Abstract
The human constitutive androstane receptor (CAR, CAR1) regulates the expression of genes involved in xenobiotic metabolism in the liver. The CAR gene uses multiple alternative splicing events during pre-mRNA processing, thereby enhancing the CAR transcriptome. Previous reports have identified two prominent human CAR variants, CAR2 and CAR3, that possess four- and five-amino acid insertions in their ligand binding domains, respectively. Unlike the constitutively active reference form of the receptor, we now demonstrate that CAR2 is a ligand-activated receptor and comprises approximately 30% of the reference transcript level in human liver tissues in human hepatocytes. Furthermore, we identify the common plasticizer, di(2-ethylhexyl) phthalate (DEHP), as a highly potent and uniquely selective agonist of CAR2. Results from reporter transactivation and mammalian two-hybrid assays reveal that DEHP activates CAR2 at low nanomolar concentrations, results further supported by analysis of CAR target gene expression in primary human hepatocytes. In addition, comparative genomic analyses show that the typical mouse, rat, and marmoset models of DEHP toxicity cannot accurately profile potential human toxicity because of these species' inability to generate a CAR2-like transcript. The discovery that CAR2 is an ultimate human DEHP receptor identifies a novel pathway modulating human DEHP toxicity with potential clinical implications for a subset of patients undergoing critical care medical interventions.
The constitutive androstane receptor (CAR, NR1I3) is a member of the nuclear receptor superfamily that is expressed primarily in the liver (Baes et al., 1994). In conjunction with the pregnane X receptor (NR1I2), CAR regulates hepatic genes involved in all three phases of xenobiotic metabolism (Wei et al., 2000). The products of these genes play a role in modulating the metabolism of a variety of pharmaceuticals, such as acetaminophen (Zhang et al., 2002) and cyclophosphamide (Roy et al., 1999), as well as endogenous compounds, including thyroid hormone (Maglich et al., 2004), bile acids (Guo et al., 2003), and steroid hormones (Xie et al., 2003).
In human livers, the CAR gene expresses a number of differentially spliced mRNA transcripts, generated through the use of alternative splice acceptor sites during pre-mRNA processing (Auerbach et al., 2003; Savkur et al., 2003; Arnold et al., 2004; Jinno et al., 2004; Lamba et al., 2004). Previous reports from our laboratory have focused on functional analysis of two prominent human CAR variants, termed CAR2 (Auerbach et al., 2007) and CAR3 (Auerbach et al., 2005). Results from these and other studies demonstrated that both CAR2 and CAR3 activate reporters containing response elements derived from the endogenous promoters of the CYP2B6 and CYP3A4 genes (Auerbach et al., 2005, 2007). Unlike CAR1, CAR3 functions as a ligand-dependent receptor (Auerbach et al., 2005), activating transcription in the presence of the human CAR ligand 6-(4-chlorophenyl)imidazo[2,1-b] [1,3]thiazole-5-carbaldehyde O-3,4-dichlorobenzyl) oxime (CITCO) (Maglich et al., 2003). Our recent study of CAR2 suggested that it may have a ligand binding profile that is distinct from both CAR1 and CAR3 (Auerbach et al., 2007).
Based on these data and results from molecular modeling analyses, we hypothesized that CAR2 possesses distinct biological properties. In this report, we demonstrate that CAR2 is a ligand-activated receptor that comprises approximately one third of the total CAR transcriptome in human hepatocytes. Furthermore, we identify the common plasticizer, di(2-ethylhexyl) phthalate (DEHP), as a highly potent and uniquely selective agonist of CAR2. Results from reporter transactivation and mammalian two-hybrid assays reveal that DEHP activates CAR2 at low nanomolar concentrations. These results are further supported by analysis of CAR target gene expression in primary human hepatocytes, where DEHP activation of CAR2 markedly induced the CYP2B6 and CYP3A4 transcripts. Furthermore, using genomic analyses, we demonstrate that the alternative splice site used to generate the CAR2 transcript is not conserved in mice, rats, or marmosets—widely used animal models of DEHP toxicity.
DEHP is a plasticizer with an annual production volume approaching 260 million pounds (Kavlock et al., 2006). Human exposure to DEHP is ubiquitous: it is used in the manufacturing of flexible vinyl found in medical tubing, other medical devices, commercial floorings, food product wraps, and many additional consumer products (Hauser and Calafat, 2005). Concerns regarding the human toxicology of DEHP are prevalent; for example, during medical interventions, patients can be exposed to relatively high doses of DEHP that leaches from plastic medical devices into infusate (United States Food and Drug Administration, 2001). Our finding that DEHP is a potent and selective activator of CAR2 is likely to have important implications for predictive human toxicity.
