Abstract
Objective
To evaluate the relation between chorioamnionitis and hypotension in very low birthweight infants.
Methods
Retrospective cohort study in infants with a birth weight of <1500 g born between January 2002 and September 2004. The placentas were examined for evidence of chorioamnionitis and funisitis. Hypotension was defined by the use of vasopressors.
Results
Of 105 infants, 37 (35%) were chorioamnionitis positive. The onset of hypotension had a skewed distribution: day 1 for 30 episodes and scattered from day 2 to day 19 for the remaining 22. Of the 30 infants who developed hypotension on day 1, 17 (57%) were chorioamnionitis positive. The mean maturity of the chorioamnionitis positive group was 27.91 weeks, marginally less than the mean maturity of 29.05 weeks of the chorioamnionitis negative group (p = 0.05). After adjustment of the effects for confounding variables (birth weight, gestation, surfactant therapy, mechanical ventilation on day 1, high frequency oscillatory ventilation, patent ductus arteriosus), chorioamnionitis was the significant factor in line with hypotension developing on day 1 (adjusted odds ratio 5.14, 95% confidence interval 1.51 to 17.50). There was no evidence that hypotension developing after day 1 was associated with chorioamnionitis.
Conclusions
There is a strong association between chorioamnionitis and hypotension developing on day 1 in very low birthweight infants.
Keywords: chorioamnionitis, funisitis, hypotension, very low birthweight infants, cytokines
Hypotension is a common problem of very low birthweight (VLBW) infants. In a study of hypotension in six neonatal intensive care units, the prevalence of hypotension ranged from 24% to 45%, and up to 39% of infants required vasopressor treatment.1 In most preterm infants, especially during the immediate postnatal period, hypotension is primarily caused by abnormal peripheral vasoregulation and/or myocardial dysfunction and not by absolute hypovolaemia.2,3 Downregulation of cardiovascular adrenergic receptors and some degree of adrenal insufficiency may explain this phenomenon.
A relation between chorioamnionitis and hypotension in the first two to four hours after preterm birth has been reported.4 Chorioamnionitis is known to be associated with an increased interleukin 6 and interleukin 1β concentration in the cord blood, and with tachycardia and hypotension. Tumour necrosis factor α and interleukin 1β were known to produce relaxation of vascular smooth muscle.5,6 Pro‐inflammatory cytokines have therefore been implicated in the development of hypotension following chorioamnionitis.
As preterm infants often require vasopressor treatment for hypotension beyond the first hours after birth, we decided to conduct a study that extended the blood pressure observations for a longer period of time. It has been reported that, after chorioamnionitis, raised concentrations of pro‐inflammatory cytokines in the respiratory tract of VLBW infants may persist for weeks and contribute to the development of chronic lung disease.7 Therefore, as pro‐inflammatory cytokines present in the airway may enter the systemic circulation causing vasodilatory shock,8 chorioamnionitis may be associated with shock beyond the first postnatal day. In this study, we set out to investigate the relation between chorioamnionitis and hypotension in VLBW infants during the first four weeks after delivery rather than only examining this relation in the first postnatal day.
Methods
This was a retrospective cohort study of VLBW infants who by definition have a birth weight <1500 g. As the placentas of VLBW infants were routinely sent for pathological section in our hospital, histological sections of these placentas were available for re‐examination. As funisitis (vasculitis of the umbilical cord) is associated with raised cord blood cytokine concentrations similar to chorioamnionitis alone,9,10 we also examined histological sections of the umbilical cord for signs of inflammation. Chorioamnionitis and funisitis were defined as the presence of tissue infiltration of more than 10 neutrophils per high power field in the chorion or amnion of haematoxyolin and eosin stained sections of the membrane roll and umbilical cord.4 Infants were divided into two groups: a chorioamnionitis positive group if there was either chorioamnionitis or funisitis, and a chorioamnionitis negative group if there was neither chorioamnionitis nor funisitis.
