Abstract
Recent studies identified novel allosteric modulators of the dopamine (DA) transporter (DAT). N-(Diphenylmethyl)-2-phenyl-4-quinazolinamine (SoRI-9804), N-(2,2-diphenylethyl)-2-phenyl-4-quinazolinamine (SoRI-20040), and N-(3,3-diphenylpropyl)-2-phenyl-4-quinazolinamine (SoRI-20041) partially inhibited [125I]3β-(4′-iodophenyl)tropan-2β-carboxylic acid methyl ester (RTI-55) binding, slowed the dissociation rate of [125I]RTI-55 from the DAT, and partially inhibited [3H]dopamine uptake. In the present study, we report that SoRI-9804 and SoRI-20040, at doses that do not alter release, partially inhibited d-amphetamine-induced DAT-mediated release of [3H]1-methyl-4-phenylpyridinium (MPP+)or[3H]dopamine from striatal synaptosomes (“DAT-mediated DA release”) in a dose-dependent manner. SoRI-20041, which does not alter DAT-mediated DA release measured with [3H]DA, reversed the effect of SoRI-20040. SoRI-20040 and SoRI-9804 also partially inhibited DAT-mediated DA release induced by DA or (±)-3,4-methylenedioxyamphetamine, demonstrating that the observed partial inhibition is not specific for a particular DAT substrate. SoRI-9804 and SoRI-20040 did not attenuate d-amphetamine-induced release of [3H]5-hydroxytryptamine from serotonergic, or [3H]MPP+ from noradrenergic, nerve terminals. Kinetic experiments demonstrated that SoRI-9804, in contrast to cocaine, slowed d-amphetamine-induced release of [3H]MPP+ from dopaminergic nerve terminals without altering the apparent rate constants. The two major findings of this study are 1) the identification of both “agonist” (SoRI-9804 and SoRI-20040) and “antagonist” (SoRI-20041) allosteric modulators of d-amphetamine-induced DAT-mediated DA release and 2) [3H]DA uptake and d-amphetamine-induced DAT-mediated efflux can be separately modulated. Such agents may have therapeutic potential for the treatment of stimulant addiction, Parkinson's disease, and other psychiatric disorders.
The biogenic amine transporters for dopamine (DAT), norepinephrine (NET), and serotonin (SERT) are important targets for a wide range of medications used to treat a variety of psychiatric conditions, such as anxiety, depression, obsessive compulsive disorder (Gorman and Kent, 1999; Zohar and Westenberg, 2000), and stimulant dependence (Grabowski et al., 2004; Rothman et al., 2006, 2008). Drugs that interact with transporters generally interact with these proteins in two distinct ways. Reuptake inhibitors bind to transporter proteins but are not transported. These drugs elevate extracellular concentrations of transmitter by blocking transporter-mediated uptake of transmitters from the synapse. Substrate-type releasers bind to transporter proteins and are subsequently transported into the cytoplasm of nerve terminals, releasing neurotransmitter via a process originally described as carrier-mediated exchange (Rudnick and Clark, 1993; Rothman and Baumann, 2006). However, the mechanism by which a substrate induces release of neurotransmitter is more complex than a simple exchange of substrate for neurotransmitter. More recent studies of the dopamine transporter have shown that the inward transport of a substrate such as amphetamine induces an inward current of sodium, which increases the concentration of internal cellular sodium at the transporter, thereby facilitating reverse transport of the dopamine (Goodwin et al., 2009; Pifl et al., 2009).
There is growing interest in the use of allosteric modulators (Christopoulos and Kenakin, 2002; Schwartz and Holst, 2007) as medications, including allosteric modulators of the biogenic amine transporters (BATs) (Sanchez, 2006). Our finding that pretreatment of guinea pig membranes with paroxetine increased the dissociation rate of [3H]cocaine from SERT provided early evidence of allosteric interactions at the biogenic amine transporters (Akunne et al., 1992). Other studies showed, using rat SERT expressed in human embryonic kidney cells, that imipramine allosterically modulated the ability of citalopram to inhibit [3H]5-HT transport (Sur et al., 1998). Others reported apparent allosteric interactions between 5-HT and [3H]paroxetine binding to human platelet SERT (Andersson and Marcusson, 1989) and between β-estradiol and SERT (Chang and Chang, 1999). More recently, we reported novel allosteric modulators of both DAT (SoRI-9804) (Rothman et al., 2002) and SERT (SoRI-6238 and TB-1-099) (Nandi et al., 2004; Nightingale et al., 2005). Moreover, Chen et al. (2005) reported evidence for allosteric modulation of [3H](S)-citalopram binding.
