backcrossed onto a CJ7BL/6J background for at least four generations. Only heterozygous offspring of crosses between Gαo(+/G184S) male and C57BL/6J female mice (N4-N5) were used in these studies because homozygous Gαo(G184S/G184S) offspring from heterozygous × heterozygous crosses were not viable. Control animals used in these studies were WT littermates [(+/+)] of the GαoG184S heterozygous (Gαo+/GS) mice.
Generation of Gαi2G184S Heterozygous (Gαi2+/GS) Knock-In Mice. Gαi2G184S heterozygous (Gαi2+/GS) knock-in mice, maintained on a pure C57BL/6J background (>10 generations), were generated at the University of Michigan, Ann Arbor, as described previously (Fu et al., 2006). All genotypes were confirmed by polymerase chain reaction. Control animals used in these studies were WT C57BL/6J mice from The Jackson Laboratory. Both the GαoG184S and Gαi2G184S heterozygous (+/GS) knock-in mice were reared at the University of Michigan and were confirmed to be pathogen-free before their shipping to the University of North Dakota at age 1 to 3 months.
Hippocampal Slice Preparation
After being deeply anesthetized with isoflurane, mice weighing 16 to 27 g were decapitated, and their brains were rapidly removed. The hippocampi were then quickly dissected from each hemisphere and placed into an ice-cold Ringer solution containing 110 mM choline chloride, 2.5 mM KCl, 7 mM MgSO4, 0.5 mM CaCl2, 1.25 mM NaH2PO4, 25 mM NaHCO3, 25 mM d-glucose, 11.6 mM sodium ascorbate, and 3.1 mM sodium pyruvate, saturated with 95% O2/5% CO2. Using a conventional tissue sectioning apparatus (Stoelting, Wood Dale, IL), the hippocampi were sliced transversely into 500-μm thick sections and transferred to ACSF consisting of 119 mM NaCl, 5 mM KCl, 1.3 mM MgSO4, 2.5 mM CaCl2, 1 mM NaH2PO4, 26.2 mM NaHCO3, and 11 mM d-glucose, which was continually aerated with 95% O2/5% CO2. The slices were incubated at 32 ± 1°C for 30 min, then transferred to room temperature (22 ± 1°C) and allowed to recover for at least 30 min before experimentation.
Electrophysiological Recordings
A single slice was transferred to the recording chamber, where it was submerged and superfused continuously at a rate of at least 4 ml/min with ACSF at room temperature. Glass microelectrodes were made using a vertical two-stage puller (PP-830; Narishige, Tokyo, Japan). Extracellular field potentials were recorded using microelectrodes filled with 3 M NaCl placed in the stratum pyramidale of the CA3 region of the hippocampus using an SZ-61 stereo microscope (Olympus, Melville, NY). Potentials were detected using either an Axoclamp 2B (Molecular Devices, Sunnyvale, CA) or BVC-700A (Dagan, Minneapolis, MN) microelectrode amplifier, amplified using a Brownlee 440 signal conditioner (Brownlee Precision, San Jose, CA), digitized with a Digidata 1322A analog-to-digital converter (Molecular Devices), and recorded using Axoscope 9.0 software (Molecular Devices).
Generation of Epileptiform Activity
Hippocampal CA3 pyramidal neurons are prone to spontaneously firing epileptiform bursts partly as a result of their extensive associational connections (Schwartzkroin, 1986). This activity was easily generated by superfusing the slice with ACSF containing 100 μM picrotoxin, a GABAA receptor blocker, to attenuate synaptic inhibition. If no burst discharges were seen after 30 min of superfusion, the slice was determined to be unresponsive and discarded. Once continuous spontaneous epileptiform burst discharges were evident, 30 min of baseline data were recorded before any exposure to an AR agonist. The ACSF also contained 0.5 μM desipramine to block NE transporters [i.e., potential reuptake of the catecholamines epinephrine (EPI) and NE] and 30 μM timolol to block any βAR-mediated excitatory effects (Jurgens et al., 2005), as well as any applicable αAR antagonist. Before being used, each AR antagonist was tested to ensure that it possessed no independent effects. Preliminary experiments also confirmed that each AR agonist concentration caused its maximal effect during an 8-min application (data not shown). Because the α2AR antagonist rauwolscine also has potent serotonergic 5-hydroxytryptamine1A receptor-mediated agonist activity (Newman-Tancredi et al., 1998), we substituted 3 μM pindolol (which blocks both βAR and 5-hydroxytryptamine1A receptors) for timolol (which only blocks βARs) when using this particular α2AR antagonist.
Data Analysis
Epileptiform burst discharge frequencies were visualized in real time (Fig. 1A) while being recorded for subsequent analysis. Postexperiment analysis was completed using Mini Analysis 6.0 software (Synaptosoft, Decatur, GA). The last interval correlating to each agonist concentration was noted, the baseline frequency was subtracted, and that value was used to plot a concentration-response expressed as a percentage of maximal response. Frequency versus agonist concentration data were then entered into Prism 5.0 software (GraphPad Software Inc., San Diego, CA), and concentration-response curves were constructed using a nonlinear least-squares curve-fitting method. Each curve was fitted with a standard (slope = unity) or variable slope, and the best fit was determined using an F test with a value of p < 0.05. The calculated EC50 value was used as a measurement of agonist potency. Significance between groups was compared statistically using the Student's t test (p < 0.05).
