Abstract
Several studies have shown marked differences in the neural localization of language functions in the brains of left‐handed individuals when compared with right‐handers. Previous experiments involving functional lateralization have demonstrated cerebral blood flow patterns that differ concordantly with subject handedness while performing language‐related tasks. The effect of handedness on function in specific stages of memory processing, however, is a largely unexplored area. We used a paired‐associates verbal memory task to elicit activation of neural areas related to declarative memory, examining the hypothesis that there are differences in activation in the medial temporal lobe (MTL) between handedness groups. 15 left‐handed and 25 right‐handed healthy adults were matched for all major demographic and neuropsychological variables. Functional and structural imaging data were acquired and analyzed for group differences within MTL subregions. Our results show that activation of the MTL during declarative memory processing varies with handedness. While both groups showed activation in left and right MTL subregions, the left‐handed group showed a statistically significant increase in the left hippocampus and amygdala during both encoding and recall. No increases in activation were found in the right‐handed group. This effect was found in the absence of any differences in performance on the verbal memory task, structural volumetric disparities, or functional asymmetries. This provides evidence of functional differences between left‐handers and right‐handers, which extends to declarative memory processes. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: laterality, cerebral dominance, language, paired‐associate learning
INTRODUCTION
Handedness is a well‐studied example of behavioral lateralization. Classic 19th century studies of handedness in aphasics have demonstrated a clear link to cerebral dominance [see historical review by Goodglass and Quadfasal,1954]. Rasmussen and Milner [1977] used the Wada intracarotid Amytal test in a sample of epilepsy patients to demonstrate the lateralization of language and speech functions. They found a pattern of right hemispheric language dominance in approximately 50% of the left‐handed patients, as opposed to only 20% of the right‐handers. Other studies, however, have demonstrated that true right hemisphere language dominance is rare and that in the absence of left‐lateralized language, bilateral representation of language is more common [Geschwind and Galaburda,1985; Loring et al.,1990; Satz,1979]. This finding was also recently corroborated by functional imaging studies [Bahn et al.,1997; Binder et al.,1996; Desmond et al.,1995; Hertz‐Pannier et al.,1997].
Although the laterality of language processes has been studied extensively in left‐handers, we know much less about the possible laterality of memory functions. A large body of research has focused on the functional localization of memory abilities and possible hemispheric asymmetries related to the type of memory encoded or the stage of memory processing. The functional localization of memory to medial temporal structures dates back to early lesion studies of amnesic patients such as H.M. and others [Scoville and Milner,1957]. It has been established that medial temporal lobe structures as well as prefrontal cortical structures play an essential role in declarative memory processes [Cohen and Eichenbaum,1993; Gabrieli et al.,1998; Poldrack and Gabrieli,1997]. Unfortunately, attempts to localize the discrete stages of memory function (such as encoding, storage, or retrieval) have produced divergent results, though most evidence indicates the existence of functional hemispheric lateralization.
Classic studies of unilateral lesions provide evidence for material‐specific memory processing and associated hemispheric lateralization, with left hemispheric lesions impairing verbal memory functions, and right hemispheric lesions impairing nonverbal (e.g., visuospatial) memory activities [see review by Milner,1972]. The onset of advanced brain imaging methods sheds new light on this distinction; using functional MRI, studies demonstrated left/right hemispheric laterality effects modulated by the stage of memory processing within the task, and not by material type. These studies implicated the left prefrontal cortex in memory encoding, and the right prefrontal cortex in retrieval, giving rise to the Hemispheric Encoding/Retrieval Asymmetry (HERA) model of prefrontal memory processes [Nyberg et al.,1996; Tulving et al.,1994].
