Abstract
Background
The long term outcome of children entered into neonatal trials of high frequency oscillatory ventilation (HFOV) or conventional ventilation (CV) has been rarely studied.
Objective
To evaluate respiratory and neurodevelopmental outcomes for children entered into the United Kingdom Oscillation Study, which was designed to evaluate these outcomes.
Methods
Surviving infants were followed until 2 years of age corrected for prematurity. Study forms were completed by local paediatricians at routine assessments, and parents were asked to complete a validated neurodevelopmental questionnaire.
Results
Paediatricians' forms were returned for 73% of the 585 surviving infants. Respiratory symptoms were common in all infants, and 41% had received inhaled medication. Mode of ventilation had no effect on frequency of any symptoms. At 24 months of age, severe neurodevelopmental disability was present in 9% and other disabilities in 38% of children, but the prevalence of disability was similar in children who received HFOV or CV (relative risk 0.93; 95% confidence interval 0.74 to 1.16). The prevalence of disability did not vary by gestational age, but boys were more likely to have overall disability. Developmental scores were unaffected by mode of ventilation (relative risk 1.13; 95% confidence interval 0.78 to 1.63) and were lower in infants born before 26 weeks gestation compared with babies born at 26–28 weeks.
Conclusions
Initial mode of ventilation in very preterm infants has no impact on respiratory or neurodevelopmental morbidity at 2 years. HFOV and CV appear equally effective for the early treatment of respiratory distress syndrome.
Keywords: premature, high frequency ventilation, development, respiratory function, disability
The effects of high frequency oscillatory ventilation (HFOV) and conventional ventilation (CV) on short term respiratory and neurological morbidity have been compared in several studies.1,2,3,4,5,6,7,8,9,10 Such short term observations may have poor predictive value for outcome in childhood, but to date only two reports have provided information about long term outcome. The HiFi trial evaluated respiratory morbidity and neurodevelopmental outcome up to 2 years of post‐term age for 77% of survivors.11 Growth and clinical respiratory status did not differ between the two groups, but neurodevelopmental outcome was worse in the HFOV group, in keeping with the excess of major cranial ultrasound abnormalities identified in the original report. This study used a low volume HFOV strategy, which is now thought to be suboptimal in preterm infants with respiratory distress syndrome.12 In the single centre PROVO study, similar neurodevelopmental outcome and prevalence of clinical respiratory morbidity at 6 years was observed between the two groups, although formal measurements of respiratory function were significantly poorer in the CV arm.13 However, the number of very immature infants included in this trial was small.
The United Kingdom oscillation study (UKOS) randomly assigned 797 infants born between 23 and 28 weeks gestation to receive either HFOV or CV within one hour of birth. No difference was found in short term outcomes: mortality, incidence of chronic lung disease, and neonatal cranial ultrasound scan appearances.10 The study was also designed to determine whether either ventilatory modality was associated with longer term respiratory or neurodevelopmental morbidity. We now report the outcome for surviving infants up to 2 years of age corrected for prematurity.
Methods
Study population
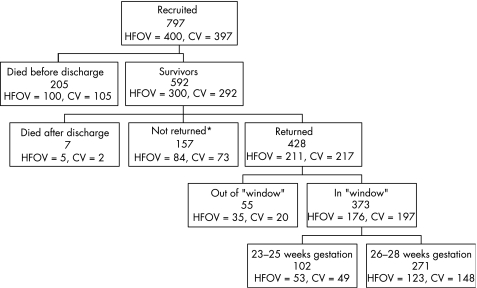
Of the 592 surviving infants who were entered into the study and discharged home, seven subsequently died, no outcome forms were returned for 164, and outcome information was available for 428 from 22 centres in the United Kingdom and one each from Australia, Ireland, and Singapore (fig 1). Infants were followed by their local paediatrician until 2 years of age corrected for prematurity. Questionnaires were mailed to the local paediatrician responsible for follow up when each infant reached 21 months post‐term age, with a request that the child be evaluated as close to 24 months post‐term age as possible and within the “window” of 22–28 months. Up to two reminders were sent to paediatricians when questionnaires had not been returned to the coordinating centre by 25 months post‐term age. If questionnaires were still not returned, in the United Kingdom the child's local health visitor was telephoned and asked to complete the forms.