In summary, in this report, we describe how the naturally occurring NR1I3 splice variant, CAR2, is unique in that it 1) is ligand activated, unlike the previously recognized constitutively active reference form of the receptor, 2) comprises ∼30% of the reference transcript level in human liver tissues, and, 3) is selectively activated by low nanomolar concentrations of DEHP, levels achieved in human serum and urine of certain patients. Furthermore, these studies yield new mechanistic insight for the toxicological effects of DEHP in human liver, findings that are particularly relevant because the conventional rodent and more recent marmoset models for DEHP toxicity do not reflect that of the human DEHP receptor, CAR2.
Materials and Methods
Chemicals. DEHP, 5α-androstan-3α-ol (androstanol), and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). CITCO was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA).
Plasmids. The vectors CMV2-CAR1, CMV2-CAR2, 3.1-RXRα, 3.1-RXRαLBD, VP16-CAR1LBD, VP16-CAR2LBD, 2B6XREM, and 3A4XREM were described previously (Auerbach et al., 2007). The vectors CMV2-CAR3 and VP16-CAR3LBD were also reported previously (Auerbach et al., 2005) as well as the pM-steroid receptor coactivator 1 (SRC1) clone (Chang et al., 1999). All clones were derived from human genes.
Bioinformatics. Protein sequences were retrieved from the NCBI database. Amino acids 212 to 348 of the human CAR1 protein (NCBI RefSeq no. NP_005113.1) were used as the reference sequence and aligned using the blosum matrix of the ClustalW2 program from the EBI website (http://www.ebi.ac.uk/Tools/clustalw2/). Genomic alignments used the University of California Santa Cruz (UCSC) genome browser (http://genome.ucsc.edu/) with the mouse CAR gene as the reference sequence.
Cell Culture. Culture conditions for the COS-1 and HepG2 cell lines were described previously (Auerbach et al., 2007), except that the cells were maintained in media containing 8% fetal bovine serum (FBS) and transfected in media containing 8% dextran/charcoal-treated FBS (HyClone, Logan, UT). Culturing conditions for primary human hepatocytes have been described previously (Olsavsky et al., 2007). Available donor information is presented in Table 1. Preparations enriched for hepatocytes were received plated in collagen-coated six-well culture dishes. Upon arrival the media was removed and replaced with 2 ml of fresh media. Matrigel was added along with a 2-ml media change within 48 h of plating. Two-milliliter changes of media occurred once 24 h after the addition of matrigel and then again approximately 48 h later. Cells were harvested 24 h subsequently if no treatment was added. For cells that underwent chemical exposures, media was replaced in the morning (72 h after Matrigel) and then again ∼4 h later with fresh media that included the chemical agents. Cells were harvested 24 h after treatments. If not specified otherwise, all culturing materials were obtained from Invitrogen (Carlsbad, CA).
TABLE 1.
Hepatocyte donor information
| Donor | Age | Sex | Race |
|---|---|---|---|
| years | |||
| A | 0.75 | Male | White |
| B | 57 | Male | White |
| C | 49 | Male | White |
| D | 61 | Male | White |
| E | 63 | Male | White |
| F | 3 | Female | White |
| G | 61 | Male | White |
| H | 60 | Female | Unknown |
| I | 69 | Female | White |
| J | 57 | Female | White |
RNA Isolation and Quantitative PCR Analysis. Total RNA was extracted from the hepatocytes using TRIzol Reagent (Invitrogen) according to the manufacturer's protocol. RNA concentrations were assessed by UV absorbance at 260 nm using a DU 800 spectrophotometer (Beckman Coulter, Fullerton, CA) and RNA integrity was ascertained using an Agilent Bioanalyzer (Agilent, Santa Clara, CA). A high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA) was used to reverse transcribe RNA into cDNA, according to the manufacturer's instructions. TaqMan Assays-on-Demand Gene Expression assays (Applied Biosystems) were performed according to manufacturer's directions for a 50-μl reaction volume subsequently divided into duplicate 25-μl reactions in a 96-well plate, and run in an real-time PCR system (7300; Applied Biosystems, Foster City, CA). Taqman probes were custom designed to detect the presence of the CAR2 insertion at the exon 6-7 junction. A multiplex reaction was performed containing the REF assay (Applied Biosystems) and CAR2 probe. The REF probe sequence is 5′-AGATGGAGCCCGTGTGGGGTTCCAG-3′ (5-carboxyfluorescein reporter). The CAR2 probe sequence is 5′-TGTATCTCCCACAGTGGG-3′ (VIC Reporter). Absolute quantification was determined through the use of a standard curve generated using plasmid DNA containing either the CAR1 (REF) or CAR2 variant. Six point standard curves were used, encompassing the range from 2 to 6000 fg of target sequence. Applied Biosystems inventoried assays were used for detection of 18S, CYP2B6, and CYP3A4.