Fig. 1.
Effects of EPI on mouse hippocampal CA3 epileptiform activity. A, continuous 150-s long chart recordings of burst discharges recorded in the hippocampal CA3 region of brain slices from WT mice. Epileptiform burst discharges were elicited by including 100 μM concentration of the GABAA receptor blocker picrotoxin in the perfusing ACSF containing 30 μM timolol and 0.5 μM desipramine. Under these conditions, bath application of EPI reduced burst frequency in a concentration-dependent manner from 10 bursts (0.067 Hz) in control Ringer solution to 7 (0.047 Hz) in 30 nM EPI, 3 (0.020 Hz) in 300 nM EPI, and 1 (0.007 Hz) in 3 μM EPI. B, frequency histogram of the number of burst discharges versus time of EPI application. Each bin represents the frequency averaged over an approximately 150-s epoch. Increasing concentrations of EPI were applied to the bath for the 8-min periods indicated. Inset, concentration-response curve derived from the frequency histogram. Data points were plotted as the percentage of maximal inhibition (decrease in epileptiform burst frequency), and the curve was constructed using a nonlinear least-squares curve-fitting method. For this experiment, the concentration-response curve was fit best by a nonvariable sigmoidal model with a calculated EC50 value for EPI of 48 nM.
Schild analysis was used to determine the apparent equilibrium dissociation constants (pKb) for selective αAR antagonists (Arunlakshana and Schild, 1959). For each experiment, cumulative concentration-response curves were performed in adjacent slices from the same mouse (one dose-response curve per slice). Dose ratios of EC50 values were calculated in the presence and absence of a selective α2AR antagonist and Schild plots constructed by graphing the log of the dose ratio - 1 versus the log of the antagonist concentration. Linear regression analysis of these points was used to determine the slope and x-intercept. Schild regression slopes are given as mean ± S.E. and were considered to be nonunity if the 95% confidence interval did not include the value of 1. The pKb values of αAR antagonists causing competitive inhibition of the EPI-mediated reduction in burst frequencies were calculated from Schild regression x-intercepts. Differences in pKb values and Schild regression slopes were determined by analysis of covariance with a p < 0.05 level of probability accepted as significant. EC50 and pKb values are expressed as the mean ± S.E. for n experiments.
Results
Effects of EPI and NE on Mouse CA3 Epileptiform Activity. We first examined the effects of EPI on mouse CA3 epileptiform burst discharges in the presence of timolol (βAR blockade) to elucidate the action of αAR activation on hippocampal activity. Picrotoxin-induced epileptiform burst discharges are shown in Fig. 1, and their frequency is reduced by application of EPI in a concentration-dependent manner. For this particular experiment, the EC50 value calculated from nonlinear regression analysis was 48 nM. Our previous work in rats has shown that this effect is most likely mediated by an α2AR in the CA3 region of the hippocampus (Jurgens et al., 2007). As illustrated in Fig. 2, the rank order of potency of the three AR agonists tested in this manner revealed that (-)EPI (31 ± 8.1 nM, n = 45 slices) > (-)NE (150 ± 45 nM, n = 15 slices) >>> (+)NE (4700 ± 3300 nM, n = 10 slices), which is consistent with our previous results (Jurgens et al., 2007) and the order expected for αARs.
Fig. 2.
Potency for EPI and NE inhibiting hippocampal CA3 epileptiform burst activity. Extracellular field potential recordings were used to generate concentration-response curves using increasing amounts of (-)EPI (▪), (-)NE (•), and (+)NE (○) in the presence of 100 μM picrotoxin, 30 μM timolol, and 0.5 μM desipramine. There was a significant difference in the potencies (EC50 values) calculated for (-)EPI (31 ± 8.1 nM, n = 45 slices from 18 animals), (-)NE (150 ± 45 nM, n = 15 slices from 7 animals), and (+)NE (○) (4700 ± 3300 nM, n = 10 slices from 4 animals). Concentration-response curves for each agonist were plotted as a percentage of decrease (reduction) in epileptiform burst frequency. Each individual experiment best fit to a nonvariable sigmoidal curve. There was no significant difference in the efficacy of (-)EPI (68 ± 2.6%), (-)NE (67 ± 3.7%), and (+)NE (58 ± 7.2%) at reducing epileptiform activity.
Effects of the Selective α2AR Antagonist Atipamezole and Subtype-Selective α2AR Antagonists on the EPI-Mediated Decrease in Burst Discharge Frequencies. Functional determination of equilibrium dissociation constant (Kb) value for selective αAR antagonists was used to characterize the type of αAR mediating decreased burst frequency in the hippocampal CA3 region. Pretreatment of hippocampal slices with 3, 10, and 30 nM atipamezole produced 2-, 6-, and 22-fold parallel rightward shifts of the fitted EPI concentration-response curve (Fig. 3A). The pKb of 8.79 (n = 5) for atipamezole (Fig. 3B) was similar to previously published binding pKi values for the mouse α2ARs (Link et al., 1992; Chruscinski et al., 1992; see also Table 1). This result suggests that the response is mediated by an α2AR.