Figure 1 Recruitment and follow up of infants. *This includes three children for whom questionnaires were returned without data. The window for follow up was 22–28 months of age corrected for prematurity. CV, Conventional ventilation; HFOV, high frequency oscillatory ventilation.
Paediatricians were asked to complete two forms. A respiratory questionnaire requested details about frequency of cough and wheeze and their relationship to infection, use of respiratory drugs, home oxygen, and hospital admissions (for both respiratory and other reasons). Social and demographic information, including family history of smoking and atopy, was also recorded. A neurodevelopmental questionnaire recorded information on health status and anthropometry. In addition, parents were separately mailed a questionnaire that included questions in three areas: non‐verbal cognitive development (derived from items in the Bayley scales of infant development14) and vocabulary and language (derived from the MacArthur language scales15). The original questionnaire was validated in a term population and modified for this study to incorporate better sensitivity at lower developmental scores.16 A total score of <49 achieved 81% sensitivity and 81% specificity for a Bayley scale mental development index of ⩽70 (more than two standard deviations below the mean).16
Statistical methods
The original trial was powered to detect a 12% difference in disability rates (estimated rate 17%), or a 14% difference in respiratory symptoms (estimates: 50% during first year; 33% during second year). We compared baseline infant, maternal, and socioeconomic variables between the two randomisation groups, to confirm that deaths or loss of children to follow up had not affected the balance. To investigate any potential bias due to the omission of subjects with missing data or data obtained outside the specified window, we compared important neonatal outcomes in the three possible groups of subjects: (a) those whose questionnaires were completed within the specified window (22–28 months post‐term); (b) those whose questionnaires were completed outside the window; (c) those whose questionnaires had not been returned.
Analysis was on an intention to treat basis using the follow up data obtained exclusively within the 22–28 month window. Relative risks with 95% confidence intervals were calculated to estimate the relative effect of HFOV compared with CV for all categorical outcomes. Adverse neurological outcomes were relatively uncommon, and so each neurological variable was dichotomised into any adverse outcome (major or minor) or normal development. For respiratory outcomes, where morbidity was common, each variable was dichotomised into the most extreme outcome—for example, cough or wheeze more than once a week—versus any other outcome where there were more than two levels of outcome. All anthropometric data were analysed as standard deviation scores to adjust for age and sex.17 To compare these data and the cognitive development score by mode of ventilation, means and 95% confidence intervals for differences in mean scores were calculated. Overall disability and anthropometry were analysed by two gestational age groups (23–25 weeks and 26–28 weeks) as used to stratify in the original randomisation.10 The effects of gestational age and sex of the child were tested by fitting an interaction term in the model. Statistical analysis was performed (LM , ESL, JLP) using Stata v7 (Stata Corporation, College Station, Texas, US).
Approval for the study was granted by the South Thames Multicentre Research Ethics Committee and by the local research ethics committee at each participating centre. Written informed consent was obtained from the parents of all participating children.
Results
Respiratory and neurodevelopmental questionnaires completed by paediatricians were returned for 428 (73%) children, of which 373 (87% of those returned) were within the specified age window. Parents returned developmental questionnaires within the specified age window for 288 children (49% of survivors to discharge) The proportion of infants with oxygen dependency at 36 weeks postmenstrual age, supplemental oxygen at discharge, or major abnormality on cranial ultrasound scanning did not differ significantly between those infants with information returned inside the follow up window, outside the window, or those without follow up data (table 1). There was a good balance in infant and maternal characteristics between the two ventilation groups among children with follow up data (table 2). Specifically, they were well matched in terms of the major determinants of outcome: birth weight, gestational age, sex of infant, or major abnormality on cranial ultrasound scan.
Table 1 Comparison of main neonatal outcomes by response status (data returned within or outside of the 22–28 month follow up window or not at all) at 2 years of age.
| Variable | Within window (N = 373) | Outside window (N = 55) | No data (N = 171*) | p Value | |||
|---|---|---|---|---|---|---|---|
| No/total | % | No/total | % | No/total | % | ||
| Chronic lung disease at 36 weeks† | 218/373 | 58 | 30/55 | 55 | 80/164 | 49 | 0.12 |
| Oxygen dependent at discharge | 83/371 | 22 | 13/55 | 24 | 26/162 | 16 | 0.22 |
| Major cranial abnormality‡ | 39/346 | 11 | 8/49 | 16 | 19/150 | 13 | 0.58 |
*Includes three questionnaires returned without any data.