A study by Kelley et al. [1998], investigating material‐type and stages of memory processing simultaneously, provided contradictory evidence to the HERA model. Kelley and colleagues used three different types of stimuli: words, line drawings of common objects, and unfamiliar faces to investigate purely verbal, mixed verbal and spatial, and purely spatial activation, respectively. Convergent with prior lesion studies, they found that hemispheric lateralization during encoding depended on the type of material being encoded, with activation of left prefrontal regions during encoding of words, and activation of right prefrontal regions during encoding of unfamiliar faces. Encoding of nameable objects yielded bilateral prefrontal activation. A similar pattern was also found in the medial temporal lobe. Wagner et al. [1998] also considered encoding and retrieval separately, and while they found no differences in the locus of activation based on stage of processing, they observed a strong link between the laterality of inferior frontal activation and material type, lending further support to the material‐specific lateralization hypothesis. Since then, Tulving's group has expanded the definition of the HERA model to show that it can be compatible with material‐specific models of encoding and retrieval [Habib et al.,2003], but it is still unclear whether this design is sufficient for explaining what seem to be contradictory results from material‐specific and task‐specific paradigms.
To accommodate these divergent findings, a more comprehensive model of the functional anatomy of memory is needed. A potentially important dimension for such a model that has not been studied extensively is handedness and its possible relationship to hemispheric asymmetry in the context of declarative memory. Since hemispheric dominance for language abilities may differ by handedness of the individual, we postulated that verbal memory, a cognitive process involving language, may be processed differently in right‐ and left‐handed individuals.
In this study, we explored the effect of handedness on declarative memory function using a verbal memory task with distinct encoding and retrieval stages. We examined functional MRI activation differences between right‐ and left‐handed individuals during both phases of the task.
MATERIALS AND METHODS
Sample Selection
Participants included in this analysis are healthy adults aged 50 and older, who serve as controls for an on‐going longitudinal study of aging and cognition [Bassett2006]. Fifteen unambiguous left‐handers were matched to 25 right‐handed individuals on age, gender, years of formal education, and IQ as measured by the New Adult Reading Test‐Revised (NART‐R) [Blair and Spreen,1989]. Handedness was assessed using the Edinburgh Handedness Inventory [Oldfield,1971]. Group differences on age, gender, education, and IQ were statistically nonsignificant by independent samples t‐tests (Table I).
Table I.
Subject demographics
| Variable | Right‐handed | Left‐handed | Significance (P) |
|---|---|---|---|
| N | 25 | 15 | N/A |
| Age | 60.2 ± 6.5 | 59.9 ± 6.4 | 0.875 |
| Sex ratio (M:F) | 18:7 | 11:4 | 0.366 |
| Years of education | 15.8 ± 2.8 | 16.7 ± 2.9 | 0.352 |
| Verbal IQ | 107.7 ± 7.6 | 108.6 ± 13.0 | 0.788 |
| Full scale IQ | 109.3 ± 6.6 | 110.1 ± 11.4 | 0.765 |
fMRI Paradigm
The word‐pair associates learning task, outlined in an earlier manuscript [Bassett et al.,2006], was employed because of its sensitivity to medial temporal lobe damage [Rausch and Babb,1993]. The paradigm was programmed in E‐prime (Psychology Software Tools, Pittsburgh, PA) and consisted of two 6 min 10 s sessions. Each session contained six trials. Each trial included an encoding phase, in which seven unrelated word‐pairs were presented verbally (e.g. food and book), and a cued recall phase, in which the first word from the pair was presented, and the subject was instructed to silently recall the second. Both encoding and recall were preceded by rest (baseline) periods. Prior to scanning, the Paired Associates Learning task from the WMS‐R battery was administered to each participant to evaluate out‐of‐scanner performance (WMS‐R: Wechsler,1987]. This task is similar to the fMRI paradigm, but uses a series of different word‐pairs to eliminate the possibility of practice effects. In‐scanner performance was evaluated by means of a free recall debriefing immediately after each session, while the subject was still in the scanner.
MRI Scanning Protocol
Functional scans were acquired on a 1.5 T Philips Intera‐NT scanner (Philips Medical Systems, Best, The Netherlands) at the F.M. Kirby Functional Imaging Research Center (Kennedy Krieger Institute, Baltimore, MD). The system is equipped with Galaxy gradients (66 mT/m at 110 mT/m/s). Two functional scans were acquired using echo‐planar imaging (EPI) and a blood oxygenation level dependent (BOLD) technique with repetition time [TR] = 1,000 ms, echo time [TE] = 39 ms, flip angle = 90o, field of view [FOV] = 230 mm, and acquisition matrix = 64 × 64. Eighteen coronal slices were acquired with a 4.5 mm thickness and an inter‐slice gap of 0.5 mm, oriented perpendicularly to the anterior‐posterior commissure (AC‐PC) line. This allowed for complete coverage of medial temporal structures, while reducing the possibility of obtaining artifacts in the images due to incomplete echo digitization.