Fig. 3.
Schild regression analysis using the selective α2AR antagonist atipamezole. A, consecutive EPI concentration-response curves demonstrate a concentration-dependent effect of the selective α2AR antagonist, atipamezole, on the EPI-mediated inhibition of hippocampal CA3 epileptiform activity in brain slices from WT mice. Pretreatment with 3 nM (○), 10 nM (▪), and 30 nM (□) concentrations of this antagonist produced consecutive parallel right-ward shifts of the EPI curve that were significantly different from control (•) (EC50 = 76 ± 3, 218 ± 30, and 762 ± 274, respectively, versus 34 ± 12 nM for control). B, using dose ratios calculated from individual experiments illustrated in A, a Schild plot was created generating a regression slope equaling 1.06 ± 0.12 and an x-intercept correlating to a pKb value of 8.79, n = 5 animals (see Table 1).
TABLE 1.
Comparisons of experimental functional pKb values with binding affinity pKi values for selective αAR antagonists for mouse α2AR subtypes
pKb values represent the negative logarithm10 of the Kb and are expressed as the mean. Schild regression slopes are expressed as the mean slope ± S.E. and were determined in three to five separate experiments using brain slices from WT mice. Reported pKi values are from binding affinity studies using recombinant mouse α2AR clones expressed in COS-7 cells. pKb value was calculated using a single 10 μM concentration of JP-1302.
Subtype-selective antagonists were then used to determine the specific subtype of α2AR mediating burst frequency reduction in the mouse hippocampal CA3 region. Apparent pKb values of subtype-selective α2AR competitive antagonists were determined using Schild regression analysis. Slices pretreated with either prazosin (α2BAR-selective), rauwolscine (α2CAR-selective), or WB-4101 (α2CAR-selective) produced parallel rightward shifts of the fitted EPI concentration-response curve in all instances (data not shown). For each of these selective α2AR antagonists, the slope of the regression line was close to the value of unity. The logs of the equilibrium dissociation constants (pKb) calculated for these α2AR antagonists were as follows: rauwolscine (7.75, n = 3), WB-4101 (6.87, n = 3), and prazosin (5.71, n = 4) (Table 1).
α2AR Antagonist Functional pKb Estimates Correlate to α2AAR pKi Values. A method often used to compare equilibrium dissociation constants of many receptor antagonists is to correlate pKb values with previously published pKi values (Bylund, 1988). Both the correlation coefficient and slope of the correlation line should be close to unity if the calculated functional values correspond to the published binding constants for a specific receptor. Illustrated in Fig. 4 are the correlations between the pKb values determined for the selective αAR antagonists used in this study and the previously published pKi values of these AR antagonists for each mouse α2AR subtype (Table 1). For the mouse α2AAR subtype, a very high correlation coefficient (r = 0.98) along with a slope similar to unity (slope = 1.07) were calculated for our experimental pKb values compared with published binding affinity values (Fig. 4A). In contrast, for the mouse α2BAR, a poor correlation coefficient (r = 0.88) and low slope (slope = 0.40) were observed when comparing our experimental pKb values with previously published pKi values (Fig. 4B). Likewise for the mouse α2CAR, a poor correlation coefficient (r = 0.89) and low slope (slope = 0.63) were seen (Fig. 4C). These results suggest that the α2AAR is the predominant subtype mediating the antiepileptic action of EPI in mouse hippocampus.
Fig. 4.
Correlation between the functional affinity estimates (pKb) to the equilibrium dissociation constants (pKi) for various selective α2AR antagonists. Using the pKb and pKi values from Table 1, correlation analyses were performed for the α2AAR (A), the α2BAR (B), and the α2CAR (C).
Effects of EPI on Epileptiform Activity in Slices from α2AAR- and α2CAR-Knockout Mice. To confirm our pharmacological results, we examined the effects of EPI on hippocampal CA3 epileptiform activity in α2AAR- and α2CAR-knockout (KO) mice. As illustrated in Fig. 5, EPI was applied in increasing concentrations to hippocampal brain slices prepared from either α2AAR- or α2CAR-KO mice. The potency of EPI in the α2CAR-KO mouse line (37 ± 12 nM, n = 15) fit best with a unity-slope sigmoidal model and was not significantly different from the WT mice (see also Fig. 2). In contrast, the effects of EPI were largely abolished in brain slices from α2AAR-KO mice with a maximum effect of less than 10% inhibition. These results demonstrate that the α2AAR is the predominant receptor subtype mediating the inhibitory effects of EPI in the mouse hippocampus.
Fig. 5.
Effects of EPI on hippocampal CA3 epileptiform activity in brain slices from α2AAR- and α2CAR-KO mice. Extracellular field potential recordings of epileptiform burst frequency were used to generate concentration-response curves using increasing amounts of EPI in the presence of 100 μM picrotoxin, 30 μM timolol, and 0.5 μM desipramine. Concentration-response curves for EPI were plotted as a percentage of decrease (reduction) in epileptiform burst frequency. For the α2AAR-KO mice, the mean concentration-response curve for 41 slices from 12 animals was fit best by a linear regression line. In contrast, the mean concentration-response curve for 39 brain slices from 15 α2CAR-KO mice was fit best by a nonvariable sigmoidal model with a calculated EC50 value of 37 ± 12 nM, which was not significantly different from the potency of 31 ± 8.1 nM calculated for EPI in slices from WT mice (see Fig. 2). The efficacy of EPI at reducing the frequency of epileptiform bursts in slices from α2CAR-KO mice was 64 ± 3.9%, which was significantly different from the 8.7 ± 3.3% inhibition for EPI in slices from α2AAR-KO mice.