†Postmenstrual age.
‡Cranial ultrasound scans after day 14.
Table 2 Characteristics at birth for children with follow up data at 2 years obtained at 22–28 months corrected gestational age by mode of ventilation.
| Variable | HFOV (N = 176) | CV (N = 197) | p Value |
|---|---|---|---|
| Birth weight (g) | 882 (208) | 914 (210) | 0.14 |
| Gestational age (weeks) | 26.7 (1.4) | 26.8 (1.3) | 0.38 |
| Birthweight SDS | −0.58 (1.01) | −0.47 (1.03) | 0.30 |
| Male | 95/176 (54%) | 107/197 (54%) | 0.95 |
| Multiple birth | 36/176 20%) | 50/197 (25%) | 0.26 |
| Mother smoked in pregnancy* | 41/149 (28%) | 43/170 (25%) | 0.65 |
| Postnatal steroids | 54/174 (31%) | 52/195 (27%) | 0.36 |
| Chronic lung disease at 36 weeks† | 103/176 (59%) | 115/197 (58%) | 0.98 |
| Oxygen dependent at discharge | 39/176 (22%) | 44/195 (23%) | 0.93 |
| Major cranial abnormality‡ | 15/158 (9%) | 24/188 (13%) | 0.34 |
Values are mean (SD) or number/total (%).
*Denominator is the number of mothers/pregnancies.
†Postmenstrual age.
‡Cranial ultrasound scans after day 14.
HFOV, High frequency oscillatory ventilation; CV, conventional ventilation.
Respiratory outcomes
The frequency of reported respiratory symptoms was high: half of parents reported that their child suffered from cough, of whom 31% coughed frequently (more than once a week); 37% reported wheezing, of whom 30% wheezed frequently. Overall 41% had received inhaled medication (table 3). Hospital admissions for respiratory problems were common: 43% of children had had a respiratory admission at some time during the first two years (table 3). Compared with those allocated to CV, the relative risks for those allocated to HFOV of frequent cough, frequent wheeze, or readmission to hospital for a respiratory diagnosis were 0.76, 1.04, and 1.01 respectively; none were significantly different (tables 3 and 4). Among infants born at 23–25 weeks gestation and allocated to HFOV, five (28%) had frequent wheeze, 11 (44%) had frequent cough, and 23 (45%) had received inhaled medication compared with seven (33%), nine (32%), and 24 (50%) respectively in the CV group. There were similarly no differences between the two groups in infants born at 26–28 weeks gestation. The prevalence of symptoms and medication was lower in this group, but not significantly (p = 0.69 for the interaction).
Table 3 Respiratory outcomes at 2 years.
| Outcome | HFOV | CV | ||||
|---|---|---|---|---|---|---|
| No/total | % | No/total | % | Relative risk* | 95% CI† | |
| Chest symptoms | ||||||
| Coughing | 84/172 | 49 | 98/194 | 51 | 0.97 | 0.79 to 1.19 |
| >Once a week | 21/81 | 26 | 33/97 | 34 | 0.76 | 0.48 to 1.21 |
| Once a week, >once a month | 17/81 | 21 | 15/97 | 15 | – | – |
| Once a month or less | 43/81 | 53 | 49/97 | 51 | – | – |
| With exercise | 15/61 | 25 | 28/76 | 37 | 0.67 | 0.39 to 1.13 |
| With infection | 68/81 | 84 | 88/98 | 90 | 0.93 | 0.83 to 1.05 |
| Wheezing | 56/167 | 34 | 75/187 | 40 | 0.84 | 0.63 to 1.10 |
| >Once a week | 16/53 | 30 | 21/72 | 29 | 1.04 | 0.60 to 1.79 |
| Once a week, >once a month | 6/53 | 11 | 12/72 | 17 | – | – |
| Once a month or less | 31/53 | 58 | 39/72 | 54 | – | – |
| With exercise | 13/42 | 31 | 26/60 | 43 | 0.71 | 0.42 to 1.22 |
| With infection | 50/56 | 89 | 66/73 | 90 | 0.99 | 0.88 to 1.11 |
| Chest medicines | ||||||
| Last 12 months | 94/171 | 55 | 115/192 | 60 | 0.92 | 0.77 to 1.10 |
| Bronchodilators | 63/171 | 37 | 82/192 | 43 | 0.86 | 0.67 to 1.11 |
| Inhaled steroids | 36/171 | 21 | 50/192 | 26 | 0.81 | 0.56 to 1.18 |
| Any inhaled drug | 63/171 | 37 | 85/192 | 44 | 0.83 | 0.65 to 1.07 |
| Other | ||||||
| On home oxygen now | 2/173 | 1 | 4/194 | 2 | 0.56 | 0.10 to 3.02 |
*Relative risk is the ratio of the risk of the most severe adverse outcome in the two groups, HFOV/CV.