Transient Transfection Assays. The details of the luciferase reporter assays were described previously (Auerbach et al., 2007). Likewise, the mammalian two-hybrid assays were conducted using 40 ng of pVP16 expression plasmid, 10 ng of pM (GAL4) expression plasmid, 100 ng of pFR-luc reporter, and 10 ng pRL-CMV (Auerbach et al., 2007). When the pcDNA3.1 expression plasmid, containing RXR-LBD, was incorporated in to the two-hybrid assay, 10 ng was used. The pM-SRC1 (Chang et al., 1999) vector was substituted in place of the pM-SRC1 RID vector in the mammalian two-hybrid experiments.
Statistical Analysis. Unless stated otherwise, statistics, and EC50 values were obtained using Prism (version 4.00; GraphPad Software, San Diego, CA); specific details are provided in the figure legends.
Results
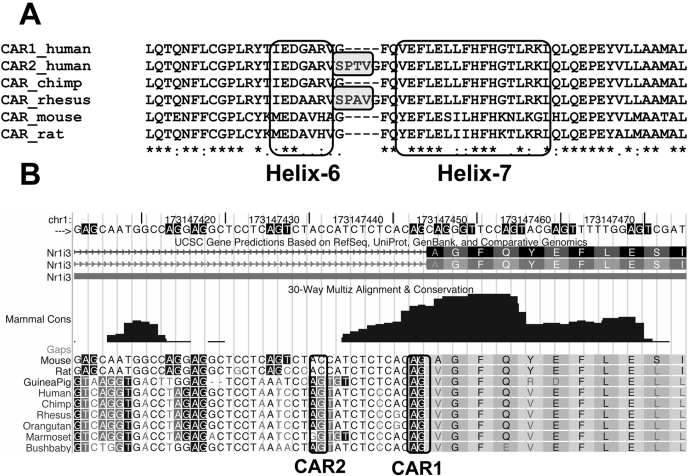
Mice, Rats, and Marmosets Cannot Generate the CAR2 Transcript. Protein sequences were retrieved from the NCBI database and aligned as described under Materials and Methods. Helix-6 and helix-7 of the CAR ligand-binding domain (Xu et al., 2004) are shown (Fig. 1A). The result shows that the rhesus monkey sequence includes a four-amino-acid insertion very similar to CAR2 (shaded boxes) suggesting that the splice variant is conserved across multiple species. To determine whether other species could generate similar transcripts a genomic alignment was performed for three species of rodents and six primates using the UCSC genome browser (Kent et al., 2002), with mouse CAR as the reference sequence (Fig. 1B). Although the splice acceptor site (AG) for CAR1 is conserved across each species, the CAR2 site is not conserved in marmoset, mouse, or rat, indicating that these latter species are incapable of generating a CAR2-like, four-amino acid insertion protein.
Fig. 1.
Rodent and primate alignments of the amino acid and genomic nucleotide sequences flanking the CAR2 insertion. A, the protein alignment of helix-6 and helix-7 of the ligand-binding domain. Protein sequences were retrieved from the NCBI database. Amino acids 212 to 348 of the human CAR1 protein (NP_005113.1) were used as the reference sequence and aligned using the blosum matrix of ClustalW2. B, CAR genomic alignment based on the mouse CAR gene using the UCSC genome browser. The intron 6/exon 7 junction is shown (relative to human CAR). The splice acceptor sites that are used to generate CAR1 or CAR2 are boxed.
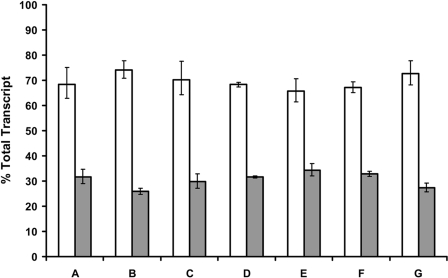
The CAR2 Insertion Is Found in ∼30.5% of Total CAR Transcripts in Human Hepatocytes. To determine the frequency of the CAR2 insertion we used a TaqMan qPCR approach as described under Materials and Methods. The frequency of the CAR2 insertion was found to be very consistent across all donors with a minimum of 25.9%, a maximum of 34.3%, and an average of 30.5% (Fig. 2). These data show that a significant portion of CAR transcripts in hepatocytes contain the CAR2 insertion.
Fig. 2.
Frequency of the CAR2 insertion in primary human hepatocytes. TaqMan qPCR was performed on total RNA obtained from seven primary human hepatocyte cultures. Hepatocytes were plated into collagen-coated six-well culture dishes and cultured for approximately six days before harvesting. Quantification of each variant was determined with standard curves generated from plasmid DNA containing either the CAR1 or CAR2 coding sequence. The data are expressed as a percentage of total transcript, defined as the sum of the REF and CAR2 probes divided by the individual probe of interest. The error bars indicate the range based on the standard deviation of the technical replicates using the square root of sum-of-squares technique.