Hypotension was diagnosed when the mean systemic blood pressure fell below the 10th centile for birth weight and postnatal age in the first week after birth.11 There is not much information about the lowest acceptable blood pressure limit in VLBW infants in the second to fourth weeks, but the value of 30 mm Hg was used in this study beyond the first postnatal week. It has been reported that there is an increased risk of severe intraventricular haemorrhage12 and loss of cerebral blood flow autoregulation below 30 mm Hg,13 during the immediate postnatal period, but there is no reliable information about the autoregulatory range of systemic blood pressure in VLBW infants beyond this period. Treatment of hypotension began with an infusion of 15–20 ml normal saline, then vasopressor treatment if hypotension persisted, followed by hydrocortisone for refractory hypotension. For the purpose of this study, hypotension is defined as the need for vasopressor treatment for systemic hypotension diagnosed with the above criteria and managed with the standard protocol. In our neonatal unit, arterial catheters were routinely inserted for all infants weighing less than 1000 g at birth or requiring continuous positive airway pressure or mechanical ventilation irrespective of birth weight. For the remaining infants in whom an arterial catheter is not inserted on admission, we routinely measure blood pressure by the oscillometric method (Dinamap) every one to two hours, reducing to every four hours after the first week when their condition stabilises. However, we do not rely on oscillometric measurements of blood pressure when it comes to a decision of using pressors for treating hypotension. When the Dinamap detects hypotension, an arterial catheter is inserted to confirm the arterial blood pressure and to enable the continuous and direct monitoring of arterial blood pressure. Hospital records were reviewed for antecedent factors that were potential causes of hypotension, in particular those that occurred within 24 hours of the onset of hypotension.
The prevalence of hypotension was compared between the chorioamnionitis positive and chorioamnionitis negative groups. Student's t test was used for continuous variates, and the Fisher exact test for discrete variates. Logistic regression analysis was used to adjust for the effects of confounding factors in the evaluation of the relations between chorioamnionitis and hypotension developing on day 1 (first 24 hours after birth) and hypotension developing after day 1. This study was approved by the hospital's ethics committee.
Results
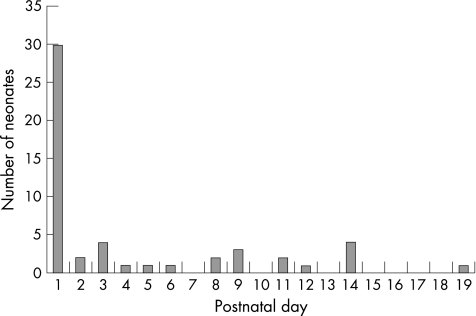
There were 112 VLBW infants admitted in the study period. Six were excluded because histological sections of the placenta were not performed, and one was excluded because the hospital record was lost. Our cohort consisted of 105 infants, of whom 37 (35%) had evidence of inflammation in their placentas (23 with both chorioamnionitis and funisitis, and 14 with chorioamnionitis only). Hypotension was diagnosed in 47 (45%) infants, who had a total of 52 episodes of hypotension. The onset of hypotension had a skewed distribution: the majority with onset on day 1 and the remaining scattered from day 2 to day 19 (fig 1). Infants with hypotension were divided into two groups: onset on day 1 and after day 1.
Figure 1 Onset of the 52 episodes of hypotension.
Table 1 shows that the chorioamnionitis positive group was born at a lower gestation than the chorioamnionitis negative group, although the difference only reached borderline significance (p = 0.05). Neither was there a significant difference between the two groups in birth weight, sex, antenatal corticosteroid or postnatal surfactant therapy, mechanical ventilation or high frequency oscillatory ventilation (HFOV) on day 1, haemodynamically significant patent ductus arteriosus (PDA) requiring indomethacin treatment, or mortality.
Table 1 Clinical data on babies born with and without chorioamnionitis.
| With chorioamnionitis (n = 37) | Without chorioamnionitis (n = 68) | p Value | |
|---|---|---|---|
| Birth weight (g) | |||
| Mean (SD) | 990 (250) | 1070 (250) | 0.105 |
| Median (interquartile range) | 930 (800–1170) | 1050 (890–1290) | – |
| Gestation (weeks) | |||
| Mean (SD) | 27.9 (3.3) | 29.1 (2.6) | 0.050 |
| Median (interquartile range) | 27.3 (25.2–29.6) | 28.8 (27.3–31.0) | – |
| Male (%) | 54 | 53 | 1.000 |
| Antenatal corticosteroids (%) | 82 | 77 | 0.594 |
| Mechanical ventilation on day 1 (%) | 73 | 67 | 0.660 |
| HFOV on day 1 (%) | 19 | 17 | 0.589 |
| Surfactant therapy (%) | 68 | 59 | 0.395 |
| PDA requiring indomethacin (%) | 27 | 26 | 1.000 |
| Mortality (%) | 8 | 9 | 1.000 |
HFOV, High frequency oscillatory ventilation; PDA, patent ductus arteriosus.