†95% confidence intervals of relative risk.
HFOV, High frequency oscillatory ventilation; CV, conventional ventilation.
Table 4 Hospital readmissions from birth to 2 years.
| Outcome | HFOV | CV | Relative risk* (95% CI)† | p Value |
|---|---|---|---|---|
| Respiratory admission ever | 118/276 (43%) | 112/264 (42%) | 1.01 (0.83 to 1.23) | – |
| Mean (SD) (range)‡ | 2.3 (2.3) (1–14) | 2.4 (2.3) (1–14) | – | 0.65 |
| Respiratory admission in last 12 months | 24/157 (15%) | 27/179 (15%) | 1.01 (0.61 to 1.68) | – |
| Mean (SD) (range)‡ | 1.4 (1.0) (1–5) | 1.3 (0.6) (1–3) | – | 0.81 |
| Surgical admission ever | 59/276 (21%) | 59/264 (22%) | 0.96 (0.70 to 1.32) | – |
| Mean (SD) (range)‡ | 1.5 (1.1) (1–7) | 1.4 (0.7) (1–4) | – | 0.82 |
| ICU admission ever | 23/276 (8%) | 25/264 (9%) | 0.88 (0.51 to 1.51) | – |
| Mean (SD) (range)‡ | 1.1 (0.5) (1–3) | 1.3 (0.6) (1–3) | – | 0.13 |
*Relative risk is the ratio of the risk of admission ever in the two groups, HFOV/CV.
†95% confidence intervals of relative risk.
‡Mean number of admissions among those who had had an admission.
HFOV, High frequency oscillatory ventilation; CV, conventional ventilation.
Developmental outcomes and growth
Overall, 9% of children had severe disability and 38% had other disabilities at 2 years. The rate of severe or other disability did not vary by allocated mode of ventilation (table 5). Rates of severe disability were similar in the two gestational age groups (9% at 23–25 weeks v 8% at 26–28 weeks; table 5). Five per cent of children were reported to have reduced vision, and 2% severe or profound hearing loss. Paediatricians reported development to be profoundly impaired (>12 months delay) in 4% of children, and as severely impaired (7–12 months delay) in 5% of children. The only statistically significant effect was for convulsions (relative risk 2.64), but this was of borderline significance (p = 0.04), and the lower limit of the 95% confidence interval was close to 1 (1.04; table 5). Boys were significantly more likely to have overall severe disability (relative risk 1.35, 95% confidence interval 1.07 to 1.70).
Table 5 Neurological outcomes at 2 years.