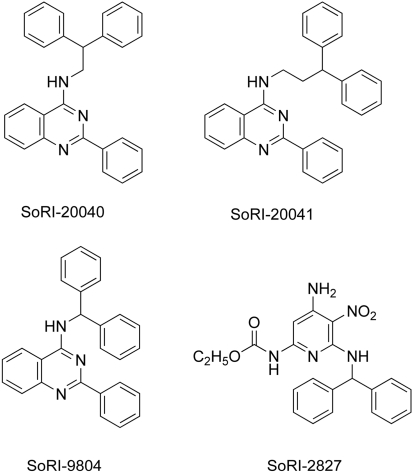
In 1999, drawing from a library of compounds (maintained by Southern Research Institute, Birmingham, AL) that possessed a diphenylmethyl (benzhydryl) group, we screened, using rat brain tissue assays, compounds for activity in binding assays for DAT, SERT, and NET (unpublished data). This effort identified several possible allosteric modulators of the BATs, and we subsequently examined in greater detail the interaction of selected agents with the BATs. SoRI-6238 and a subsequent compound that was not part of the SoRI library (TB-1-099) were shown to allosterically modulate SERT (Nandi et al., 2004; Nightingale et al., 2005). Three other compounds were identified as possible allosteric modulators of DAT. SoRI-9804 (Fig. 1) partially inhibited [125I]RTI-55 binding to DAT and partially inhibited [3H]DA uptake by rat brain synaptosomes (Rothman et al., 2002). More recently (Pariser et al., 2008), we reported that SoRI-20040 and SoRI-20041 had several properties consistent with an allosteric modulator of DAT. A third compound, SoRI-2827, was studied, but the evidence for it being an allosteric modulator of DAT was less compelling. As observed with SoRI-9804, both compounds partially inhibited [125I]RTI-55 binding to DAT and partially inhibited [3H]DA uptake by rat brain striatal synaptosomes. In [125I]RTI-55 DAT binding assays, both SoRI-20040 and SoRI-20041 decreased the Bmax in a dose-dependent manner, and both compounds decreased the Vmax of [3H]DA uptake in a dose-dependent manner. It is noteworthy that SoRI-20040 and SoRI-20041 slowed the dissociation rate of [125I]RTI-55 prebound to DAT (Pariser et al., 2008). Similar data were obtained with SoRI-9804 (unpublished data).
Fig. 1.
Compound structures.
Interestingly, SoRI-9804 and SoRI-20040 attenuated the ability of d-amphetamine to release [3H]MPP+ from striatal dopaminergic synaptosomes at doses that produced little or no effect by themselves. In dose-response experiments, both agents decreased the Emax of the d-amphetamine-induced [3H]MPP+ release. Thus, in the present study, we further characterized the modulatory effect of SoRI-9804, SoRI-20040, and SoRI-20041 on DAT-mediated DA release.
Materials and Methods
Animals. Male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA), weighing 300 to 400 g, were used as subjects in these experiments. Rats were housed in standard conditions (lights on from 7:00 AM to 7:00 PM) with food and water freely available. Animals were maintained in facilities fully accredited by the American Association of the Accreditation of Laboratory Animal Care, and experiments were performed in accordance with the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse.
Drugs and Reagents. [3H]MPP+ (SA = 85 Ci/mmol), [3H]5-HT (SA = 27.5 Ci/mmol), and [3H]DA (SA = 31.8 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). (±)-Methylenedioxymethamphetamine (Ecstasy) and (±)-MDA were obtained from the Drug Supply Program, National Institute on Drug Abuse. The sources of other reagents are published (Rothman et al., 2001; Pariser et al., 2008).
In Vitro Release Methods. Transporter-mediated release assays were carried out as described previously, with minor modifications (Rothman et al., 2003). Rats were sacrificed by CO2 asphyxiation. Tissue from caudate (for DAT assay), or from whole brain minus cerebellum and caudate (for SERT and NET assay), was homogenized in ice-cold 10% sucrose containing 1 μM reserpine. For DAT-mediated release assays, either [3H]1-methyl-4-phenylpyridinium ([3H]MPP+)or[3H]DA was used as the radiolabeled substrate; 100 nM desipramine and 100 nM citalopram were added to prevent uptake of [3H]MPP+ into NE and 5-HT nerves. For SERT-mediated release assays, [3H]5-HT was used as the radiolabeled substrate; 100 nM nomifensine and 50 nM GBR12935 were added to the sucrose solution to prevent uptake of [3H]5-HT into NE and DA nerve terminals. For the NET-mediated release assay, 50 nM GBR12935 and 100 nM citalopram were added to block [3H]MPP+ uptake into DA and 5-HT nerves. Synaptosomal preparations were incubated to steady state with 5 nM [3H]MPP+ (60 min), 5 nM [3H]DA (30 min), or 5 nM [3H]5-HT (60 min) in Krebs-phosphate buffer, pH 7.4, plus 1 μM reserpine. Subsequently, 850 μl of synaptosomes preloaded with [3H]ligand was added to polystyrene test tubes that contained 150 μl of test drug in assay buffer plus 1 mg/ml bovine serum albumin. After 5 min ([3H]5-HT and [3H]DA) or 30 min ([3H]MPP+), the release reaction was terminated by dilution with 4 ml of wash buffer followed by rapid vacuum filtration. Nonspecific values were measured by incubations in the presence of either 100 μM tyramine ([3H]5-HT release assay) or 10 μM tyramine ([3H]DA and [3H]MPP+ release assays). The retained tritium was counted by a TopCount liquid scintillation counter (PerkinElmer Life and Analytical Sciences). For the sake of brevity, we use these terms “DAT-mediated DA ([3H]DA) release” and “DAT-mediated DA ([3H]MPP+) release” to indicate which radioligand was used in the DA release assays.