Effects of Subtype-Selective α2AAR Antagonist Oxymetazoline on the EPI-Mediated Decrease in Burst Discharge Frequencies in α2CAR-KO Mice. To further evaluate a potential role for α2BARs and confirm that the response was primarily an α2AAR response, the selective α2AAR antagonist oxymetazoline was used in brain slices made from α2CAR-KO mice. α2CAR-KO mouse slices that had been pretreated with 100, 300, and 1000 nM oxymetazoline produced 6-, 22-, and 70-fold parallel rightward shifts of the fitted EPI concentration-response curve (Fig. 6A). The Schild regression slope was 1.16 ± 0.12 and the x-intercept correlating to a pKb value of 7.50 (n = 7 animals) (Fig. 6B). The mouse α2AAR reported a pKi value of 7.49 matched closely to our pKb value, whereas the α2BAR and α2CAR reported pKi values of 5.92 (Chruscinski et al., 1992) and 6.96 (Link et al., 1992) did not. If the α2BAR made a significant contribution, the slope of the Schild plot should have been less than 1. These results further confirm that this response is primarily mediated by an α2AAR.
Fig. 6.
Schild regression analysis using the selective α2AAR ligand oxymetazoline in slices from α2CAR-KO mice. A, consecutive EPI concentration-response curves demonstrate a concentration-dependent effect of the selective α2AAR ligand, oxymetazoline, on the EPI-mediated inhibition of hippocampal CA3 epileptiform activity in brain slices from α2CAR-KO mice. Pretreatment with 100 nM (○), 300 nM (▪), and 1000 nM (□) concentrations of this antagonist produced consecutive parallel rightward shifts of the EPI curve that were significantly different from control (•) (EC50 = 205 ± 48, 738 ± 267, and 2317 ± 980 nM, respectively, versus 33 ± 9 nM for control). B, using dose ratios calculated from individual experiments illustrated in A, a Schild plot was created generating a regression slope equaling 1.16 ± 0.12 and an x-intercept correlating to a pKb value of 7.50, n = 7 animals. This pKb value matched the binding affinity of oxymetazoline (pKi = 7.49) for the mouse α2AAR, but not the mouse α2BAR (pKi = 5.92) (Chruscinski et al., 1992) or mouse α2CAR (pKi = 6.96) (Link et al., 1992).
Effects of Pertussis Toxin on EPI-Mediated Inhibition of CA3 Epileptiform Activity. Pertussis toxin (PTX) blocks the receptor-mediated activation of Gi/o proteins. We used PTX to assess which G protein types are involved in the inhibitory effects of EPI. Extracellular field potential recordings of epileptiform burst frequency were used to generate concentration-response curves using increasing amounts of EPI in untreated control slices or in slices treated with 5 μg/ml PTX for 7 to 8 h. As illustrated in Fig. 7, the mean concentration-response curve for nontreated control slices was fit best by a unity-slope sigmoidal model with a calculated EC50 value of 12 ± 3.9 nM and a maximum effect of 74 ± 6.1% (n = 13 slices). Conversely, for PTX-treated slices from the same mice, the mean concentration-response curve showed minimal inhibition (<25%) (n = 12 slices). These results indicate that inhibition of mouse hippocampal CA3 epileptiform activity in response to EPI is mediated by either Gi or Go proteins and not Gs or Gq proteins.
Fig. 7.
PTX reduces the EPI-mediated inhibition of hippocampal CA3 epileptiform bursts. Extracellular field potential recordings of epileptiform burst frequency were used to generate concentration-response curves using increasing amounts of EPI in untreated control slices (•) or slices treated (○) with 5 μg/ml PTX for 7 to 8 h. Concentration-response curves for EPI were plotted as a percentage of decrease (reduction) in epileptiform burst frequency. The mean concentration-response curve for nontreated control slices was fit best by a nonvariable sigmoidal model with a calculated EC50 value of 12 ± 3.9 nM and an efficacy of 74 ± 6.1% (n = 13 slices from 6 animals). In contrast, for PTX-treated slices from these same mice, the mean concentration-response curve was fit best by a linear regression line and had an efficacy of 24 ± 13% (n = 12 slices from 6 animals).