| Outcome | HFOV | CV | Relative risk or difference in means | 95% CI |
|---|---|---|---|---|
| Neuromotor | ||||
| No head control | 0/170 (0%) | 1/189 (1%) | * | |
| Poor head control | 0/170 (0%) | 0/189 (0%) | ||
| Normal head control | 170/170 (100%) | 188/189 (99%) | ||
| Unable to sit unsupported | 3/168 (2%) | 1/185 (1%) | 1.47 | 0.33 to 6.46 |
| Sits unsupported, less well than others | 1/168 (1%) | 2/185 (1%) | ||
| Sits unsupported | 164/168 (98%) | 182/185 (98%) | ||
| Unable to stand | 5/168 (3%) | 7/189 (4%) | 0.96 | 0.46 to 2.03 |
| Requires support to rise, stands | 7/168 (4%) | 7/189 (4%) | ||
| Stands in one movement | 156/168 (93%) | 175/189 (93%) | ||
| Unable to walk | 11/169 (7%) | 14/190 (7%) | 0.78 | 0.43 to 1.43 |
| Walks, non‐fluent gait | 5/169 (3%) | 9/190 (5%) | ||
| Walks normally | 153/169 (91%) | 167/190 (88%) | ||
| Unable to use left hand | 3/165 (2%) | 1/179 (1%) | 1.55 | 0.60 to 3.98 |
| Picks up with left hand not pincer grip | 7/165 (4%) | 6/179 (3%) | ||
| Picks up with left hand, pincer grip | 155/165 (94%) | 172/179 (96%) | ||
| Unable to use right hand | 1/167 (1%) | 2/185 (1%) | 1.11 | 0.36 to 3.37 |
| Picks up with right hand not pincer grip | 5/167 (3%) | 4/185 (2%) | ||
| Picks up with right hand, pincer grip | 161/167 (96%) | 179/185 (97%) | ||
| Unable to do bimanual tasks | 5/168 (3%) | 1/188 (1%) | 2.24 | 0.93 to 5.41 |
| Difficulty using both hands together | 9/168 (5%) | 6/188 (3%) | ||
| Uses both hands well | 154/168 (92%) | 181/188 (96%) | ||
| ⩾1convulsion/month on treatment | 1/167 (1%) | 0/189 (0%) | 2.64 | 1.04 to 6.72 |
| <1/month on treatment | 3/167 (2%) | 0/189 (0%) | ||
| Convulsions, no treatment | 10/167 (6%) | 6/189 (3%) | ||
| No convulsions | 153/167 (92%) | 183/189 (97%) | ||
| Vision | ||||
| Squint | 22/171 (13%) | 23/189 (12%) | 1.06 | 0.61 to 1.83 |
| Parental report of visual problems; reduced vision | 5/163 (3%) | 14/189 (7%) | 0.41 | 0.15 to 1.12 |
| Abnormal eye movements | 8/165 (5%) | 7/188 (4%) | 1.30 | 0.48 to 3.51 |
| Hearing | ||||
| Profound hearing loss despite aids | 2/170 (1%) | 0/188 (0%) | 0.81 | 0.38 to 1.72 |
| Hearing loss helped by aids | 3/170 (2%) | 2/188 (1%) | ||
| Hearing loss not severe enough for aids | 3/170 (2%) | 10/188 (5%) | ||
| Suspected hearing loss | 3/170 (2%) | 3/188 (2%) | ||
| No hearing loss | 159/170 (94%) | 173/188 (92%) | ||
| Other domains | ||||
| Does not understand signs or words | 3/168 (2%) | 0/185 (0%) | * | |
| Tube feeding | 1/172 (1%) | 4/191 (2%) | 0.28 | 0.03 to 2.46 |
| Disability grading | ||||
| Overall | ||||
| Severe disability | 15/172 (9%) | 16/191 (8%) | 0.93 | 0.74 to 1.16 |
| Other disability | 62/172 (36%) | 76/191 (40%) | ||
| No disability | 95/172 (55%) | 99/191 (52%) | ||
| 23–25 weeks gestation | ||||
| Severe disability | 5/51 (10%) | 4/47 (9%) | 0.88 | 0.60 to 1.31 |
| Other disability | 19/51 (37%) | 21/47 (45%) | ||
| No disability | 27/51 (53%) | 22/47 (47%) | ||
| 26–28 weeks gestation | ||||
| Severe disability | 10/121 (8%) | 12/144 (8%) | 0.94 | 0.72 to 1.23 |
| Other disability | 43/121 (36%) | 55/144 (38%) | ||
| No disability | 68/121 (56%) | 77/144 (53%) | ||
| Cognitive development | ||||
| Parent report composite score <49† | 41/137 (30%) | 40/151 (26%) | 1.13 | 0.78 to 1.63 |
| Parent report composite | 75 (38) | 76 (37) | −1.7 | −10.4 to 7.0 |
| Growth | ||||
| 23–25 weeks gestation | ||||
| Height SDS | −0.76 (1.03) | −0.67 (0.98) | −0.09 | −0.50 to 0.33 |
| Weight SDS | −0.90 (1.16) | −0.80 (1.41) | −0.10 | −0.63 to 0.43 |
| Head circumference SDS | −1.46 (1.28) | −1.59 (1.44) | 0.13 | −0.45 to 0.70 |
| 26–28 weeks gestation | ||||
| Height SDS | −0.40 (1.09) | −0.53 (1.10) | 0.13 | −0.16 to 0.42 |
| Weight SDS | −0.54 (1.26) | −0.73 (1.24) | 0.19 | −0.13 to 0.51 |
| Head circumference SDS | −1.14 (1.42) | −1.28 (1.50) | 0.15 | −0.25 to 0.54 |
Values are number/total (%) or mean (SD). Relative risk is the ratio of the risk of any adverse outcome in the two groups. Severe disability is at least one extreme response in one of the following clinical domains: neuromotor, vision, hearing, communication, or other physical disabilities. No disability is a normal (or missing) response to all clinical domains.