For [3H]MPP+ efflux experiments, synaptosomes were preloaded with [3H]MPP+ for 60 min. Test drugs were then added, and samples were filtered at various times up to 60 min. Control samples were also filtered at the same times. The specific “efflux” was then calculated by subtracting the cpm in the presence of test drug from the cpm in the absence of test drug.
Data Analysis and Statistics. Dose-response curves were generated using eight concentrations of test drug. The data of three experiments, expressed as percentage of inhibition, were then fit to a dose-response curve model: Y = Emax × ([D]/([D] + EC50) for the best fit estimates of the Emax and EC50 values using either KaleidaGraph version 3.6.4 (Abelbeck/Synergy, Reading, PA) or MLAB-PC (Nightingale et al., 2005). For the [3H]MPP+ efflux experiments, the data of three independent experiments were pooled and fit to one- and two-component dissociation models using MLAB-PC as described previously (Rothman et al., 1991). Graphs were generated with KaleidaGraph 3.6 software. For most experiments, statistical significance was determined with ANOVA with a post hoc Bonferroni test (Prism version 4.0; GraphPad Software Inc., San Diego, CA). For certain kinetic experiments, two sets of data (data set α and data set β) were simultaneously fit (using MLAB-PC) to the two-component dissociation model using the following equations: Y = A1 × e(-K1 × t) + A2 × e(-K2 × t) and Y = A3 × e(-K3 × t) + A4 × e(-K4 × t).
Four different constraint conditions were used: 1) unconstrained, 2) four parameters of set α= four parameters of set β (A1 = A3, A2 = A4, K1 = K3, K2 = K4), 3) two kinetic constants of set α= two kinetic constants of set β (K1 = K3, K2 = K4), and 4) two “A” values of set α= two A values of set β (A1 = A3, A2 = A4). An F-test was calculated based on the sum-of-squares for each of the constraint conditions. The threshold for significance was set at P < 0.01(Nandi et al., 2004).
Results
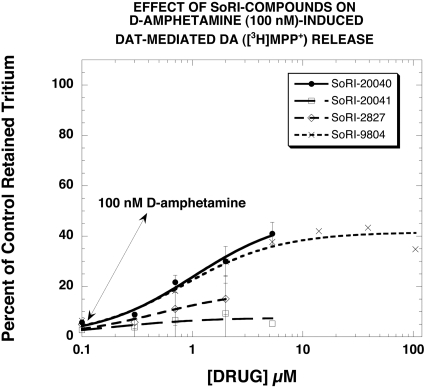
Figure 1 reports the structures of SoRI-9804, SoRI-20040, SoRI-20041, and SoRI-2827. In an initial experiment, we determined the effect of SoRI-20040, SoRI-20041, SoRI-2827, and SoRI-9804 on control and DAT-mediated DA ([3H]MPP+) release. In the absence of d-amphetamine (control), only SoRI-9804 had no effect on DA release over the entire concentration range examined (0.1–100 μM). The other compounds induced DA release (SoRI-2827 > SoRI-20041 > SoRI-20040) (data not shown). Figure 2 reports the reversal of the effects of 100 nM d-amphetamine on DAT-mediated DA ([3H]MPP+) release by the SoRI compounds at concentrations that did not by themselves significantly affect DA release (<10%). It is apparent that SoRI-20040 (EC50 = 1.0 ± 0.2 μM; Emax = 48 ± 4%) and SoRI-9804 (EC50 = 0.84 ± 0.21 μM; Emax = 41 ± 2%) had the largest effects, producing a fairly substantial reversal of d-amphetamine (100 nM)-induced DA release. SoRI-20041 (EC50 = 0.18 ± 0.17 μM; Emax = 7.6 ± 1.6%) had a much smaller, barely detectable effect. Likewise, although SoRI-2827 had a somewhat larger effect than SoRI-20041 (EC50 = 0.49 ± 0.22 μM; Emax = 18.6 ± 3.1%), the limited concentration range that did not alter control DA release made further study of this compound difficult. Based on these observations, we focused subsequent experiments on SoRI-9804, SoRI-20040, and SoRI-20041.