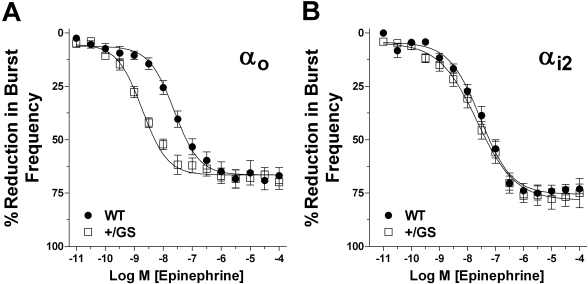
EPI-Mediated Inhibition of CA3 Epileptiform Activity in Slices from GαoG184S Heterozygous (Gαo+/GS) and Gαi2G184S Heterozygous (Gαi2+/GS) Knock-in Mice. To determine a potential role of RGS proteins in the regulation of this response and which type of inhibitory G protein may be involved, we used mice with a knock-in Gα subunit mutation (G184S) that renders Gαo and Gαi2 proteins incapable of binding to the RGS protein. This results in the loss of RGS-mediated inhibition of the Gαo and Gαi2 protein and enhances Gα-specific effects in tissues with responses under RGS control. An increase in response with one of these RGS-insensitive G proteins would implicate that G protein as contributing to the response and RGS proteins as negative regulators. As before, WT control, Gαo+/GS, or Gαi2+/GS slices were pretreated with the GABA blocker picrotoxin, βAR blocker timolol, and NE transporter reuptake inhibitor desipramine. Extracellular field potential recordings were used to generate concentration-response curves using increasing amounts of EPI. Inhibition of frequency burst discharges was significantly more potent in brain slices from Gαo mice, with an EC50 of 2.5 ± 0.9 nM (n = 23 slices) versus litter mate control mice (EC50 = 19 ± 5 nM, n = 21 slices) (Fig. 8A). In contrast, there was no significant difference in Gαi2 mice (EC50 = 19 ± 5 nM, n = 32 slices) compared with the WT controls (EC50 = 23 ± 7 nM, n = 22 slices) (Fig. 8B). These results indicate the EPI-mediated inhibition of mouse hippocampal CA3 epileptiform activity involves a Gαo mechanism under strong negative regulation by RGS proteins.
Fig. 8.
EPI-mediated inhibition of hippocampal CA3 epileptiform bursts is significantly enhanced in brain slices from Gαo+/GS mice but not Gαi2+/GS mice. Extracellular field potential recordings were used to generate concentration-response curves using increasing amounts of EPI (•) in the presence of 30 μM timolol and 0.5 μM desipramine. Concentration-response curves for EPI were plotted as a percent reduction in epileptiform burst frequency. Each individual experiment best fit to a nonvariable sigmoidal curve. A, there was a significant difference in the potencies (EC50 values) calculated for EPI in brain slices from Gαo+/GS mice (□) (2.5 ± 0.9 nM, n = 23 slices from 6 animals) versus litter mate control mice (•) (19 ± 5 nM, n = 21 slices from 6 animals). B, in contrast, the EPI-mediated inhibition of epileptiform activity was unchanged in brain slices from Gαi2+/GS mice (□) (19 ± 5 nM, n = 32 slices from 6 animals) compared with WT control mice (•) (23 ± 7 nM, n = 22 slices from 8 animals). There was no significant difference in the efficacy of EPI among these four groups (Gαo+/GS, 74 ± 3.8%; Gαo litter mate control, 74 ± 4.4%; Gαi2+/GS, 73 ± 2.8%; Gαi2 WT control, 75 ± 3.6%).
Discussion
The role of catecholamines in seizures and epilepsy is complicated, but it is clear that endogenous EPI and NE can protect against many types of seizures (Weinshenker and Szot, 2002). Agonists at all three types of AR (β, α1, and α2) can be antiepileptic, but the most consistent findings show that α2AR agonists are generally anticonvulsant, and selective α2AR antagonists are proconvulsant (Weinshenker and Szot, 2002). Consequently, we focused the current study on the hippocampus, which plays an important role in the common clinical condition of temporal lobe seizures, to begin to dissect mechanisms underlying the antiepileptic actions of α2AR agonists. We used both pharmacological and mouse genetic models to define the receptor and G protein involved in the EPI-mediated antiepileptiform activity in the hippocampus. We have confirmed the role of the α2AAR in inhibition of hippocampal CA3 epileptiform activity in mice, as shown previously by a pharmacological approach in rats (Jurgens et al., 2007). We built upon these findings by demonstrating that this involves a PTX-sensitive Gi/o-type G protein. Furthermore, using RGS-insensitive Gα subunit mutant knock-in mice, we show that endogenous RGS protein action on Gαo strongly suppresses this signal, implicating Gαo as a mediator of the response. In contrast, Gαi2 seems not to be involved. These findings enhance our understanding of the mechanism underlying α2AAR-mediated inhibition of hippocampal epileptiform activity by NE and suggest a novel approach to antiepileptic drug therapies.
The α2AAR is the predominant α2AR in the CNS, and it has been implicated as the primary anticonvulsant α2AR in rat hippocampus in vitro (Jurgens et al., 2007) and in mouse in vivo (Janumpalli et al., 1998). A previous study using dopamine β-hydroxylase, α2AAR, and α2CAR-KO mice showed that the proconvulsant effects of α2AR agonists were mediated by the α2AAR autoreceptor, which decreases NE release, whereas the anticonvulsant effects of α2AR agonists were mediated by α2AARs on target neurons (Szot et al., 2004). In the present study, we confirm the results of these findings in mouse using both pharmacological (antagonist pKb) and genetic (α2AAR- and α2CAR-KO) approaches. Despite expression of the α2CAR in hippocampus, it does not seem to contribute at all to the antiepileptiform activity of EPI and NE (Fig. 5). Likewise, the α2BAR does not seem to play a role (Fig. 6). Neither were α1ARs involved in this particular response (Fig. 3 and Table 1). This level of receptor subtype-specificity does not, however, provide any significant therapeutic advance on its own, because the α2AAR is also the major receptor involved in the antihypertensive therapeutic effect of α2AR agonists and in their major sedative side effect as well (MacMillan et al., 1998). Thus, we pursued subsequent steps in the downstream signaling.