A high‐resolution anatomical image of the brain was obtained using a high resolution T1‐weighted, 3D MP‐RAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) sequence [Mugler and Brookeman,1990]: with the following parameters: TR = 8.6 ms, TE = 3.9 ms, FOV = 240 mm, flip angle = 8°, matrix size = 256 × 256, slice thickness = 1.5 mm, 124 slices.
Structural MRI Processing and Analysis
Volumetric measures were completed using locally developed custom graphics software [MEASURE, Barta et al.,1997] and neuroanatomical measurement protocols. Volumes for total brain, hippocampus, amygdala, and entorhinal cortex were measured, with a rater (JLC for total brain volume or NAH for medial temporal lobe structures) blind to group status, according to previously described protocols [Barta et al.,1997; Honeycutt et al.,1998]. The locally developed protocols for these measurements have been shown to be highly reliable, with intraclass correlation coefficients of 0.99 for total brain volume, 0.95 for hippocampus, 0.88 for amygdala, and 0.96 for entorhinal cortex. An analysis of covariance (ANCOVA) was used to assess volumetric differences of MTL structures, treating total brain volume as a nuisance variable.
fMRI Processing and Analysis
Functional data processing was conducted on Windows XP workstations, using Statistical Parametric Mapping (SPM99, Wellcome Department of Imaging Neuroscience, UCL, London, UK) running under MATLAB 6.1 (The Mathworks, Sherborn, MA). Scans were corrected for head motion using a 6‐parameter rigid‐body transformation, and normalized to standard space using a 12 parameter affine transform, followed by the application of nonlinear basis functions. A high resolution EPI scan (Montreal Neurologic Institute, McGill University, Montreal, Canada) was used as a reference template for normalization. Normalized scans were resliced to isotropic voxels (2 mm3) and spatially smoothed with a full‐width at half‐maximum (FWHM) Gaussian kernel of 5 mm3.
The analysis of individual time series was conducted using the general linear model within the framework of statistical parametric mapping. Blocks of interest were identified as the encoding and recall phases and summed over both sessions. Baseline blocks were subtracted from the blocks of interest. Individual contrast maps were generated and used for second order, random effects analyses. This was used to investigate both within group activation (1 sample t‐tests), and between group differences in activation (independent samples t‐tests) during the encoding and recall phases.
Regions of Interest (ROI) Analysis
Structural scans acquired for all subjects were registered (using SPM's nonlinear registration tool) to the MNI single subject template and used to create an averaged anatomical template. Outlines of the medial temporal lobe that encompassed the hippocampi, parahippocampal gyri, entorhinal cortices, and the amygdale were manually placed on this template using a robust method established in our laboratory [Honeycutt et al.,1998]. Statistical images from the group analyses were masked with the medial temporal lobe regions of interest using SPM's small volume correction utility. This ensured that the statistical significance values produced were corrected only for the appropriate volumes of interest and not for the entire brain. Only results within the regions of interest were considered for this analysis.
Functional ROI masks were constructed to evaluate possible activation asymmetry in the temporal lobes. These masks were generated in MarsBaR Toolbox for SPM (MARSeille Boîte À Région d'Intérêt Toolbox 0.38, The MarsBaR Team, Marseilles, France) by creating a composite of the left and right hemisphere activations. This resulted in two sets of symmetrical masks, corresponding to the areas of greatest activation in the Encode and Recall conditions. The ROI data for these masks were acquired through MarsBaR by extracting the values of the beta files for the Encode and Recall conditions.
RESULTS
Functional MRI Activation
Within group analysis found both right‐ and left‐handers demonstrating similar activation patterns in response to this memory paradigm (Fig. 1). During the encoding phase both groups showed increased activation in the parahippocampal and superior temporal regions (Brodmann areas 22, 28, 38) with T values ranging from 5.15 to 2.85. During recall both showed activation in the superior and middle temporal regions, with T values ranging from 10.03 to 3.22. The left‐handers also showed activation in the right parahippocampal gyrus.