Fig. 2.
Effect of SoRI compounds on d-amphetamine (100 nM)-induced DAT-mediated DA ([3H]MPP+) release. Dose-response curves were generated in the absence and presence of 100 nM d-amphetamine. The effect of test drugs on d-amphetamine (100 nM)-induced release of [3H]MPP+ is graphed for doses that did not affect [3H]MPP+ release in the absence of d-amphetamine. Each value is the mean ± S.D. (n = 3).
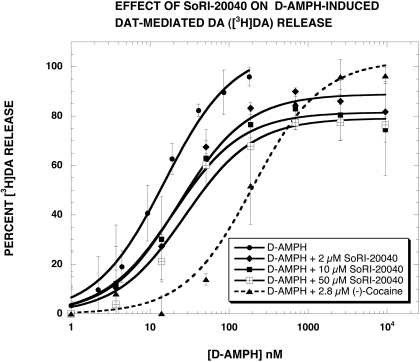
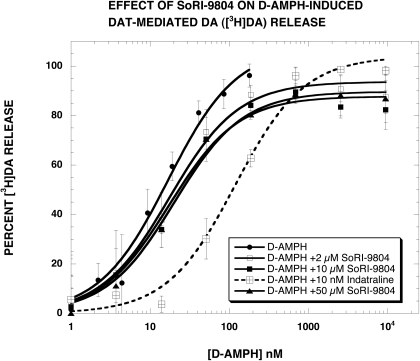
In our previous study (Pariser et al., 2008), we reported that SoRI-20040 and SoRI-9804 partially inhibited d-amphetamine-induced DAT-mediated DA ([3H]MPP+) release. To ascertain whether this finding resulted from the use of [3H]MPP+, compared with [3H]DA, we conducted similar experiments using [3H]DA. Control experiments indicated that SoRI-20040, SoRI-20041, and SoRI-9804 did not alter DAT-mediated DA ([3H]DA) release at concentrations up to 100 μM (data not shown). As reported in Fig. 3 and Table 1, SoRI-20040 (2, 10, and 50 μM) partially inhibited d-amphetamine-induced DAT-mediated DA ([3H]DA) release in a dose-dependent manner. The maximal decrease in the Emax value was ∼20%, less than observed with [3H]MPP+ (∼40%). In contrast, cocaine (2.8 μM) shifted the d-amphetamine curve to the right and did not alter the Emax value. SoRI-9804 (2, 10, and 50 μM) also partially inhibited d-amphetamine-induced DA release (Fig. 4) but to a much lesser extent (∼10%) than observed with [3H]MPP+ (∼25%). As observed for cocaine, indatraline (10 nM) shifted the d-amphetamine curve to the right and did not alter the Emax value. Along with the data in Fig. 2, these experiments indicate that SoRI-9804 and SoRI-20040 modulate DAT-mediated DA release at concentrations that are otherwise without effect and that the modulation is readily apparent when the endogenous substrate for DAT ([3H]DA) is used in the assay.
Fig. 3.
Effect of SoRI-20040 on d-amphetamine-induced DAT-mediated DA ([3H]DA) release. d-Amphetamine dose-response curves were generated in the absence and presence of the indicated concentrations of SoRI-20040 and cocaine. The dose-response curves were fit to the dose-response equation, and the results are reported in Table 1. Each value is the mean± S.D. (n = 3).
TABLE 1.
Effect of test agents on d-amphetamine-induced dopaminergic release Eight point dose-response curves were generated using either [3H]MPP+ or [3H]DA in the absence and presence of the indicated test agents. The data of three independent experiments were pooled (30 data points) and fit to the dose-response equation for the best-fit estimate of the EC50 and Emax (± S.D.) values.
| Drug | EC50 [Ke] | Emax |
|---|---|---|
| nM | % | |
| Experiment 1 ([3H]MPP+)a | ||
| d-Amph | 5.8 ± 0.4 | 102 ± 2 |
| + SoRI-9804 (2.0 μM) | 8.3 ± 1.0 | 86 ± 2* |
| + SoRI-9804 (10 μM) | 11.5 ± 0.4 | 73 ± 0.5* |
| + cocaine (2.8 μM) | 82 ± 9* [0.21 μM] | 102 ± 2 |
| Experiment 2 (Fig. 4), [3H]DA) | ||
| d-Amph | 17 ± 3 | 108 ± 5 |
| + SoRI-9804 (2.0 μM) | 18 ± 2 | 94 ± 2* |
| + SoRI-9804 (10 μM) | 17 ± 3 | 88 ± 3* |
| + SoRI-9804 (50 μM) | 20 ± 3 | 90 ± 2* |
| + indatraline (10 nM) | 120 ± 21* [1.6 nM] | 104 ± 4 |
| Experiment 3 ([3H]MPP+)a | ||
| d-Amph | 6.5 ± 0.3 | 103 ± 1 |
| + SoRI-20040 (2.0 μM) | 11.6 ± 1.0 | 78 ± 2* |
| + SoRI-20040 (10 μM) | 15.7 ± 2.5* | 60 ± 2* |
| + indatraline (66 nM) | 116 ± 14* [3.9 nM] | 101 ± 3 |
| Experiment 4 (Fig. 3), [3H]DA) | ||
| d-Amph | 15 ± 2 | 107 ± 4 |
| + SoRI-20040 (2.0 μM) | 23 ± 5 | 89 ± 3* |
| + SoRI-20040 (10 μM) | 20 ± 3 | 81 ± 2* |
| + SoRI-20040 (50 μM) | 27 ± 68 | 79 ± 3* |
| + cocaine (2.8 μM) | 200 ± 39* [0.23 μM] | 103 ± 4 |
P < 0.05 compared with the value of the d-Amph condition (ANOVA with a post hoc Bonferroni test)
Data for experiments 1 and 3 are data taken from Pariser et al. (2008)
Fig. 4.
