Abstract
Background
There have been only a few reports on the renin‐angiotensin system in low birthweight infants; in particular, plasma angiotensin II concentrations have not been studied.
Aim
To investigate plasma angiotensin II concentrations in early neonatal infants including low birthweight infants.
Methods
Forty six patients were studied, of whom 14 weighed not less than 2500 g (normal birth weight), 16 weighed less than 2500 g but not less than 1500 g (moderately low birth weight), and 16 weighed less than 1500 g (very low birth weight). Blood samples were collected twice, on day 0 and day 7. Angiotensin II concentration was assayed using an enzyme immunoassay kit with a microplate.
Results
Geometric means of angiotensin II concentrations on day 7 were 19 pg/ml in the normal birthweight group, 28 pg/ml in the moderately low birthweight group, and 76 pg/ml in the very low birthweight group. The concentrations on day 7 in the very low birthweight group were significantly higher than those in the normal birthweight and moderately low birthweight groups (p = 0.005, p = 0.031). There were significant correlations between angiotensin II concentration on day 7 and gestational age (rs = −0.4, p = 0.007) and birth weight (rs = −0.36, p = 0.016).
Conclusions
Specific physiological conditions associated with a very low birth weight are thought to be responsible for the increased concentration of angiotensin II on day 7. It is necessary to measure angiotensin II concentration for a longer period after birth and study the factors that could influence it.
Keywords: low birth weight, renin‐angiotensin system, angiotensin II
It has been reported that the renin‐angiotensin system is more highly activated in the neonatal or infant period than in later childhood.1 Such reports were based on data from normal birthweight (NBW) infants. However, there have been only a few reports on the renin‐angiotensin system in low birthweight infants, particularly the concentration of angiotensin II (AII). In this study, therefore, we examined plasma AII concentrations in earl neonatal infants including low birthweight infants.
Subjects and methods
The study subjects were 46 infants who were admitted soon after birth to Wakayama Medical University neonatal intensive care unit between March 2003 and March 2004. These subjects included both patients who were born in our hospital and also infants admitted within 24 hours of birth. Exclusion criteria were: (a) congenital heart disease and renal anomaly diagnosed by echocardiography on admission; (b) lethal malformations; (c) suspected malformation syndrome; (d) chromosomal abnormalities. The study protocol was approved by the review board of Wakayama Medical University. Written informed consent was obtained from the patients' parents. Of the 46 patients, 14 weighed not less than 2500 g (NBW), 16 weighed less than 2500 g but not less than 1500 g (moderately low birth weight (MLBW)), and 16 weighed less than 1500 g (very low birth weight (VLBW)). Blood samples were collected twice, once on day 0 and once on day 7 (median 7, range 4–11; n = 46). On admission, blood samples are generally taken after echocardiographic examination, and we collected an extra sample at that time. Then, when blood was sampled for screening for congenital metabolic disorders on day 7, we collected an extra sample for this study. On day 7, all the patients had been supine since midnight. All blood samples were taken at 0800–1000. The samples were collected in tubes containing EDTA and kept on ice at 4°C. The collected plasma samples were stored in a freezer at −30°C. Before assay, we purified the plasma samples with Amprep Mini‐Columns PH Phenyl (Amersham Biosciences, Amersham, Bucks, UK) after thawing them. We then assayed the AII concentrations using an Angiotensin II Enzyme Immunoassay Kit (SPI‐BIO, Massy, France) with a microplate.2 For statistical analysis, AII data were logarithmically transformed because of the skew distribution of the raw data. The t test was applied for comparison between two groups, analysis of variance for comparison among three groups, and Fisher's protected least significant difference for multiple comparisons. Spearman's rank correlation coefficient was used for the correlation coefficient test. Statistical analysis was performed using StatView J‐5.0 software (SAS Institute, Cary, North Carolina, USA). Differences at p<0.05 were considered significant.
Results
The mean (SD) gestational age and birth weight in the NBW (14 cases), MLBW (16 cases), and VLBW (16 cases) groups were 38.9 (1.6) weeks and 2948 (287) g, 33.8 (1.5) weeks and 2014 (249) g, and 27.9 (2.9) weeks and 1001 (307) g respectively. Table 1 shows the AII concentrations on day 0 and day 7 in each group. Geometric means on day 0 were 74 pg/ml in the NBW group, 43 pg/ml in the MLBW group, and 34 pg/ml in the VLBW group, and the corresponding values on day 7 were 19, 28, and 76 pg/ml respectively.
Table 1 Plasma angiotensin II concentrations (pg/ml) in the study groups.
| NBW | MLBW | VLBW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Geometric mean | Range | n | Geometric mean | Range | n | Geometric mean | Range | |
| Day 0 | 14 | 74 | 3–443 | 16 | 43 | 5–438 | 16 | 34 | 5–201 |
| Day 7 | 14 | 19 | 1–127 | 16 | 28 | 5–254 | 16 | 76 | 7–1041 |
NBW, normal birth weight; MLBW, moderately low birth weight; VLBW, very low birth weight.