Figure 1.
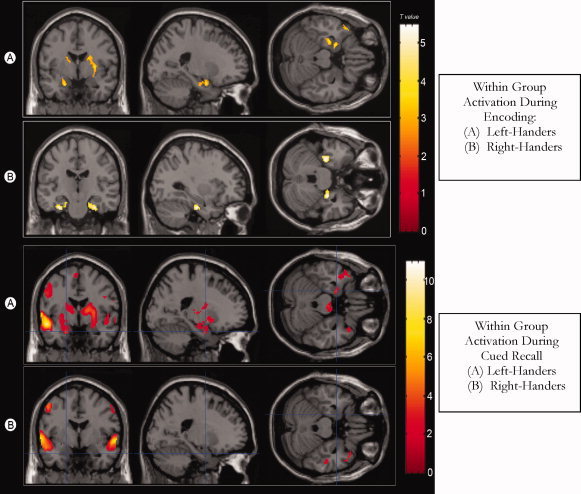
Within‐Group Comparisons for Encoding (top) and Recall (bottom).
Comparison of group activation during the encoding phase revealed a single cluster (43 voxels; Talairach coordinates −20, 3, −20; T = 4.03; P < 0.05 corrected for family‐wise error) in the left medial temporal lobe that demonstrated increased activation in left‐handed individuals when compared with right‐handed individuals (Fig. 2). The cluster was further localized using medial temporal lobe subdivision masks (hippocampus, entorhinal cortex, amygdala). The locus of over‐activation was mapped to the left amygdala and the left hippocampus. The reverse contrast (increased activation in right‐handers compared to left‐handers) did not yield any significant findings. Comparison of group activation during the recall phase revealed similar results; left‐handed individuals showed greater activation in the exact same locus as during encoding (T = 3.71; P < 0.05 corrected for family‐wise error). Once again, the reverse comparison (Right‐handers minus left‐handers) did not show any differences. Examination of functional asymmetry in the medial temporal lobe did not find differences between the groups. During the encode phase there was no significant functional asymmetry in either the left‐handers (P = 0.166) or right‐handers (P = 0.697). During recall, both left‐ and right‐handers demonstrated significant asymmetry, with greater activation in the left hemisphere. The degree of asymmetry was similar in both groups (left‐handed: P = 0.009; right‐handed: P = 0.018; Fig. 3).
Figure 2.
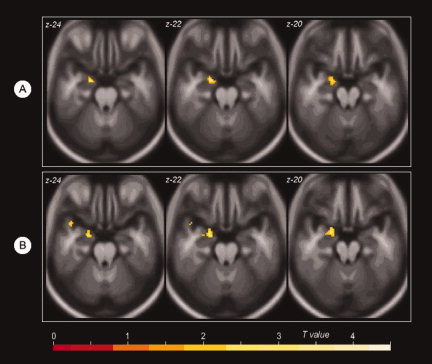
Functional Activation Group Differences During Encoding (A) and Recall (B). A: Increased activation in the left medial temporal lobe in left‐handed (LH) individuals compared to right‐handed (RH) individuals during the encoding phase. B: Increased activation in the same locus of the left medial temporal lobe in LH individuals compared to RH individuals during the cued recall phase. Results are overlaid on a custom averaged template. The Z axis coordinate of each axial slice is noted in the top left corner of each image.
Figure 3.
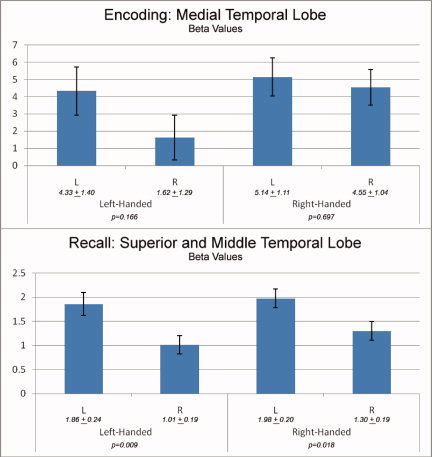
Functional Asymmetry Measures Beta values and asymmetry during encoding (top) and recall (bottom) in their respective maxima.