Effect of SoRI-9804 on d-amphetamine-induced DAT-mediated DA ([3H]DA) release. d-Amphetamine dose-response curves were generated in the absence and presence of the indicated concentrations of SoRI-9804 and indatraline. The dose-response curves were fit to the dose-response equation and the results are reported in Table 1. Each value is the mean ± S.D. (n = 3).
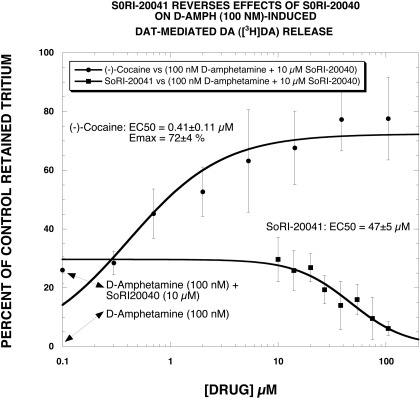
As noted above (Fig. 2), SoRI-20041 had a minimal effect on DAT-mediated DA release (measured with [3H]MPP+ release) in the absence or presence of 100 nM d-amphetamine. However, SoRI-20041 allosterically modulates both DAT binding and [3H]DA uptake (Pariser et al., 2008). This raised the possibility that SoRI-20041 could reverse the effect of SoRI-20040 on [3H]DA release. An experiment designed to test this hypothesis was performed using [3H]DA, rather than [3H]MPP+, because SoRI-20041 has minimal effect on [3H]DA release. As reported in Fig. 5, d-amphetamine (100 nM) complete released [3H]DA (reduced retained [3H]DA to ∼ 0%). The addition of SoRI-20040 (10 μM) reversed this effect to ∼25% of control. SoRI-20041 then reduced the combined effect of SoRI-20040 + d-amphetamine back to 0% in a dose-dependent manner (EC50 = 47 ± 5 μM). In contrast, cocaine had the opposite effect, increasing the percentage of control in a dose-dependent manner.
Fig. 5.
SoRI-20041 reverses the effect of SoRI-20040 on DAT-mediated DA ([3H]DA) release. SoRI-20041 and cocaine dose-response curves were generated in the presence of 100 nM d-amphetamine + SoRI-20040 (10 μM). SoRI-20041 then reduced the combined effect of SoRI-20040 + d-amphetamine back to 0% in a dose-dependent manner (EC50 = 47 ± 5 μM). In contrast, cocaine had the opposite effect, increasing the percentage of control in a dose-dependent manner (EC50 = 0.41 ± 0.11 μM). Each value is the mean ± S.D. (n = 3).
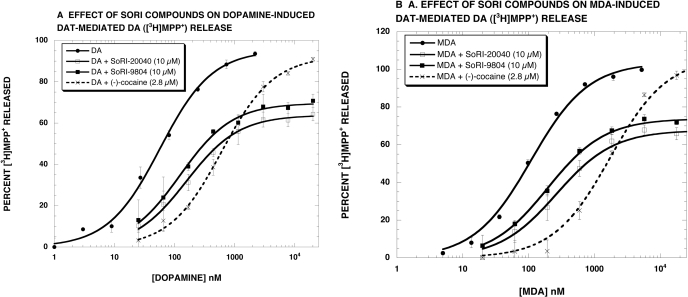
To establish whether the ability of SoRI-9804 and SoRI-20040 to modulate DA release was specific for d-amphetamine, we determined the effect of these compounds on DAT-mediated DA ([3H]MPP+) release induced by other DAT substrates (DA and MDA). The results show that SoRI-9804 and SoRI-20040 partially inhibited both DA-induced (Fig. 6A) and MDA-induced (Fig. 6B) DAT-mediated DA ([3H]MPP+) release. In contrast, cocaine, a competitive DAT inhibitor, shifted the dose-response curves to the right and did not alter the Emax value. These results, summarized in Table 2, were similar to those obtained when d-amphetamine was used as the substrate (Table 1).