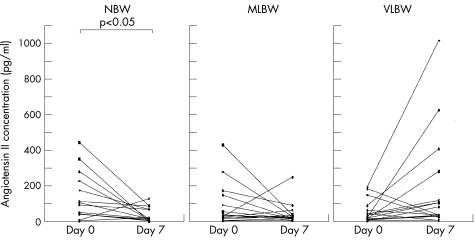
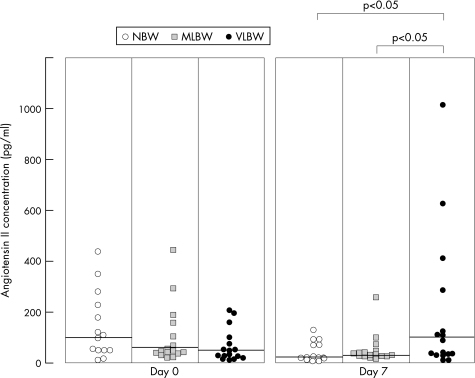
Figure 1 shows comparisons among the groups on day 0. AII concentrations were significantly higher on day 0 than on day 7 in the NBW group (p = 0.033). On the other hand, those on day 7 tended to be increased compared with those on day 0 in the VLBW group, but there was no significant difference in the concentrations between day 0 and day 7 (p = 0.06). Figure 2 shows a comparison of the AII concentrations among the three groups on day 0 and day 7. No significant intergroup differences were evident on day 0. However, the AII concentrations on day 7 in the VLBW group were significantly higher than those in the NBW and MLBW groups (p = 0.005, p = 0.031).
Figure 1 Plasma angiotensin II concentrations in the study groups. NBW, normal birth weight; MLBW, moderately low birth weight; VLBW, very low birth weight.
Figure 2 Comparison of the angiotensin II concentrations among the three groups on day 0 and day 7. NBW, normal birth weight; MLBW, moderately low birth weight; VLBW, very low birth weight.
In addition, we examined possible factors that might affect the AII concentrations. These were gestational age, birth weight, Apgar score (one and five minutes), mean blood pressure, serum potassium concentration, serum sodium concentration, and percentage of body weight loss (table 2). There were significant correlations between the AII concentration on day 7 and gestational age (rs = −0.4, p = 0.007) and the AII concentration on day 7 and birth weight (rs = −0.36, p = 0.016).
Table 2 Correlation of angiotensin II concentrations with various factors.
| Day 0 | Day 7 | |||
|---|---|---|---|---|
| n | rs | n | rs | |
| Gestational age (weeks) | 46 | 0.24 | 46 | −0.4** |
| Birth weight (g) | 46 | 0.24 | 46 | −0.36* |
| Apgar score (1 min) | 46 | −0.12 | – | – |
| Apgar score (5 min) | 46 | −0.05 | – | – |
| Mean blood pressure (mm Hg) | 45 | 0.14 | 33 | 0.08 |
| K+ (mEq/l) | 46 | 0.19 | 36 | −0.06 |
| Na+ (mEq/l) | 46 | −0.04 | 36 | −0.07 |
| Body weight loss (%) | – | – | 46 | 0.06 |
*p<0.05, **p<0.01.
Discussion
AII concentrations on day 0 showed no significant differences among the three different birth weight groups. AII concentrations on day 7 were lower in the NBW group than those on day 0, and higher in the VLBW group than in the MLBW or NBW group. Specific physiological factors associated with VLBW were therefore thought to be responsible for the increase in the AII concentrations.
A few limited reports have focused on AII concentrations in childhood, and have indicated that AII concentrations are high in early childhood and then decrease gradually with age.3,4
There have been several reports on angiotensin converting enzyme (ACE) activity in the neonatal period. One study showed that ACE activity in 2–4 day old infants with respiratory distress syndrome was higher than that in healthy immature or mature infants, or mature infants with other acute illnesses.5 Another study has shown that ACE activity in immature infants with respiratory distress syndrome was equivalent to that in healthy immature infants on day 0, and a negative association was observed between birth weight and ACE activity.6 The same study also showed that ACE activity in immature infants was higher than in mature infants, whereas ACE activity in immature infants was equivalent to that in their mothers or in normal adult controls.
Another study that investigated plasma aldosterone concentrations in children aged from 2 hours to 15 years showed a maximum concentration at 2 hours of age, and then a gradual decrease during the first year of life.7 After 1 year of age, the plasma aldosterone concentration stabilised, but remained higher than in adults. Other studies of the renin‐angiotensin system in mature infants or children have shown that plasma aldosterone concentrations are not associated with potassium or sodium concentrations in serum.8,9 Our study also showed that AII concentrations were not associated with serum potassium or sodium concentrations.