*Impossible to calculate as one group has no adverse outcomes.
†Parental questionnaire composite score of non‐verbal development, sentence complexity, and vocabulary; 49 is the cut off for cognitive delay equivalent to Bayley mental development index ⩽70.16
HFOV, High frequency oscillatory ventilation; CV, conventional ventilation; SDS, standard deviation score.17
The mean standard deviation scores for height, weight, and head circumference were all below expected values after correction for prematurity. There were no significant differences when analysed by allocated mode of ventilation. Growth was poorer in babies born at 23–25 weeks gestation than for those born at 26–28 weeks for height, weight, and head circumference but the differences were not significant (p = 0.09, 0.15, 0.15 respectively; table 5).
Parent completed developmental questionnaires were returned for 288 children (68% of children assessed). Total scores varied by gestational age; mean (SD) parent report composite scores for births at 23–25 weeks gestation was 67 (38) compared with 79 (37) at 26–28 weeks; mean difference 12 (95% confidence interval 3 to 22). Developmental scores were higher in girls (mean difference from boys 24; 95% confidence interval 16 to 33). Overall, 28% had a score of <49, the cut off for cognitive delay. Of babies born at 23–25 weeks gestation, 41% had parent report composite scores <49 compared with 23% of children born at 26–28 weeks. The mean scores or the proportion scoring <49 did not differ significantly by allocated mode of ventilation.
We compared the findings from this dataset where information was obtained between 22 and 28 months with the findings from the whole dataset. These were very similar and also showed no evidence of association between outcome at age 2 years and mode of ventilation (data not shown). In particular, the relative risk for convulsions was similar, 2.40, but not significant (p = 0.07). The observed difference in neurological outcome by sex was also seen in the full dataset.
Discussion
This study has provided evidence that there is no difference in the effects of HFOV and CV on respiratory and neurological outcome at 2 years. The UKOS trial is unique in that study entrants received their allocated mode of ventilation within one hour of birth. In our previous report, we found no improvement in short term respiratory or neurological morbidity associated with the use of HFOV.10 In this comprehensive evaluation, we show that our earlier findings are complemented by the observation that neurology, development, and respiratory morbidity at 2 years of age corrected for prematurity are similarly distributed in the two allocation groups.
Because of the size of the cohort for whom we required outcome evaluation, we elected to use current hospital based follow up systems to identify disabilities in survivors. Many of the children in this study were transferred before birth in order to be able to receive neonatal intensive care. Their follow up was thus by their local paediatricians and not by the team who had recruited the cohort. Despite agreeing to complete the follow up assessments, we were disappointed to only obtain information on 73% of these survivors. There is concern that low response rates may produce a significant response bias, leading to fewer responses from parents of children with disability.18 To demonstrate as far as possible that we had not introduced such bias, the distribution of perinatal variables between responders and non‐responders was evaluated and found to be similar. Thirteen per cent of the assessments were carried out outside the identified age range, but sensitivity analyses indicated that this had no effect on our conclusions.