There was a strong correlation between gestation and birth weight (r = 0.698, p<0.001), mechanical ventilation on day 1 and surfactant therapy (Fisher exact test, p<0.001), HFOV (Fisher exact test, p = 0.001), and PDA (Fisher exact test, p<0.001). To minimise the effect of possible multicollinearity, a logistic regression model of hypotension and a Cox survival model for survival were constructed with the following independent variables: gestation (as proxy for birth weight), mechanical ventilation on day 1 (as proxy for surfactant therapy, HFOV, and PDA), sex, and chorioamnionitis.
Thirty infants developed hypotension on day 1, of whom 17 were in the chorioamnionitis positive group. After adjustment for potential confounding variables, chorioamnionitis with or without funisitis was found to be an independent risk factor for hypotension on the first postnatal day. Table 2 shows that the prevalence of hypotension on day 1 was 46% (17 of 37) in the chorioamnionitis positive group and 19% (13 of 68) in the chorioamnionitis negative group (adjusted odds ratio 5.14, 95% confidence interval 1.70 to 18.79).
Table 2 Association between chorioamnionitis and hypotension.
| Hypotension onset | Chorioamnionitis positive (n = 37) | Chorioamnionitis negative (n = 68) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI)* |
|---|---|---|---|---|
| Day 1 | 17 (46%) | 13 (19%) | 3.60 (1.48 to 8.71) | 5.65 (1.70 to 18.79) |
| After day 1 | 8 (22%) | 14 (21%) | 1.12 (0.42 to 3.00) | 0.66 (0.20 to 2.19) |
*Additional variables entered into the logistic regression model: antenatal corticosteroid therapy, gestation, sex, and mechanical ventilation on day 1.
Twenty two infants developed hypotension after day 1, but this was not found to be associated with chorioamnionitis (table 2). Logistic regression analysis showed that low gestation was the only factor that increased the risk of hypotension developing after day 1. Cox regression revealed that mechanical ventilation on day 1 and gestation, but not chorioamnionitis, were significantly associated with hypotension developing at anytime during the neonatal period.
Five infants had hypotension first diagnosed and treated on day 1, followed by resolution, but a second episode requiring treatment occurred. The time from resolution of the first episode of hypotension to the occurrence of the second episode ranged from 2 to 11 days (median five days). Both episodes were included in the analysis. When the later episodes were excluded from the analysis in these five infants, the results were similar.
No obvious antecedent factors were identified for hypotension developing on day 1. Although 12 infants in this group had a haemodynamically significant PDA, the median time of diagnosis was day 4, and it was unlikely that the PDA contributed to hypotension on day 1 in these infants. For the 22 infants who developed hypotension after day 1, antecedent factors were identified in 10. Five infants had sepsis, one with necrotising enterocolitis. Three infants had a haemodynamically significant PDA, one of whom had trisomy 13. Two infants developed hypotension as a terminal event: one with congenital herpes simplex infection and another with congenital cystic adenomatous malformation.
Discussion
In the previous study showing an association between chorioamnionitis and hypotension,4 the infant's blood pressure was measured only two to four hours after birth, and the number of infants who were on vasopressor treatment during this period was not reported. That study had also evaluated cardiac performance and measured cardiac output, and the findings suggested that VLBW infants with chorioamnionitis have good cardiac contractility and increased cardiac output. These observations suggest that vasodilation, and not myocardial dysfunction, is the predominant pathogenic factor for hypotension in this patient population.