Volumetric and Behavioral Results
Volumes of bilateral MTL sub‐regions including the hippocampus, entorhinal cortex, and amygdala did not significantly differ between the right and left‐handers (Table II) Group differences in performance on paired associate learning tasks both inside and outside of the scanner were statistically nonsignificant by independent samples t‐tests (P > 0.05, Table III).
Table II.
MTL volume comparisons
| Region | Right‐handed | Left‐handed | Significance (P) |
|---|---|---|---|
| Left hippocampus | 3.196 ± 0.080 | 3.320 ± 0.102 | 0.352 |
| Right hippocampus | 3.213 ± 0.116 | 3.283 ± 0.148 | 0.716 |
| Left amygdala | 1.373 ± 0.054 | 1.460 ± 0.069 | 0.334 |
| Right amygdala | 1.552 ± 0.077 | 1.516 ± 0.098 | 0.779 |
All means were adjusted for total brain volume. Reported volumes are in cubic centimeters.
Table III.
Subject verbal memory performance
| Variable | Right‐handed | Left‐handed | Significance (P) |
|---|---|---|---|
| Paired associates recall (outside scanner) | 12.24 ± 2.84 | 12.36 ± 2.32 | 0.346 |
| Word pair free recall (inside scanner) | 7.47 ± 2.71 | 6.08 ± 2.75 | 0.177 |
DISCUSSION
Previous studies have localized declarative memory functions to the medial temporal (MTL) and frontal lobes; however, specific hemispheric localization of these functions has not been consistent. Lesion studies, as well as some brain imaging studies, suggest that functional hemispheric asymmetry depends on the type of stimulus being remembered (verbal vs. nonverbal), while other fMRI studies indicate that distinct stages of processing are localized in different hemispheres (encoding vs. retrieval). To date, no study has explored the effects of handedness on declarative memory function in the MTL.
Our findings suggest that left‐handed individuals tend to recruit more neural resources in the left medial temporal lobe during both the encoding and recall stages of this declarative memory task, though this heightened activation is not associated with improved performance. It has been hypothesized that such increased activation reflects the recruitment of alternative neural mechanisms that compensate for impairment. This view is supported by studies of those with cognitive impairments who demonstrate a pattern of increased activation to successfully complete memory tasks [Dickerson et al.,2005; Grady et al.,2003]. Theories of cerebral dominance including the developmental model of Geschwind [Geschwind and Behan,1982] and the genetic “right shift” theory of Annett [Annett and Kilshaw,1983] posit suboptimal verbal processing skills in left‐handed individuals when compared with right‐handers. This might explain the increased activation seen among the left‐handers; however, these theories have not addressed memory function as assessed here and neither has been substantially supported [Bryden et al., 1994; McManus et al., 1993].
Studies of brain morphology have determined that there are often variations in the structural development of language areas according to handedness. The most consistent finding is a lack of expected leftward asymmetry in the planum temporale of left‐handers [Herve et al.,2006; Shapleske et al.,1999; Steinmetz et al.,1991]. Handedness‐related differences have also been discovered in the subregions of Broca's area, which has long been accepted as a primary center for language processing and production. In the pars triangularis region of right‐handed people, a leftward asymmetry is typical, whereas the opposite finding is more common in the brains of left‐handers. A general leftward asymmetry was found in the pars opercularis in both right and left‐handers, but the effect was significantly weaker in the brains of left‐handed people [Foundas et al.,1995,1998]. Although the pars triangularis is thought to be associated with lexical retrieval, a dimension involved in this particular fMRI task, we did not find any inter‐group activation differences in the pars triangularis, or in any other cortical area associated with language function.
A typical pattern of asymmetry in hippocampal structures has been noted in many studies, with volumes of right hippocampi tending to be larger than left hippocampi in children [Giedd et al.,1996] and healthy adult samples [Pedraza et al.,2004, meta‐analysis]. Several studies examining handedness report temporal lobe asymmetries to be much smaller or nonexistent in left‐handers in comparison to the significant asymmetries seen in right‐handers [Szabo et al., 2001; Watkins et al.,2001]. We did not find any evidence of structural asymmetry in our sample of older adults, which is in concordance with results from Anstey et al. [2004], who found that the expected hippocampal asymmetry was not identified in a large group of adults, aged 60–64.