Fig. 6.
Effect of SoRI-20040 and SoRI-9804 on substrate-induced DAT-mediated DA ([3H]MPP+) release. Dopamine (A) and MDA (B) dose-response curves were generated in the absence and presence of SoRI-20040 (10 μM), SoRI-9804 (10 μM), and cocaine (2.8 μM). The dose-response curves were fit to the dose-response equation, and the results are reported in Table 2. Each value is the mean ± S.D. (n = 3).
TABLE 2.
Effect of test agents on substrate-induced [3H]MPP+ release Eight point dose-response curves were generated using [3H]MPP+ in the absence and presence of the indicated test agents. The data of three independent experiments were pooled (30 data points) and fit to the dose-response equation for the best-fit estimate of the EC50 and Emax (± S.D.) values.
| Drug | EC50 [Ke] | Emax |
|---|---|---|
| nM | % | |
| Experiment 1 (Fig. 6A) | ||
| Dopamine | 57 ± 5 | 95 ± 2 |
| + SoRI-20040 (10 μ M) | 158 ± 12* | 64 ± 1* |
| + SoRI-9804 (10 μ M) | 124 ± 9* | 70 ± 1* |
| + (–)-cocaine (2.8 μM) | 608 ± 40* [0.29 μM] | 92 ± 1 |
| Experiment 2 (Fig. 6B) | ||
| MDA | 110 ± 10 | 104 ± 2 |
| + SoRI-20040 (10 μ M) | 265 ± 39* | 68 ± 2* |
| + SoRI-9804 (10 μ M) | 195 ± 12* | 74 ± 1* |
| + (–)-cocaine (2.8 μM) | 1744 ± 225* [0.19 μM] | 106 ± 3 |
P < 0.05 compared with the value of the control condition (ANOVA with a post hoc Bonferroni test)
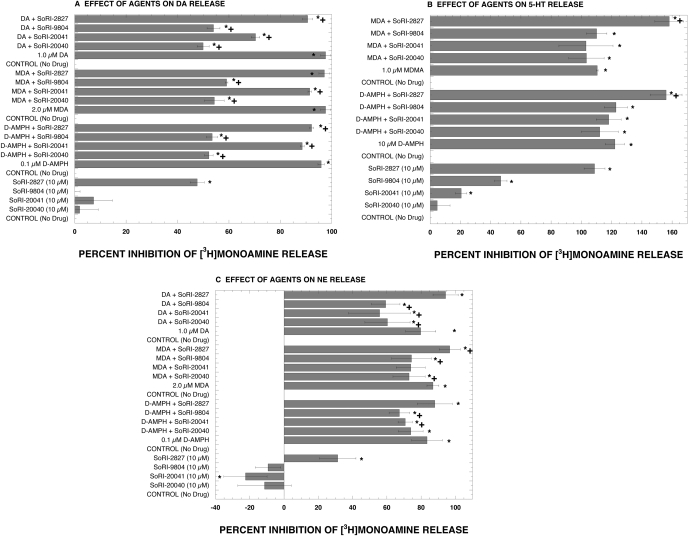
The next series of experiments determined the effect of SoRI-9804 and SoRI-20040 on d-amphetamine-induced release mediated by SERT (using [3H]5-HT) and by NET (using [3H]MPP+). As reported in Fig. 7A, consistent with our previous experiments, SoRI-9804 and SoRI-20040 attenuated d-amphetamine-induced DAT-mediated DA ([3H]MPP+)release. SoRI-20041 and SoRI-2827 had very small effects on d-amphetamine-induced DAT-mediated DA ([3H]MPP+)release that reached the level of statistical significance. In contrast, SoRI-20040, SoRI-9804, and SoRI-20041 did not affect d-amphetamine-induced 5-HT release (Fig. 7B). SoRI-2827 enhanced d-amphetamine-induced 5-HT release but that was because of the substantial effect this compound had by itself. Interestingly, SoRI-20041 and SoRI-9804 significantly attenuated d-amphetamine-induced NE release (Fig. 7C). The degree of attenuation was substantially less than observed for d-amphetamine-induced DA release. With some exceptions, a similar profile of results was observed when different substrates (dopamine and MDA) were used to induce neurotransmitter release. The ineffectiveness of these compounds in the SERT release assay demonstrates that the modulatory effects of SoRI-9804 and SoRI-20040 are selective for DAT. The weaker effect of these same compounds in the NE release assay suggests that similar phenomena might be observed at NET. However, the signal-to-noise ratio of this assay (2:1), as currently implemented, precludes more detailed study.
Fig. 7.