The α2ARs are known to couple primarily to Gi/o family G proteins with subsequent actions on several effector systems, including inhibition of adenylyl cyclase, inhibition of voltage-gated calcium channels, and activation of G protein-coupled inwardly rectifying K+ currents (Offermanns, 2003). The Gi/o protein family is also strongly regulated by the 20-plus member RGS protein family (Neubig and Siderovski, 2002), which has been implicated as a potential drug target (Zhong and Neubig, 2001; Roman et al., 2007). We first confirmed that the α2AAR response in hippocampus was PTX-sensitive, indicating a role for the Gi/o family. The small residual effect after PTX treatment (<1/3 of the control response) could be due to incomplete modification of the Gi/o proteins during the 7- to 8-h pretreatment period, because many studies use an overnight (>12 h) treatment with PTX. Alternatively, a non-PTX-sensitive protein like Gz could play a small role.
To further examine which Gi/o subtype(s) can mediate EPI's effect, mice with knock-in mutant RGS-insensitive Gαo or Gαi2 were used. The knock-in mice differ from WT only in the presence of the G184S mutation, which prevents RGS binding to the Gα subunit and the subsequent GTPase acceleration (Fu et al., 2004; Huang et al., 2006). Consequently, this mutation results in prolonged and enhanced activation of the modified G protein, which increases signal transduction by both the α and βγ subunits derived from that G protein. The heterozygous Gαo RGS-insensitive [Gαo(+/G184S)] knock-in animals showed a 7-fold leftward shift of the EPI dose-response curve (2.5 versus 19 nM), whereas there was no significant difference in potency between the heterozygous Gαi2 RGS-insensitive mouse (19 nM) and its control (23 nM). The pronounced effect even in the heterozygous mouse is not surprising. RGS proteins can accelerate G protein deactivation nearly 1000-fold (Mukhopadhyay and Ross, 1999; Lan et al., 2000), dramatically suppressing G protein signaling. The G184S mutation eliminates this negative regulatory effect, so it produces a gain-of-function phenotype in which even half of the G protein removed from this suppression could produce a marked increase in signaling. Previous studies with the Gαi2G184S knock-in mutants have also shown significant effects in heterozygous mice (Huang et al., 2006). Thus, these results show that RGS proteins play a key role in regulating the α2AAR-mediated hippocampal CA3 antiepileptiform effect and suggest that the Gαo subtype of Gi/o proteins is involved in the signaling mechanism, whereas Gαi2 seems not to be. At this stage, we cannot rule out a contribution from other pertussis toxin-sensitive G proteins such as Gαi1 or Gαi3, but the evidence clearly indicates that Gαo does play a role.
Several important questions remain. Although the Gαo RGS-insensitive mouse shows that an RGS protein is involved in this system, it does not reveal which of the 20-plus RGS proteins (Hollinger and Hepler, 2002; Neubig and Siderovski, 2002) are important. Given that nearly 15 different RGS proteins can function as a GTPase-activating protein for Gαo, it may be difficult to establish which one(s) are involved. Furthermore, it is possible that more than one RGS protein may work in a redundant manner in this system. That said, the RGS7 family of RGS proteins (RGS6, 7, 9, and 11) represent intriguing candidates because they are relatively selective for Gαo in vitro (Lan et al., 2000). A second question is whether the same enhancement of α2AAR agonist anticonvulsant actions will be seen in vivo. Studies are currently underway to assess this question.
The present study suggests two strategies that may provide improved therapeutics for adrenergic agonist anticonvulsants. First, an α2AAR agonist that can selectively activate Gαo versus Gαi2 or other Gi family members could lead to improved potency and/or reduced side effects. It would also be important for such a compound to preferentially activate the α2AARs on target neurons over α2AAR autoreceptors that would decrease NE release. This could be achieved by a “functionally selective” (Urban et al., 2007) α2AAR agonist. Second, RGS proteins have been implicated as potential therapeutic targets. Several peptide (Jin et al., 2004; Young et al., 2004; Roof et al., 2006) and nonpeptide (Roman et al., 2007) RGS inhibitors have been described. To date, none are active in vivo for pharmacological studies, but the identification of the involved RGS protein and the creation of an inhibitor that could target it could either produce anticonvulsant effects through endogenous NE or potentially reduce side effects on the treatment with α2AAR-selective agonists in patients with epilepsy.
In summary, we have defined the receptor (α2AAR), a G protein (Gαo), and a regulatory mechanism (RGS proteins) that are important for the antiepileptiform actions of NE and EPI in the hippocampus, a key site of seizure activity in many patients. These advances provide a theoretical rationale for future, novel therapeutic approaches.