Respiratory morbidity was high; 50% had had cough (in 15% this occurred more than once a week), and 37% had had wheeze (10% more than once a week). In addition, 43% had had at least one admission for a respiratory illness, 15% in the last 12 months. Data were collected using a modified version of a questionnaire previously used to assess infant respiratory outcome following antenatal invasive procedures.19 The hospital admission rate in the previous 12 months for lower respiratory tract infection (15%) was the same as that previously reported in very premature infants (15%).20 No significant differences were found with regard to the proportions of children with cough, wheeze, taking respiratory drugs, or having been admitted to hospital for a lower respiratory tract infection between those allocated to HFOV or CV. These results are in keeping with the findings of the HiFi Study Group, who also reported no significant differences in the incidence of respiratory tract infections, episodes of wheezing or hospital admission according to mode of ventilation.21 In the PROVO study, which only included 21 children <1000 g birth weight, a high volume HFOV strategy was also used and, although there were some significant differences in the results of certain lung function tests, the incidences of asthma and respiratory illness did not differ significantly between those who had been allocated to HFOV or by CV.13 Furthermore, the lack of evidence for any differences in respiratory morbidity at age 2 years in our study is consistent with the finding of similar lung function results in the two groups when examined at 1 year.22 Lung function results at 1 year in our laboratory have been shown to be predictive of respiratory morbidity during the preschool years.23
What is already known on this topic
Studies of high frequency ventilation for the prevention of chronic lung disease have shown inconsistent effects on neonatal brain injuries
One neurodevelopmental follow up suggested worse outcomes for babies treated with HFOV, and one small respiratory follow up showed potential benefit from neonatal HFOV
What this study adds
This study provides reassurance that there is no long term neurodevelopmental sequelae from neonatal HFOV
Respiratory outcomes are similar between those treated conventionally and those with HFOV
The rate of neurodevelopmental disability was lower than might be expected from contemporary population studies.24 The outpatient assessment did not include a formal developmental assessment; cerebral palsy, blindness, and profound hearing loss were recorded as severe disabilities. The prevalence of these disorders is consistent with those in other recent reports.25,26,27 The most common disability in very preterm babies, however, is developmental impairment, which requires a more accurate assessment. To assess this, we used a parent report questionnaire, adapted for use with children of lower developmental scores.16 Total scores <49 (predictive of a Bayley scales mental development index of greater than two standard deviations below the mean) were observed in 28% of the children for whom questionnaires were returned. Scores <49 were found in 40% of children of 23–25 weeks gestational age and 23% in children 26–28 weeks. The recent EPICure study reported equivalent rates of developmental impairment for 51% of assessed children born at 22–25 weeks gestation in 1995 at 30 months age corrected for prematurity.25 The lower rates of disability in our study therefore encourage the belief that outcome for such extremely preterm populations, particularly in terms of neurosensory disability, may have improved over the last decade.
Although short term neonatal outcomes such as cerebral ultrasound abnormalities, duration of mechanical ventilation, and use of supplemental oxygen are important outcomes, their predictive value for later function is not good. The evaluation of clinical progress and determination of functional outcomes is thus an important end point for a study such as this where there is a clear expectation that the intervention may affect such outcomes.1,7 In our study, we have previously concluded that the early use of HFOV is equally as effective a management strategy for the early treatment of respiratory distress syndrome in preterm babies as CV. We further conclude that there is no difference in the effects of HFOV and CV on respiratory and neurological outcome at age 2 years.
Acknowledgements
This study was funded by the Medical Research Council, London. We thank all the neonatal units and their staff and all of the UKOS parents and their children for participating in this study.
Abbreviations
CV - conventional ventilation
HFOV - high frequency oscillatory ventilation
UKOS - United Kingdom oscillation study
Footnotes
Competing interests: none declared
References
- 1.Hi Fi Study Group High frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. N Engl J Med 198932088–93. [DOI] [PubMed] [Google Scholar]
- 2.Clark R H, Gerstmann D R, Null D M.et al Prospective randomised comparison of high frequency oscillatory and conventional ventilation in respiratory distress syndrome. Pediatrics 1992894–12. [PubMed] [Google Scholar]