AII has been reported to be related to organ fibrosis.10 A previous study that used immunohistochemical staining using polyclonal antibody to human AII type 1 receptor in human fetal lung fibroblasts (HFL‐1) showed that the receptor was stained.11 In the present study, the AII concentrations on day 7 were significantly higher in the VLBW group than in the other two heavier birthweight groups. Taken together, these results indicate that an increased AII concentration may affect AII type 1 receptor in the lungs of VLBW infants and promote lung fibrosis. Furthermore, this may be implicated in the progression of chronic lung disease in neonates.
On the other hand, it has been reported that the increase in plasma AII may play a role in organogenesis and neonatal growth in rats.12,13 Furthermore, the increased AII concentration in the VLBW group on day 7 may reflect a defence mechanism in infants. VLBW infants tend to be in a salt‐losing state. Furthermore, VLBW infants with respiratory disturbances often have limited water intake and are administered diuretics at the same time. Therefore, to maintain circulating blood volume and blood pressure concentrations, the AII concentration may rise. However, no obvious factors affecting the increase in the AII concentration could be identified in our study. It will therefore be necessary to measure the AII concentration for a longer period after birth and study the factors that mainly influence it.
What is already known on this topic
The renin‐angiotensin system is more activated in the neonatal or infantile period than in later childhood
Angiotensin II concentrations have not been studied in low birthweight infants
What this study adds
Angiotensin II concentrations on day 7 in VLBW infants were significantly higher than those in NBW and MLBW infants
There were significant correlations between angiotensin II concentration on day 7 and gestational age, and angiotensin II concentration on day 7 and birth weight
Abbreviations
ACE - angiotensin converting enzyme
AII - angiotensin II
NBW - normal birth weight
MLBW - moderately low birth weight
VLBW - very low birth weight
Footnotes
Competing interests: none declared
References
- 1.Fiselier T, Monnens L, van Munster P.et al The renin‐angiotensin‐aldosterone system in infancy and childhood in basal conditions and after stimulation. Eur J Pediatr 198414318–24. [DOI] [PubMed] [Google Scholar]
- 2.Volland H, Pradelles Ph, Ronco P.et al A solid‐phase immobilized epitope immnoassay (SPIE‐IA) permitting very sensitive and specific measurment of angiotensin II in plasma. J Immunol Methods 199922837–47. [DOI] [PubMed] [Google Scholar]
- 3.Broughton Pipkin F, Smales O R, O'Callaghan M. Renin and angiotensin levels in children. Arch Dis Child 198156298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiselier T J, Lijnen P, Monnens L.et al Levels of renin, angiotensin I and II, angiotensin‐converting enzyme and aldosterone in infancy and childhood. Eur J Pediatr 19831413–7. [DOI] [PubMed] [Google Scholar]
- 5.Mattioli L, Zakheim R M, Mullis K.et al Angiotensin‐I‐converting enzyme activity in idiopathic respiratory distress syndrome of the newborn infant and in experimental alveolar hypoxia in mice. J Pediatr 19758797–101. [DOI] [PubMed] [Google Scholar]
- 6.Bender J W, Davitt M K, Jose P. Angiotensin‐I‐converting enzyme activity in term and premature infants. Biol Neonate 19783419–23. [DOI] [PubMed] [Google Scholar]
- 7.Sippell W G, Dorr H G, Bidlingmaier F.et al Plasma levels of aldosterone, corticosterone, 11‐deoxycorticosterone, progesterone, 17‐hydroxyprogesterone, cortisol, and cortisone during infancy and childhood. Pediatr Res 19801439–46. [DOI] [PubMed] [Google Scholar]
- 8.Varga F, Sulyok E, Nemeth M.et al Activity of the renin‐angiotensin‐aldosterone system in full‐term newborn infants during the first week of life. Acta Paediatr Acad Sci Hung 198122123–130. [PubMed] [Google Scholar]
- 9.Garcia del Rio C, Acuna D, Bustamante M.et al Increased activity of the renin‐angiotensin‐aldosterone system during the perinatal period. Rev Esp Fisiol 198238171–176. [PubMed] [Google Scholar]
- 10.Horiuchi M, Akishita M, Dzau V J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 199933613–621. [DOI] [PubMed] [Google Scholar]
- 11.Marshall R P, McAnulty R J, Laurent G J. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med 20001611999–2004. [DOI] [PubMed] [Google Scholar]
- 12.Shanmugam S, Corvol P, Gasc J M. Angiotensin II type 2 receptor mRNA expression in the developing cardiopulmonary system of the rat. Hypertension 19962891–97. [DOI] [PubMed] [Google Scholar]
- 13.Goodfriend T L, Elliott M E, Catt K J. Angiotensin receptors and their antagonists. N Engl J Med 1996201649–1654. [DOI] [PubMed] [Google Scholar]