DEHP Is a Contaminant of Fetal Bovine Serum and a Potent Agonist of CAR2. Previous studies conducted in our laboratory used media containing untreated FBS. Results from these studies implied that CAR2 was constitutively active, like CAR1, whereas CAR3 was uniquely ligand-activated (Auerbach et al., 2003, 2005, 2007). Although no previous reports indicated an effect caused by using dextran/charcoal-treated FBS for the study of CAR activity, our initial data suggested that CAR2 may possess a unique ligand-binding profile (Auerbach et al., 2007). To examine this property further, we tested charcoal/dextran-treated FBS to reduce the likelihood of potential background effects that may be contributed from endogenous compounds.
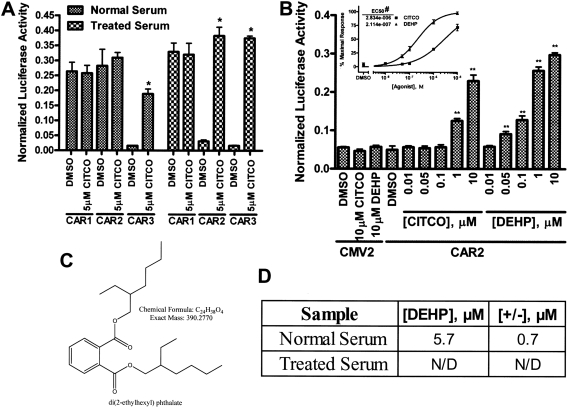
HepG2 cells were transfected in media containing either normal FBS or charcoal/dextran-treated FBS and reporter assays were conducted for activation of the 2B6XREM reporter by the CAR variants (Fig. 3A). In cells that were cultured using media containing the untreated FBS, CAR1, and CAR2 activated the reporter both in the presence and absence of the specific CAR ligand CITCO, whereas CAR3 was ligand-activated as detected previously (Auerbach et al., 2005). We were surprised to find that the use of dextran/charcoal-treated FBS resulted in CAR2 activation only in the presence of CITCO, whereas CAR1 and CAR3 were relatively unaffected by the differences in media formulation. This result suggested that FBS contains a specific CAR2 agonist that is removed by dextran/charcoal treatment.
Fig. 3.
Differential activation of the CAR variants in response to dextran/charcoal-treated FBS and activation of CAR2 by DEHP. All transfections included the 3.1-RXRα expression vector, the 2B6XREM reporter and the pRL-CMV vector for normalization of transfection efficiency. Data are represented as normalized luciferase values and each data point represents the mean (± S.D.) of four separate transfections. A, HepG2 cells were transfected with CMV2-CAR1, CMV2-CAR2, or CMV2-CAR3 in media containing 8% FBS or 8% charcoal/dextran-treated FBS and then treated for 24 h. B, COS-1 cells were transfected with CMV2 or CMV2-CAR2 and treated for 24 h in media containing 8% charcoal/dextran-treated FBS. The inset shows the CAR2 data plotted as a dose-response curve. The DMSO group defined the 0% response and the 10 μM DEHP group was set to 100% response. C, chemical structure and properties of DEHP. *, data points that were significantly different from their respective DMSO control as determined by unpaired two-tailed t test (p < 0.0001). **, data points that were significantly different from their respective DMSO control as determined by ANOVA in combination with a Dunnett's multiple comparison post test (p < 0.01). #, the difference in the LogEC50 values was statistically significant as determined by F test (p < 0.0001). D, DEHP concentrations ascertained using quantitative mass spectroscopy.
It should be noted that the data in Fig. 3A were obtained using HepG2 cells, a human hepatoma cell line. We have previously shown that the HepG2 and COS-1 cell lines obtain similar results in our system (Auerbach et al., 2007). In our hands, COS-1 cells generally exhibit a much higher transfection efficiency and consistency than HepG2 cells, therefore further experiments were conducted using the COS-1 cell line.
Using methods to be described elsewhere, we identified DEHP as a contaminant of FBS that is efficiently removed by charcoal/dextran treatment. We assessed the ability of both DEHP and CITCO to activate CAR2 using the 2B6XREM reporter (Fig. 3B). DEHP (Fig. 3C) activated the reporter through CAR2 with an EC50 of 211 nM, more than 10-fold greater than the EC50 of CITCO, a highly potent CAR ligand.