The functional differences seen with this verbal memory task do not reflect differential activation of cortical language areas, nor do they appear to be the result of underlying structural differences. In addition, the lack of significant asymmetry during encoding and the presence of similar asymmetry during recall preclude ascribing the disparity in activation of the left medial temporal lobes between groups to a difference in functional asymmetry. It should be noted that despite the lack of significance, there is a trend suggesting greater asymmetry during the encoding phase in left‐handed subjects, a point which could be explored with a larger sample of left‐handed individuals.
It remains unclear whether increased activation in response to this task among left‐handers is a marker for less efficient cognitive processing, or reflects the less lateralized pattern of language function seen in some left‐handers, which is significant as this paradigm employs verbal word pairs. For example, in an fMRI study of language, Pujol et al. [1999] found left‐hemisphere dominance to be less frequent in left‐handers (76%) compared to right‐handers (96%). It is possible that our left‐handed subjects were demonstrating evidence of less lateralized left hemisphere dominance rather than deficit; however, the increased activation was not associated with improved performance on the memory task. Left‐handed individuals showed more activation in the left medial temporal lobe, yet this did not correspond to higher scores on free recall of word pairs. This suggests that they may be less efficient at processing declarative memories than right‐handed controls, by virtue of having to recruit more extensive neural resources.
Somewhat surprisingly, there were no right hemispheric differences in activation between groups. Most studies investigating sinistrality conclude that a significant percentage of left‐handed individuals have a bilateral network of linguistic neural processing [Geschwind and Galaburda,1985; Loring et al.,1990; Pujol et al.,1999]. Although our results may seem contradictory, our sample size is perhaps too small to detect right hemispheric differences that may only be present in a subset of the sample. It is also possible that hemispheric functional asymmetry is reduced in our subjects because of their older age. A large body of evidence indicates that lateralization of function decreases with increasing age in the prefrontal cortex, which is the basis of the Hemispheric Asymmetry Reduction in Older Adults (HAROLD) model of cognitive performance [Cabeza,2002]. Cabeza postulated that this difference is compensatory in nature; older adults may need to employ both hemispheres to perform tasks that usually require the use of only one hemisphere in younger adults. Although the HAROLD model is generally used to describe activity in the frontal lobes, it is possible that this pattern of increased bilateral activity extends to associated memory processes in the medial temporal lobes. Recent reports of differences in activation during both encoding and retrieval found when contrasting younger and older individuals [Morcum et al.,2003,2007] precludes the application of these findings across the age spectrum.
CONCLUSION
This study proposes yet another dimension that influences memory function, in addition to the established determinants of material type and distinct memory processing stage. Our findings emphasize the importance of taking handedness into consideration when studying declarative memory, at least in those 50 years of age and over. Future brain imaging work should continue to investigate the neural correlates of handedness and its relationship to memory and other cognitive abilities.