Acknowledgments
We thank Sarah J. Boese, Chris W. D. Jurgens, Brandi A. Kaster, Jasmine J. O'Brien, and Danielle D. Schlosser for help with the experiments, Karen L. Cisek for assistance in editing the manuscript, and Dr. James E. Porter for advice about data acquisition and Schild regression analysis.
This work was supported by the North Dakota Experimental Program to Stimulate Competitive Research through the National Science Foundation (NSF) [Grant EPS-0447679]; NSF Faculty Early Career Development Award [Grant 0347259]; NSF Research Experience for Undergraduates Site [Grant 0639227]; NSF Research Experience for Teachers [Grant 0639227]; National Institutes of Health National Institute on Drug Abuse [Grant 5-R01-DA17963]; National Institutes of Health National Institute of General Medical Sciences [Grant 5-R01-GM039561]; and National Institutes of Health National Center for Research Resources INBRE Program [Grant P20-RR016741].
Preliminary reports of these findings were presented at the 2007 annual meeting of the American Society for Biochemistry and Molecular Biology (ASBMB) Northwest Regional Undergraduate Affiliate Network; 2007 October 26-27; Moorhead, MN; and the 2008 annual meetings of the ASBMB and the American Society for Pharmacology and Experimental Therapeutics, 2008 April 5-9, San Diego, CA.
B.L.G. and B.W.N. contributed equally to this work.
ABBREVIATIONS: CNS, central nervous system; ACSF, artificial cerebral spinal fluid; AR, adrenergic receptor; CA3, cornu ammonis 3; EPI, epinephrine; GPCR, G-protein coupled receptor; KO, knockout; NE, norepinephrine; RGS, regulator of G-protein signaling; WB-4101, 2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride; WT, wild type; PTX, pertussis toxin; JP-1302, N-[4-(4-methyl-1-piperazinyl)phenyl]-9-acridinamine dihydrochloride.
References
- Arunlakshana O and Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14 48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DM, Wilkie TM, and Gilman AG (1996) GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell 86 445-452. [DOI] [PubMed] [Google Scholar]
- Bylund DB (1988) Subtypes of α2-adrenoceptors: pharmacological and molecular evidence converge. Trends Pharmacol Sci 9 356-361. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR Jr, and Trendelenburg U (1994) International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev 46 121-136. [PubMed] [Google Scholar]
- Chruscinski AJ, Link RE, Daunt DA, Barsh GS, and Kobilka BK (1992) Cloning and expression of the mouse homolog of the human α2-C2 adrenergic receptor. Biochem Biophys Res Commun 186 1280-1287. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Harrison C, Zhong H, Neubig RR, and Traynor JR (2003) Endogenous RGS protein action modulates μ-opioid signaling through Gαo. J Biol Chem 278 9418-9425. [DOI] [PubMed] [Google Scholar]
- Clark MJ, Linderman JJ, and Traynor JR (2008) Endogenous regulators of G protein signaling differentially modulate full and partial μ-opioid agonists at adenylyl cyclase as predicted by a collision coupling model. Mol Pharmacol 73 1538-1548. [DOI] [PubMed] [Google Scholar]
- Devauges V and Sara SJ (1991) Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by β-receptors. Behav Brain Res 43 93-97. [DOI] [PubMed] [Google Scholar]
- Fu Y, Huang X, Zhong H, Mortensen RM, D'Alecy LG, and Neubig RR (2006) Endogenous RGS proteins and Gα subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ Res 98 659-666. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhong H, Nanamori M, Mortensen RM, Huang X, Lan K, and Neubig RR (2004) RGS-insensitive G-protein mutations to study the role of endogenous RGS proteins. Methods Enzymol 389 229-243. [DOI] [PubMed] [Google Scholar]
- Gilman AG (1987) G proteins: Transducers of receptor-generated signals. Annu Rev Biochem 56 615-649. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Pizzanelli C, Biagioni F, Murri L, and Fornai F (2004) The role of norepinephrine in epilepsy: from the bench to the bedside. Neurosci Biobehav Rev 28 507-524. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Knudson CA, Carr PA, Doze VA, and Porter JE (2005) Adrenergic receptor characterization of CA1 hippocampal neurons using real time single cell RT-PCR. Brain Res Mol Brain Res 139 267-276. [DOI] [PubMed] [Google Scholar]
- Hollinger S and Hepler JR (2002) Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev 54 527-559. [DOI] [PubMed] [Google Scholar]
- Hopkins WF and Johnston D (1988) Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J Neurophysiol 59 667-687. [DOI] [PubMed] [Google Scholar]
- Huang X, Fu Y, Charbeneau RA, Saunders TL, Taylor DK, Hankenson KD, Russell MW, D'Alecy LG, and Neubig RR (2006) Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol Cell Biol 26 6870-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC.