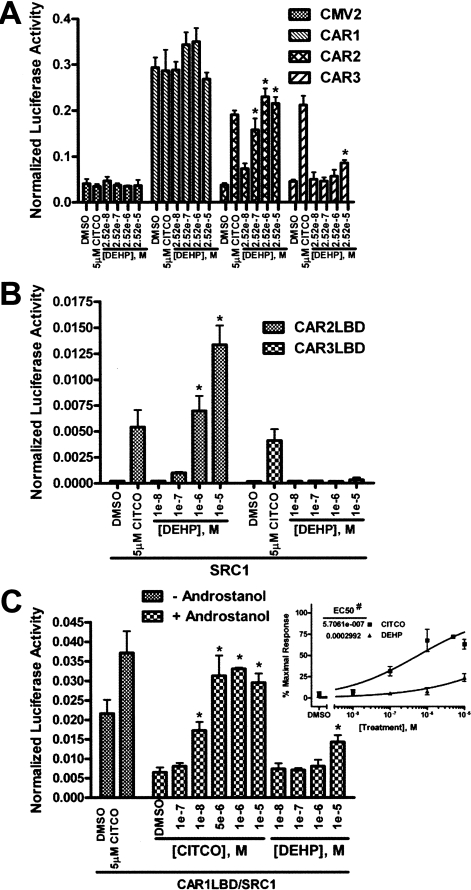
DEHP Shows Strong Specificity for CAR2 Compared with CAR1 and CAR3. Activity of the CAR variants was assessed by 2B6XREM reporter activity after treatment (Fig. 4A). CAR1 is active under all conditions tested because of its constitutive nature and ligand-independent nuclear accumulation after overexpression in cell lines. Under normal physiologic conditions, CAR1 is sequestered in the cytoplasm and translocates to the nucleus upon activation (Kawamoto et al., 1999). This complex mechanism of CAR1 makes in vitro assessment of ligand activation difficult. The CAR3 insertion is predicted not to significantly alter the ligand binding pocket; therefore, CAR3 is probably an accurate proxy of CAR1 ligand interactions (Faucette et al., 2007). CAR3 shows only a minimal response to DEHP at a dose of 25.2 μM but is markedly activated by 5 μM CITCO, as previously reported (Auerbach et al., 2005). CAR2 again displayed a strong dose-response to DEHP; no effect is seen in the empty expression vector (CMV2) control group.
Fig. 4.
Transactivation and coactivator recruitment by CAR1, CAR2, and CAR3 in response to DEHP. All transfections were done in COS-1 cells using media containing 8% charcoal/dextran-treated FBS and included the 3.1-RXRα expression vector (A) or the 3.1-RXRαLBD expression vector (B and C) as well as the pRL-CMV vector for normalization of transfection efficiency. Data are represented as normalized luciferase values, and each data point represents the mean (± S.D.) of four separate transfections. A, cells were transfected with the 2B6XREM reporter and CMV2 or CMV2-CAR1, 2, or 3 and treated for 24 h. B, cells were transfected with pFR-Luciferase, pM-SRC1, and VP16-CAR2LBD or VP16-CAR3LBD expression vectors and treated for 24 h. C, cells were transfected with pFR-Luciferase, pM-SRC1, and VP16-CAR1LBD expression vector and treated for 24 h. The inset shows the “+ androstanol” data plotted as a dose-response curve. A 100% response was defined as the reporter activity in the presence of 5 μM CITCO (without androstanol) and a 0% response was defined as the reporter activity in the presence 10 μM androstanol (without CITCO or DEHP). *, data points that were significantly different from their respective DMSO control as determined by ANOVA in combination with a Dunnett's multiple comparison post test (p < 0.01). #, difference in the LogEC50 values was statistically significant as determined by F test (p < 0.0001).
Transactivation of target genes by CAR occurs through the recruitment of coactivators when the receptor is in its active conformation. SRC1 has been shown previously to be recruited by CAR1, CAR2, and CAR3 (Moore et al., 2000; Auerbach et al., 2005, 2007). To assess the interaction of SRC1 with CAR2 and CAR3 in the presence of DEHP, we employed a mammalian two-hybrid assay as described under Materials and Methods. Both CAR2 and CAR3 interact with SRC1 in the presence of 5 μM CITCO (Fig. 4B). CAR2 also shows a dose-dependent interaction with SRC1 in response to DEHP, an effect that is not seen with CAR3. No effect resulted when CAR2 or CAR3 were cotransfected with a pM vector that did not include SRC1 (data not shown).
To better evaluate the interaction between CAR1 and DEHP, we applied the mammalian two-hybrid system used in Fig. 4B to CAR1. The dynamic range of this system is greater than that obtained using the 2B6XREM reporter construct, allowing a more sensitive approach for evaluating physical interactions of chemical entities with the ligand binding pocket of CAR1, as determined by their abilities to compete away the inverse agonist androstanol (Forman et al., 1998). The interaction between the CAR1LBD and SRC1 was measured with and without 10 μM androstanol. In the presence of 10 μM androstanol, the reporter activity is approximately 30% of the constitutive activity seen in the absence of androstanol (Fig. 4C). When CITCO is titrated into the experiment reporter activity is readily restored, with a clearly pronounced effect at 100 nM. On the other hand, DEHP acts as only a weak competitor of androstanol at 10 μM. Similarly weak competition of androstanol was also observed in separate analysis of mCAR (data not shown). To better illustrate this effect, dose-response curves were generated using the data in Fig. 4C, inset. The result shows an EC50 of 571 nM for CITCO and 299 μM for DEHP. In a previous report, using a CAR-Förster resonance energy transfer assay, the EC50 for CITCO was published as 49 nM (Maglich et al., 2003), 8.6% of that determined in our competition study. If the competition among CITCO, androstanol, DEHP, and androstanol is linear, we estimate the EC50 of DEHP for CAR1 to be ∼25 μM, an estimate that is consistent with the data obtained for the CAR3 variant (Fig. 4A).