REFERENCES
- Annett M,Kilshaw D ( 1983): Right‐ and left‐handed skill II: Estimating the parameters of the distribution of L‐R differences in males and females. Br J Psychol 74: 269–283. [DOI] [PubMed] [Google Scholar]
- Anstey KJ,Maller JJ,Meslin C,Christensen H,Jorm AF,Wen W,Sachdev P ( 2004): Hippocampal and amygdalar volumes in relation to handedness in adults aged 60–64. NeuroReport 15: 2825–2829. [PubMed] [Google Scholar]
- Bahn MM,Lin W,Silbergeld DL,Miller JW,Kuppusamy K,Cook RJ,Hammer G,Wetzel R,Cross D III ( 1997): Localization of language cortices by functional MR imaging compared with intracarotid amobarbital hemispheric sedation. Am J Roentgenol 169: 575–579. [DOI] [PubMed] [Google Scholar]
- Barta PE,Dhingra L,Royall R,Schwartz E ( 1997): Improving stereological estimates for the volume of structures identified in three‐dimensional arrays of spatial data. J Neurosci Methods 75: 111–118. [DOI] [PubMed] [Google Scholar]
- Bassett SS,Yousem DM,Cristinzio C,Kusevic I,Yassa MA,Caffo BS,Zeger SL ( 2006): Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain 129: 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR,Swanson SJ,Hammeke TA,Morris GL,Mueller WM,Fischer M,Benbadis S,Frost JA,Rao SM,Haughton VM ( 1996): Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology 46: 978–984. [DOI] [PubMed] [Google Scholar]
- Blair JR,Spreen O ( 1989): Predicting premorbid IQ: A revision of the national adult reading test. Clin Psychol 3: 129–136. [Google Scholar]
- Bryden MP,McManus IC,Bulman‐Fleming MB ( 1994): Evaluating the empirical support for the Geschwind‐Behan‐Galaburda model of cerebral lateralization. Brain Cogn 26: 103–167. [DOI] [PubMed] [Google Scholar]
- Cabeza R ( 2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Cohen NJ,Eichenbaum HE ( 1993): Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press. [Google Scholar]
- Desmond JE,Sum JM,Wagner AD,Demb JB,Shear PK,Glover GH,Gabrieli JD,Morrell MJ ( 1995): Functional MRI measurement of language lateralization in Wada‐tested patients. Brain 118 (Part 6): 1411–1419. [DOI] [PubMed] [Google Scholar]
- Dickerson BC,Salat DH,Greve DN,Chua EF,Rand‐Giovannetti E,Rentz DM,Bertram L,Mullin K,Tanzi RE,Blacker D,Albert MS,Sperling RA ( 2005): Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL,Leonard CM,Heilman KM ( 1995): Morphologic cerebral asymmetries and handedness. The pars triangularis and planum temporale. Arch Neurol 52: 501–508. [DOI] [PubMed] [Google Scholar]
- Foundas AL,Eure KF,Luevano LF,Weinberger DR ( 1998): MRI asymmetries of Broca's area: The pars triangularis and pars opercularis. Brain Lang 64: 282–296. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD,Brewer JB,Poldrack RA ( 1998): Images of medial temporal lobe functions in human learning and memory. Neurobiol Learn Mem 70: 275–283. [DOI] [PubMed] [Google Scholar]
- Geschwind N,Behan P ( 1982): Left‐handedness: Association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci USA 79: 5097–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N,Galaburda AM ( 1985): Cerebral lateralization. Biological mechanisms, associations, and pathology. I. A hypothesis and a program for research. Arch Neurol 42: 428–459. [DOI] [PubMed] [Google Scholar]
- Giedd JN,Vaituzis AC,Hamburger SD,Lange N,Rajapakse JC,Kaysen D,Vauss YC,Rapoport JL ( 1996): Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4–18 years. J Comp Neurol 366: 223–230. [DOI] [PubMed] [Google Scholar]
- Goodglass H,Quadfasal FA ( 1954): Language laterality in left‐handed aphasics. Brain 77: 521–548. [DOI] [PubMed] [Google Scholar]
- Grady CL,McIntosh AR,Beig S,Keightley ML,Burian H,Black SE ( 2003): Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci 23: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R,Nyberg L,Tulving E ( 2003): Hemispheric asymmetries of memory: The HERA model revisited. Trends Cogn Sci 7: 241–245. [DOI] [PubMed] [Google Scholar]
- Hertz‐Pannier L,Gaillard WD,Mott SH,Cuenod CA,Bookheimer SY,Weinstein S,Conry J,Papero PH,Schiff SJ,Le Bihan D,Theodore WH ( 1997): Noninvasive assessment of language dominance in children and adolescents with functional MRI: A preliminary study. Neurology 48: 1003–1012. [DOI] [PubMed] [Google Scholar]