- Janumpalli S, Butler LS, MacMillan LB, Limbird LE, and McNamara JO (1998) A point mutation (D79N) of the α2A adrenergic receptor abolishes the antiepileptogenic action of endogenous norepinephrine. J Neurosci 18 2004-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Zhong H, Omnaas JR, Neubig RR, and Mosberg HI (2004) Structure-based design, synthesis, and pharmacologic evaluation of peptide RGS4 inhibitors. J Pept Res 63 141-146. [DOI] [PubMed] [Google Scholar]
- Jurgens CW, Boese SJ, King JD, Pyle SJ, Porter JE, and Doze VA (2005) Adrenergic receptor modulation of hippocampal CA3 network activity. Epilepsy Res 66 117-128. [DOI] [PubMed] [Google Scholar]
- Jurgens CW, Hammad HM, Lichter JA, Boese SJ, Nelson BW, Goldenstein BL, Davis KL, Xu K, Hillman KL, Porter JE, et al. (2007) α2A Adrenergic receptor activation inhibits epileptiform activity in the rat hippocampal CA3 region. Mol Pharmacol 71 1572-1581. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, and Gilbert P (2004) A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci 15 333-351. [DOI] [PubMed] [Google Scholar]
- Lan KL, Sarvazyan NA, Taussig R, Mackenzie RG, DiBello PR, Dohlman HG, and Neubig RR (1998) A point mutation in Gαo and Gαi1 blocks interaction with regulator of G protein signaling proteins. J Biol Chem 273 12794-12797. [DOI] [PubMed] [Google Scholar]
- Lan KL, Zhong H, Nanamori M, and Neubig RR (2000) Rapid kinetics of regulator of G-protein signaling (RGS)-mediated Gαi and Gαo deactivation. J Biol Chem 275 33497-33503. [DOI] [PubMed] [Google Scholar]
- Link R, Daunt D, Barsh G, Chruscinski A, and Kobilka B (1992) Cloning of two mouse genes encoding α2-adrenergic receptor subtypes and identification of a single amino acid in the mouse α2-C10 homolog responsible for an interspecies variation in antagonist binding. Mol Pharmacol 42 16-27. [PubMed] [Google Scholar]
- MacMillan LB, Lakhlani PP, Hein L, Piascik M, Guo TZ, Lovinger D, Maze M, and Limbird LE (1998) In vivo mutation of the α2A-adrenergic receptor by homologous recombination reveals the role of this receptor subtype in multiple physiological processes. Adv Pharmacol 42 493-496. [DOI] [PubMed] [Google Scholar]
- Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AA, and Seidman JG (1992) Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol 12 2391-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S and Ross EM (1999) Rapid GTP binding and hydrolysis by Gq promoted by receptor and GTPase-activating proteins. Proc Natl Acad Sci U S A 96 9539-9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig RR and Siderovski DP (2002) Regulators of G-protein signaling as new central nervous system drug targets. Nat Rev Drug Discov 1 187-197. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verrièle L, Touzard M, Chaput C, Richard N, and Millan MJ (1998) Actions of α2 adrenoceptor ligands at α2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexme-detomidine, are highly selective for α2A adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 358 197-206. [DOI] [PubMed] [Google Scholar]
- Offermanns S (2003) G-proteins as transducers in transmembrane signaling. Prog Biophys Mol Biol 83 101-130. [DOI] [PubMed] [Google Scholar]
- Pupo AS and Minneman KP (2001) Adrenergic pharmacology: Focus on the central nervous system. CNS Spectr 6 656-662. [DOI] [PubMed] [Google Scholar]
- Roman DL, Talbot JN, Roof RA, Sunahara RK, Traynor JR, and Neubig RR (2007) Identification of small-molecule inhibitors of RGS4 using a high-throughput flow cytometry protein interaction assay. Mol Pharmacol 71 169-175. [DOI] [PubMed] [Google Scholar]
- Roof RA, Jin Y, Roman DL, Sunahara RK, Ishii M, Mosberg HI, and Neubig RR (2006) Mechanism of action and structural requirements of constrained peptide inhibitors of RGS proteins. Chem Biol Drug Des 67 266-274. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA (1986) Hippocampal slices in experimental and human epilepsy. Adv Neurol 44 991-1010. [PubMed] [Google Scholar]
- Szot P, Lester M, Laughlin ML, Palmiter RD, Liles LC, and Weinshenker D (2004) The anticonvulsant and proconvulsant effects of α2-adrenoreceptor agonists are mediated by distinct populations of α2A-adrenoreceptors. Neuroscience 126 795-803. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320 1-13. [DOI] [PubMed] [Google Scholar]
- Watson N, Linder ME, Druey KM, Kehrl JH, and Blumer KJ (1996) RGS family members: GTPase-activating proteins for heterotrimeric G-protein α-subunits. Nature 383 172-175. [DOI] [PubMed] [Google Scholar]
- Weinshenker D and Szot P (2002) The role of catecholamines in seizure susceptibility: new results using genetically engineered mice. Pharmacol Ther 94 213-233. [DOI] [PubMed] [Google Scholar]
- Young KH, Wang Y, Bender C, Ajit S, Ramirez F, Gilbert A, and Nieuwenhuijsen BW (2004) Yeast-based screening for inhibitors of RGS proteins. Methods Enzymol 389 277-301. [DOI] [PubMed] [Google Scholar]
- Zhong H and Neubig RR (2001) Regulator of G protein signaling proteins: novel multifunctional drug targets. J Pharmacol Exp Ther 297 837-845. [PubMed] [Google Scholar]