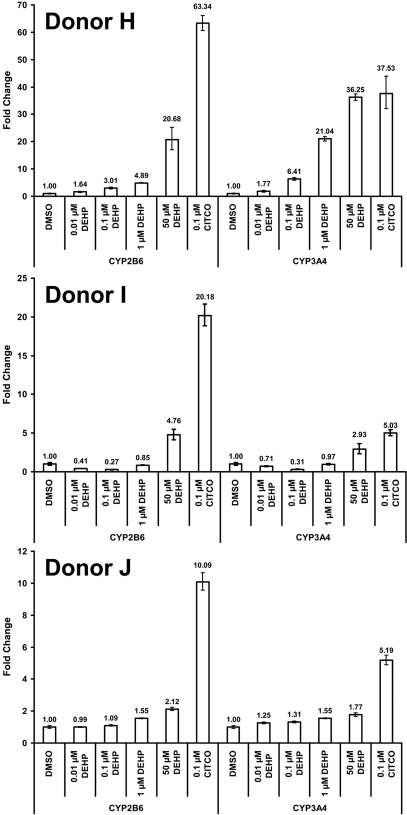
DEHP Increases CYP2B6 and CYP3A4 Transcript Levels in Primary Human Hepatocytes. Hepatocytes were isolated from three separate donors and cultured as described under Materials and Methods. Cells were treated with 100 nM CITCO or increasing concentrations of DEHP for 24 h and total RNA was isolated for use in qPCR analysis for activation of two CAR target genes, CYP2B6 and CYP3A4 (Fig. 5). All three donors showed an increase in CYP2B6 and CYP3A4 transcripts in response to 50 μM DEHP. Donor H displayed a strong dose-response to DEHP with an increase in transcript seen at a 10 nM dose for both CYP2B6 and CYP3A4. The other two donors exhibited more moderate responses to DEHP, although the responses from all donors indicated a dose-response enhancement of transcript levels with DEHP. The more modest responses noted for donors I and J probably reflect either interindividual differences in effect, or the apparent higher basal level of expression for both CYP2B6 and CYP3A4 existing before treatments.
Fig. 5.
Relative levels of CYP2B6 and CYP3A4 transcript in primary human hepatocytes after treatment with DEHP or CITCO. Hepatocytes were plated into collagen-coated six-well culture dishes and cultured for approximately 5 days before being treated for 24 h, after which they were harvested for TaqMan qPCR analysis. All data have been normalized to the expression of 18s RNA and are presented as the -fold change in transcript relative to the DMSO-treated group. The error bars indicate the range based on the standard deviation of the technical replicates using the square root of sum-of-squares technique.
Discussion
The report of the crystal structure of CAR1 demonstrated that helix-6 and helix-7 line a large portion of the ligand binding pocket and that a F234A mutation of CAR1 results in loss of the receptor's constitutive activity (Xu et al., 2004). CAR2 contains a four-amino acid insertion that resides in the highly conserved loop between helix-6 and helix-7 of the ligand binding domain, and lies just 1 amino acid upstream of Phe234 (Fig. 1A). These data provide support for a hypothesis that CAR2 lacks constitutive activity and may possess a unique ligand binding profile. We now demonstrate that CAR2, unlike CAR1, is a ligand-dependent receptor and that DEHP is a highly sensitive and selective activator of this variant CAR. In separate experiments (DeKeyser et al., manuscript in preparation), we demonstrated that no other member of the human NR1I subfamily possess a similar affinity for DEHP, establishing a potency of DEHP for CAR2 that is 40-fold greater than that of any other human NR1I receptor.
As a consequence of their widespread use and environmental prevalence, there has been a great deal of concern noted for potential human toxicity resulting from exposure to phthalates, concerns that have been focused primarily on reproductive toxicity, stemming from the antiandrogenic properties of DEHP. A recent report from the National Toxicology Program Center for the Evaluation of Risks to Human Reproduction (NTP-CERHR) underscores the issues over potential adverse effects of DEHP on the developing male reproductive tract, with infants, particularly those who have faced critical illness with associated medical device interventions, at the greatest risk (Kavlock et al., 2006). It has been suggested that blood phthalate levels in patients undergoing critical care procedures may approach micromolar concentrations (McKee, 2004), with DEHP levels of 1.19 μg/ml reported in cord blood (Latini et al., 2003). DEHP exposure in male subjects may compromise sperm quality and quantity (Hoppin, 2003; Duty et al., 2005; Kavlock et al., 2006) and disrupt circulating levels of thyroid hormone (Meeker et al., 2007). It is noteworthy that the metabolism of thyroid hormone is enhanced through CAR regulation (Maglich et al., 2004). Furthermore, the human CYP2B6 and CYP3A4 enzymes metabolize testosterone (Imaoka et al., 1996). It is interesting to speculate that phthalate levels in certain tissues and perhaps hepatocytes may actually exceed circulating blood levels, implying that CAR2 may be activated by DEHP at even lower blood concentrations than achieved in critical care interventions, and that CAR1 or CAR3 may additionally take on a target role under such circumstances.