Effect of test drugs (10 μM) on substrate-induced release mediated by DAT (A), SERT (B), and NET (C). Release assays were conducted as described under Materials and Methods using [3H]MPP+ for DAT- and NET-mediated release and [3H]5-HT for SERT-mediated release. Each value is mean ± S.D. (n = 3 for DAT and SERT; n = 6 for NET). *, P < 0.05 compared with the no drug control. †, P < 0.05 compared with the substrate alone (ANOVA with post hoc Student-Newman-Keuls multiple comparison test).
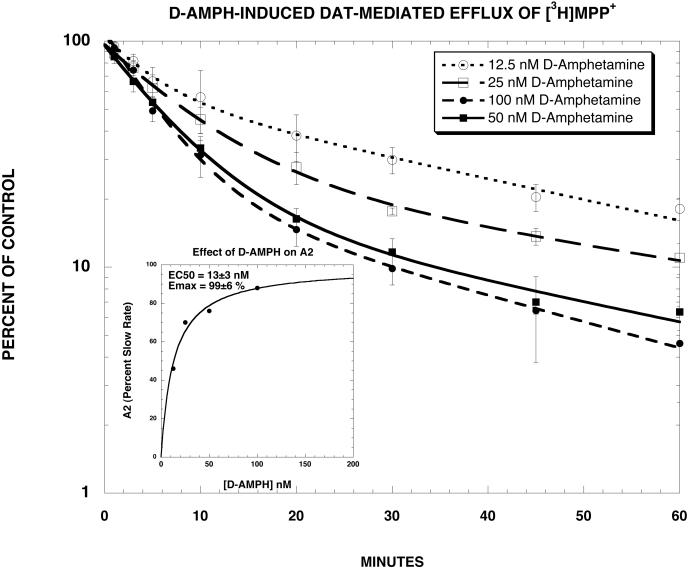
The goal of the next series of experiments was to ascertain the effect of SoRI-9804 on the kinetics of d-amphetamine-induced efflux of [3H]MPP+. SoRI-9804 was used rather than SoRI-20040 or SoRI-20041 because this compound does not directly alter [3H]MPP+ efflux (Fig. 2). For these experiments, synaptosomes were preloaded with [3H]MPP+ for 60 min. Test drugs were then added, and samples were filtered at various times up to 60 min later. As reported in Fig. 8 and Table 3, d-amphetamine-induced [3H]MPP+ efflux was well described by a two-component dissociation model, composed of a fast (A2 and K2) and slow component (A1 and K1), where A1 and A2 are the proportion of efflux attributed to each component, and K1 and K2 are the corresponding rate constants. With 100 nM d-amphetamine, the fast component (A2) contributed 88% to the efflux and had a rate constant of 0.19 ± 0.02 min-1. The slow component (A1) contributed 22% to the efflux and had a rate constant of 0.027 ± 0.01 min-1. Statistical analysis of the kinetic data showed that d-amphetamine significantly increased the value of A2 and decrease the value of A1 in a dose-dependent manner, yielding an estimated EC50 value of 13 nM (Fig. 8, inset), a value somewhat higher than the EC50 value for d-amphetamine-induced DAT-mediated DA ([3H]MPP+) release (∼6 nM). There were no significant changes in the value of rate constants K1 and K2.
Fig. 8.
DAT-mediated [3H]MPP+ efflux experiments. As described under Materials and Methods, synaptosomes were preloaded with [3H]MPP+ for 60 min. Various concentrations of d-amphetamine were then added, and samples were filtered at various times up to 60 min later. Each value is the mean ± S.D. The data of three independent experiments were pooled and fit to one- and two-component dissociation experiments using MLAB-PC. In all cases, the two-component model fit significantly better than the one-component model (F-test, P < 0.002). The best-fit estimates of the kinetic parameters are reported in Table 3.
TABLE 3.
Effect of d-amphetamine on [3H]MPP+ efflux [3H]MPP+ efflux experiments were conducted as described under Materials and Methods. The data of three (12.5, 25, and 50 nM d-Amph) or 10 (100 nM d-Amph) independent experiments were pooled and fit to one- and two-component dissociation experiments using MLAB-PC. The parameter values are ± S.D. In all cases, the two-component model fit significantly better than the one-component model (F-test, P < 0.002).