The proportions of infants receiving antenatal corticosteroid therapy or HFOV were similar in the chorioamnionitis positive and chorioamnionitis negative groups. This lack of bias is important, as antenatal corticosteroid therapy improves blood pressure,14 but HFOV can compromise blood pressure.15
The strong association found in this study between chorioamnionitis and hypotension developing on day 1 is consistent with the finding of a previous study that more infants had hypotension two to four hours after birth following chorioamnionitis.4 In our chorioamnionitis group developing hypotension on day 1, the mean duration of hypotension was 5.4 days, which is consistent with the finding of another study that hypotension after funisitis and/or fetal vasculitis lasted for one week.16 In the episodes of hypotension developing after day 1, antecedent factors were identified, including sepsis, a haemodynamically significant PDA, and terminal events in patients.
What is already known on this topic
Chorioamnionitis is associated with hypotension two to four hours after birth in preterm infants
What this study adds
In VLBW infants, chorioamnionitis is associated with hypotension developing on day 1, but not when it develops after day 1, when other antecedent factors are present
In conclusion, this study has shown that chorioamnionitis is an important antecedent factor for hypotension developing on day 1, but not for hypotension developing after day 1.
Acknowledgements
We thank CH Chan, statistician, Department of Paediatrics, Kwong Wah Hospital.
Abbreviations
HFOV - high frequency oscillatory ventilation
PDA - patent ductus arteriosus
VLBW - very low birthweight
Footnotes
Competing interests: none declared
References
- 1.Al‐Aweel I, Pursley D M, Rubin L P.et al Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J Perinatol 200121272–278. [DOI] [PubMed] [Google Scholar]
- 2.Seri I. Circulatory support of the sick preterm infant. Semin Neonatol 2001685–95. [DOI] [PubMed] [Google Scholar]
- 3.Subhedar N V. Treatment of hypotension in newborns. Semin Neonatol 20038413–423. [DOI] [PubMed] [Google Scholar]
- 4.Yanowitz T D, Jordan J A, Gilmour C H.et al Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res 200251310–316. [DOI] [PubMed] [Google Scholar]
- 5.Abbas A, Lichtman A, Pober J.Cellular and molecular immunology. 2nd ed. Philadelphia: WB Saunders, 1994245–251.
- 6.Kuhl S, Rosen H. Nitric oxide and septic shock. West J Med 1998168176–181. [PMC free article] [PubMed] [Google Scholar]
- 7.Jobe A H. Antenatal factors and the development of bronchopulmonary dysplasia. Semin Neonatol 200389–17. [DOI] [PubMed] [Google Scholar]
- 8.Kramer B W, Ikegami M, Jobe A. Intratracheal endotoxin causes systemic inflammation in ventilated preterm lambs. Am J Respir Crit Care Med 2002165463–469. [DOI] [PubMed] [Google Scholar]
- 9.Kim C J, Yoon B H, Park S S.et al Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol 200132623–629. [DOI] [PubMed] [Google Scholar]
- 10.Pacora P, Chaiworapongsa T, Maymon E.et al Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Mat Fetal Neonatal Med 20021118–25. [DOI] [PubMed] [Google Scholar]
- 11.Nuntrarumit P, Yan W, Bado‐Ellzey H S. Blood pressure measurements in newborn. Clin Perinatol 199926981–996. [PubMed] [Google Scholar]
- 12.Miall‐Allen V M, de Vries L S, Whitelaw A G. Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child 1987621068–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munro M J, Walker A M, Barfield C P. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics 20041141591–1596. [DOI] [PubMed] [Google Scholar]
- 14.Demarini S, Dollberg S, Hoath S B.et al Effects of antenatal corticosteroids on blood pressure in very low birth weight infants during the first 24 hours of life. J Perinatol 199919(6 Pt1)419–425. [DOI] [PubMed] [Google Scholar]
- 15.Osborn D A, Evans N. Randomized trial of high‐frequency oscillatory ventilation versus conventional ventilation: effect on systemic blood flow in very preterm infants. J Pediatr 2003143192–198. [DOI] [PubMed] [Google Scholar]
- 16.Yanowitz T D, Baker R W, Roberts J M.et al Low blood pressure among very‐low‐birth‐weight infants with fetal vessel inflammation. J Perinatol 200424299–304. [DOI] [PubMed] [Google Scholar]