Mono(ethylhexyl) phthalate (MEHP), a major metabolite of DEHP, has been implicated as a mediator of the reproductive and developmental toxicity associated with DEHP (Heindel and Powell, 1992; Kavlock et al., 2006). We tested MEHP as a CAR2 activator, but even at 10 μM, this agent exhibited only weak activity in our in vitro reporter assays (data not shown), results that further support our conclusion that the DEHP parent compound itself is the most active modulator of CAR2. It is noteworthy that most of the previous conclusions regarding phthalate toxicology (Kavlock et al., 2006) have been drawn from high-dose studies in rodent or marmoset models, species that are not capable of generating a CAR2-like transcript (Fig. 1B). Furthermore, it has been suggested that DEHP is metabolized to MEHP to a lesser degree in primates compared with rodents (Ito et al., 2005; Rusyn et al., 2006). In light of these considerations, the potential role of CAR2 as a mediator of the developmental and reproductive toxicities associated with DEHP in humans warrants further investigation.
In this report, we demonstrate that the CAR2 transcript comprises ∼30% of the CAR reference level in human hepatocytes (Fig. 2), and that exposure to DEHP induces transcription of the CAR target genes, CYP2B6 and CYP3A4. In particular, one donor exhibited a DEHP response at a dose that is likely to activate CAR2 exclusively (Fig. 5). The discovery that low levels of DEHP can induce xenobiotic metabolism in human hepatocytes may have immediate clinical relevance for some patients, especially those receiving large amounts of intravenous fluids or blood transfusions, as well as medications that have contraindications associated with CAR target genes.
Furthermore, the results of our study strongly imply that CAR2 has the capacity to interact with a set of ligands that are highly distinct from that of other nuclear receptors. This finding will probably serve to redirect future evaluation of drug and chemical safety data and should set new directions underscoring the importance of CAR in human biology, including for the emerging role of CAR as a regulator of hepatic energy and lipid metabolism (Konno et al., 2008; Masson et al., 2008).
Acknowledgments
We acknowledge the expert technical assistance of Denise Weyant and thank Matthew DeKeyser for graphical assistance.
This work was supported, in part, by the National Institutes of Health National Institute of General Medical Sciences [Grant GM66411]; by the Intramural Research program of the National Institutes of Health National Institute of Environmental Health Sciences; and by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Contract N01-DK-7-0004/HHSN267200700004c]. The Quattro Premier XE mass spectrometer was purchased with funds from the National Science Foundation [Grant DBI-0619489].
ABBREVIATIONS: CAR, constitutive androstane receptor; CITCO, 6-(4-chlorophenyl)imidazo[2,1-b] [1,3]thiazole-5-carbaldehyde O-3,4-dichlorobenzyl) oxime; DEHP, di(2-ethylhexyl) phthalate; DMSO, dimethyl sulfoxide; RXR, retinoid X receptor; CMV, cytomegalovirus; FBS, fetal bovine serum; SRC1, steroid receptor coactivator 1; MEHP, mono(ethylhexyl) phthalate.