- Herve PY,Crivello F,Perchey G,Mazoyer B,Tzourio‐Mazoyer N ( 2006): Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage 29: 1066–1079. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA,Smith PD,Aylward E,Li Q,Chan M,Barta PE,Pearlson GD ( 1998): Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Res 83: 85–94. [DOI] [PubMed] [Google Scholar]
- Kelley WM,Miezin FM,McDermott KB,Buckner RL,Raichle ME,Cohen NJ,Ollinger JM,Akbudak E,Conturo TE,Snyder AZ,Petersen SE ( 1998): Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron 20: 927–936. [DOI] [PubMed] [Google Scholar]
- Loring DW,Meador KJ,Lee GP,Murro AM,Smith JR,Flanigin HF,Gallagher BB,King DW ( 1990): Cerebral language lateralization: Evidence from intracarotid amobarbital testing. Neuropsychologia 28: 831–838. [DOI] [PubMed] [Google Scholar]
- McManus IC,Shergill S,Bryden MP ( 1993): Annett's theory that individuals heterozygous for the right shift gene are intellectually advantaged: Theoretical and empirical problems. Br J Psychol 84 (Pt 4): 517–537. [DOI] [PubMed] [Google Scholar]
- Milner B ( 1972): Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 19: 421–446. [DOI] [PubMed] [Google Scholar]
- Morcom AM,Good CD,Frackowiak RS,Rugg MD ( 2003): Age effects on the neural correlates of successful memory encoding. Brain 126 (Part 1): m213–m229. [DOI] [PubMed] [Google Scholar]
- Morcom AM,Li J,Rugg MD ( 2007): Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb Cortex 17: 2491–2506. [DOI] [PubMed] [Google Scholar]
- Mugler JP III,Brookeman JR ( 1990): Three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MP RAGE). Magn Reson Med 15: 152–157. [DOI] [PubMed] [Google Scholar]
- Nyberg L,McIntosh AR,Cabeza R,Habib R,Houle S,Tulving E ( 1996): General and specific brain regions involved in encoding and retrieval of events: What, where, and when. Proc Natl Acad Sci USA 93: 11280–11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pedraza O,Bowers D,Gilmore R ( 2004): Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc 10: 664–678. [DOI] [PubMed] [Google Scholar]
- Poldrack RA,Gabrieli JD ( 1997): Functional anatomy of long‐term memory. J Clin Neurophysiol 14: 294–310. [DOI] [PubMed] [Google Scholar]
- Pujol J,Deus J,Losilla JM,Capdevila A ( 1999): Cerebral lateralization of language in normal left‐handed people studied by functional MRI. Neurology 52: 1038–1043. [DOI] [PubMed] [Google Scholar]
- Rasmussen T,Milner B ( 1977): The role of early left‐brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci 299: 355–369. [DOI] [PubMed] [Google Scholar]
- Rausch R,Babb TL ( 1993): Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol 50: 812–817. [DOI] [PubMed] [Google Scholar]
- Satz P ( 1979): A test of some models of hemispheric speech organization in the left‐ and right‐handed. Science 203: 1131–1133. [DOI] [PubMed] [Google Scholar]
- Scoville W,Milner B ( 1957): Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapleske J,Rossell SL,Woodruff PW,David AS ( 1999): The planum temporale: A systematic quantitative review of its structural, functional and clinical significance. Brain Res Rev 29: 26–49. [DOI] [PubMed] [Google Scholar]
- Steinmetz H,Volkmann J,Jancke L,Freund HJ ( 1991): Anatomical left‐right asymmetry of language‐related temporal cortex is different in left‐ and right‐handers. Ann Neurol 29: 315–319. [DOI] [PubMed] [Google Scholar]
- Tulving E,Kapur S,Craik FI,Moscovitch M,Houle S ( 1994): Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci USA 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD,Poldrack RA,Eldridge LL,Desmond JE,Glover GH,Gabrieli JD ( 1998): Material‐specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport 9: 3711–3717. [DOI] [PubMed] [Google Scholar]
- Watkins KE,Paus T,Lerch JP,Zijdenbos A,Collins DL,Neelin P,Taylor J,Worsley KJ,Evans AC ( 2001): Structural asymmetries in the human brain: A voxel‐based statistical analysis of 142 MRI scans. Cereb Cortex 11: 868–877. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 1987): Wechsler Memory Scale‐Revised manual. San Antonio, TX: Psychological Corporation. [Google Scholar]