- 3.Ogawa Y, Miyasaka K, Kawano T.et al A multicenter randomized trial of high frequency oscillatory ventilation as compared with conventional mechanical ventilation in preterm infants with respiratory failure. Early Hum Dev 1993321–10. [DOI] [PubMed] [Google Scholar]
- 4.Gerstmann D R, Minton S D, Stoddard R A.et al The Provo Multicenter Early High‐ frequency Oscillatory Ventilation Trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics 1996981044–1057. [PubMed] [Google Scholar]
- 5.Plavka R, Kopecky P, Sebron V.et al A prospective randomized comparison of conventional mechanical ventilation and very early high frequency oscillatory ventilation in extremely premature newborns with respiratory distress syndrome. Int Care Med 19992568–75. [DOI] [PubMed] [Google Scholar]
- 6.Thome U, Kossel H, Lipowsky G.et al Randomized comparison of high frequency ventilation with high rate intermittent positive pressure ventilation in preterm infants with respiratory failure. J Pediatr 199913539–46. [DOI] [PubMed] [Google Scholar]
- 7.Moriette G, Paris‐Llado J, Walti H.et al Prospective, randomized multicenter comparison of high frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics 2001107363–372. [DOI] [PubMed] [Google Scholar]
- 8.Rettwitz‐Volk W, Veldman A, Roth B, A prospective, randomized, multicentre trial of high frequency oscillatory ventilation in preterm infants with respiratory distress syndrome receiving surfactant et alJ Paediatr 1998132249–254. [DOI] [PubMed] [Google Scholar]
- 9.Courtney S E, Durand D J, Asselin J M.et al Neonatal ventilation study group. HFO versus conventional mechanical ventilation for VLBW infants. N Engl J Med 2002347643–652. [DOI] [PubMed] [Google Scholar]
- 10.Johnson A H, Peacock J L, Greenough A.et al High‐frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med 2002347633–642. [DOI] [PubMed] [Google Scholar]
- 11. HiFi Study Group High‐frequency oscillatory ventilation compared with conventional intermittent mechanical ventilation in the treatment of respiratory failure in preterm infants: neurodevelopmental status at 16 to 24 months of post term age. J Pediatr 199017939–946. [DOI] [PubMed] [Google Scholar]
- 12.Froese A B, Bryan A C. Reflections on the HIFI trial. Pediatrics 199187565–567. [PubMed] [Google Scholar]
- 13.Gerstmann D R, Wood K, Milner A.et al Childhood outcome after high frequency oscillatory ventilation for neonatal respiratory distress syndrome. Pediatrics 2001108617–623. [DOI] [PubMed] [Google Scholar]
- 14.Bayley N.Bayley scales of infant development. 2nd ed. San Antonio, TX: The Psychological Corporation, 1993
- 15.Fenson L, Pethick S, Renda C.et al Short‐form versions of the MacArthur Communicative Development Inventories. Applied Psycholinguistics 20002195–115. [Google Scholar]
- 16.Johnson S, Marlow N, Wolke D.et al Validation of a parent report measure of cognitive development in very preterm infants. Dev Med Child Neurol 200446389–397. [DOI] [PubMed] [Google Scholar]
- 17.Cole T J, Freeman J V, Preece M A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalised likelihood. Stat Med 199817407–429. [PubMed] [Google Scholar]
- 18.Tin W, S Fritz, Wariyar U, et al Outcome of very preterm birth: children reviewed with ease at 2 years differ from those followed up with difficulty. Arch Dis Child Fetal Neonatal Ed 199879F83–F87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenough A, Yuksel B, Naik S.et al First trimester invasive procedures: effects on symptom status and lung volume in very young children. Pediatr Pulmonol 199724415–422. [DOI] [PubMed] [Google Scholar]
- 20.Lacaze‐Masmonteil T, Truffert P, Pinguier D.et al Lower respiratory tract illness at RSV prophylaxis in very premature infants. Arch Dis Child 200489562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HiFi Study Group High frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants: assessment of pulmonary function at 9 months corrected age. J Pediatr 1990116933–941. [DOI] [PubMed] [Google Scholar]
- 22.Thomas M R, Rafferty G F, Limb E S.et al Pulmonary function at follow up of very preterm infants from the UK Oscillation Study. Am J Respir Crit Care Med 20041691–5. [DOI] [PubMed] [Google Scholar]
- 23.Giffin F, Greenough A, Yuksel B. Relationship between lung function results in the first year of life and respiratory morbidity in early childhood in patients born prematurely. Pediatr Pulmonol 199418290–294. [DOI] [PubMed] [Google Scholar]
- 24.Marlow N. Neurocognitive outcome after very preterm birth. Arch Dis Child Fetal Neonatal Ed 200489F224–F228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood N S, Marlow N, Costeloe K.et al Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med 2000343378–384. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs S E, O'Brien K, Inwood S.et al Outcome of infants 23–26 weeks' gestation pre and post surfactant. Acta Paediatr 200089959–965. [DOI] [PubMed] [Google Scholar]
- 27.Victorian Infant Collaborative Study Group Outcome at 2 years of children 23–27 weeks' gestation born in Victoria in 1991–92. J Paediatr Child Health 199733161–165. [PubMed] [Google Scholar]