References
- Arnold KA, Eichelbaum M, and Burk O (2004) Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nucl Recept 2 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, Dekeyser JG, Stoner MA, and Omiecinski CJ (2007) CAR2 displays unique ligand binding and RXRα heterodimerization characteristics. Drug Metab Dispos 35 428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, Ramsden R, Stoner MA, Verlinde C, Hassett C, and Omiecinski CJ (2003) Alternatively spliced isoforms of the human constitutive androstane receptor. Nucleic Acids Res 31 3194-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, Stoner MA, Su S, and Omiecinski CJ (2005) Retinoid x receptor-α-dependent transactivation by a naturally occurring structural variant of human constitutive androstane receptor (NR1I3). Mol Pharmacol 68 1239-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, and Moore DD (1994) A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol 14 1544-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Norris JD, Grøn H, Paige LA, Hamilton PT, Kenan DJ, Fowlkes D, and McDonnell DP (1999) Dissection of the LXXLL nuclear receptor-coactivator interaction motif using combinatorial peptide libraries: discovery of peptide antagonists of estrogen receptors alpha and beta. Mol Cell Biol 19 8226-8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Ryan L, and Hauser R (2005) Phthalate exposure and reproductive hormones in adult men. Hum Reprod 20 604-610. [DOI] [PubMed] [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, and Wang H (2007) Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 320 72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, and Moore DD (1998) Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature 395 612-615. [DOI] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB Jr, Kliewer SA, Gonzalez FJ, and Sinal CJ (2003) Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem 278 45062-45071. [DOI] [PubMed] [Google Scholar]
- Hauser R and Calafat AM (2005) Phthalates and human health. Occup Environ Med 62 806-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ and Powell CJ (1992) Phthalate ester effects on rat sertoli cell function in vitro: effects of phthalate side chain and age of animal. Toxicol Appl Pharmacol 115 116-123. [DOI] [PubMed] [Google Scholar]
- Hoppin JA (2003) Male reproductive effects of phthalates: an emerging picture. Epidemiology 14 259-260. [PubMed] [Google Scholar]
- Imaoka S, Yamada T, Hiroi T, Hayashi K, Sakaki T, Yabusaki Y, and Funae Y (1996) Multiple forms of human P450 expressed in Saccharomyces cerevisiae. Systematic characterization and comparison with those of the rat. Biochem Pharmacol 51 1041-1050. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yokota H, Wang R, Yamanoshita O, Ichihara G, Wang H, Kurata Y, Takagi K, and Nakajima T (2005) Species differences in the metabolism of di(2-ethylhexyl) phthalate (DEHP) in several organs of mice, rats, and marmosets. Arch Toxicol 79 147-154. [DOI] [PubMed] [Google Scholar]
- Jinno H, Tanaka-Kagawa T, Hanioka N, Ishida S, Saeki M, Soyama A, Itoda M, Nishimura T, Saito Y, Ozawa S, et al. (2004) Identification of novel alternative splice variants of human constitutive androstane receptor and characterization of their expression in the liver. Mol Pharmacol 65 496-502. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, Gaido K, Hodgson E, Marcus M, Shea K, et al. (2006) NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 22 291-399. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, and Negishi M (1999) Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol 19 6318-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, and Haussler D (2002) The human genome browser at UCSC. Genome Research 12 996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno Y, Negishi M, and Kodama S (2008) The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet 23 8-13. [DOI] [PubMed] [Google Scholar]
- Lamba JK, Lamba V, Yasuda K, Lin YS, Assem M, Thompson E, Strom S, and Schuetz E (2004) Expression of constitutive androstane receptor splice variants in human tissues and their functional consequences. J Pharmacol Exp Ther 311 811-821. [DOI] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, and Mazzeo P (2003) In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect 111 1783-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, et al. (2003) Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem 278 17277-17283. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, and Moore JT (2004) The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem 279 19832-19838. [DOI] [PubMed] [Google Scholar]
- Masson D, Qatanani M, Sberna AL, Xiao R, Pais de Barros JP, Grober J, Deckert V, Athias A, Gambert P, Lagrost L, et al. (2008) Activation of the constitutive androstane receptor decreases HDL in wild-type and human apoA-I transgenic mice. J Lipid Res 49 1682-1691. [DOI] [PubMed] [Google Scholar]
- McKee RH (2004) Phthalate exposure and early thelarche. Environ Health Perspect 112 A541-A543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, and Hauser R (2007) Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 115 1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, et al. (2000) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275 15122-15127. [DOI] [PubMed] [Google Scholar]
- Olsavsky KM, Page JL, Johnson MC, Zarbl H, Strom SC, and Omiecinski CJ (2007) Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol Appl Pharmacol 222 42-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Yu LJ, Crespi CL, and Waxman DJ (1999) Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on CDNA-expressed activities and liver microsomal P-450 Profiles. Drug Metab Dispos 27 655-666. [PubMed] [Google Scholar]
- Rusyn I, Peters JM, and Cunningham ML (2006) Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol 36 459-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savkur RS, Wu Y, Bramlett KS, Wang M, Yao S, Perkins D, Totten M, Searfoss G 3rd, Ryan TP, Su EW, et al. (2003) Alternative splicing within the ligand binding domain of the human constitutive androstane receptor. Mol Genet Metab 80 216-226. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration (2001) Safety Assessment of Di(2-ethylhexyl) Phthalate (DEHP) Released from PVC Medical Devices. Center for Devices and Radiological Health, US Food and Drug Administration, Rockville, MD. http://www.fda.gov/cdrh/ost/dehp-pvc.pdf
- Wei P, Zhang J, Egan-Hafley M, Liang S, and Moore DD (2000) The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 407 920-923. [DOI] [PubMed] [Google Scholar]
- Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, Cabrera GY, Tukey RH, and Evans RM (2003) Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci U S A 100 4150-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RX, Lambert MH, Wisely BB, Warren EN, Weinert EE, Waitt GM, Williams JD, Collins JL, Moore LB, Willson TM, et al. (2004) A structural basis for constitutive activity in the human CAR/RXRalpha heterodimer. Mol Cell 16 919-928. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, and Moore DD (2002) Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science 298 422-424. [DOI] [PubMed] [Google Scholar]