| Condition | K1 | A1 | K2 | A2 |
|---|---|---|---|---|
| min–1 | % | min–1 | % | |
| d-Amph,† 12.5 nM | 0.021 ± 0.005 | 57 ± 11* | 0.18 ± 0.07 | 46 ± 10* |
| d-Amph,† 25 nM | 0.015 ± 0.010 | 26 ± 1* | 0.12 ± 0.03 | 70 ± 13* |
| d-Amph,† 50 nM | 0.020 ± 0.010 | 19 ± 11* | 0.15 ± 0.03 | 77 ± 10* |
| d-Amph, 100 nM | 0.027 ± 0.010 | 22 ± 6 | 0.19 ± 0.02 | 88 ± 5 |
The A values of the curve (constraint 4; see Materials and Methods) were significantly different (F test, P < 0.01) from the 100 nM d-Amph curve. The kinetic constants of each curve did not significantly differ from those of the 100 nM d-Amph curve
The entire curve (constraint 1; see Materials and Methods) was significantly different (F-test, P < 0.01) from the 100 nM d-Amph curve
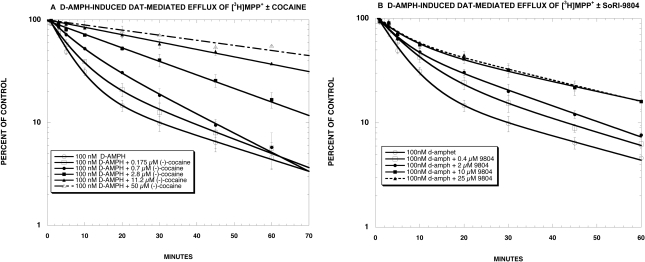
The next experiments compared the effects of various concentrations of cocaine and SoRI-9804 on [3H]MPP+ efflux induced by 100 nM d-amphetamine. As reported in Fig. 9A, cocaine produced a dose-dependent reduction in the rate of d-amphetamine-induced [3H]MPP+ efflux. In contrast, SoRI-9804 (Fig. 9B) reduced the rate of d-amphetamine-induced [3H]MPP+ efflux in an asymptotic manner, with a similar reduction observed with the 10 and 25 μM dose of SoRI-9804.
Fig. 9.
Effect of cocaine and SoRI-9804 on d-amphetamine induced DAT-mediated efflux of [3H]MPP+. As described under Materials and Methods, synaptosomes were preloaded with [3H]MPP+ for 60 min. d-Amphetamine (100 nM) was added in the absence and presence of various concentrations of cocaine (A) or SoRI-9804 (B), and samples were filtered at various times up to 60 min later. Each value is the mean ± S.D. The data of three independent experiments were pooled and fit to one- and two-component dissociation experiments using MLAB-PC. The best-fit estimates of the kinetic parameters are reported in Table 4 for cocaine and in Table 5 for SoRI-9804.
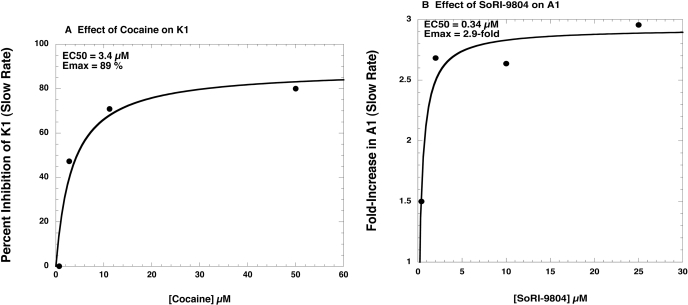
The efflux experiments (Fig. 9, A and B) were fit to one- and two-component dissociation models using MLAB-PC. As reported in Table 4, the main effect of the lowest dose of cocaine (0.175 μM) was to increase A1 from 22% in the presence of 100 nM d-amphetamine alone, to 36%. Although the individual parameter values in the presence of cocaine did not differ significantly from the values in the absence of cocaine, the two overall curves were significantly different (F-test, P < 0.001). At cocaine doses greater than 0.175 μM, a one-component model fit the data as well as the two-component model, and cocaine significantly decreased the apparent rate of the slow component (K1) in a dose-dependent manner. The EC50 value for cocaine reducing K1 was 3.4 μM (Fig. 10A). Thus, the main effect of cocaine was to first eliminate d-amphetamine-induced [3H]MPP+ efflux, i.e., reduce A2 to 0, and at higher doses to reduce the apparent rate (K1) of the slow [3H]MPP+ efflux. In contrast, the d-amphetamine-induced [3H]MPP+ efflux curves in the presence of SoRI-9804 were best fit by the two-component model (Table 5). SoRI-9804 significantly increased A1 (and decreased A2) in a dose-dependent manner without producing significant changes in the rate constants. The EC50 value for SoRI-9804 increasing A1 was 0.34 μM (Fig. 10B).
TABLE 4.
Effect of cocaine on D-amphetamine-induced [3H]MPP+ efflux [3H]MPP+ efflux experiments were conducted as described under Materials and Methods to determine the effect of cocaine on d-amphetamine-induced (100 nM) [3H]MPP+ efflux. The data of 3 (0.175, 0.7, 2.8, 11.2, and 50 μ M cocaine + 100 nM d-Amph) or 10 (100 nM d-Amph) independent experiments were pooled and fit to one- and two-component dissociation experiments using MLAB-PC. The two-component model fit better than the one-component model only for the lowest cocaine dose (0.175 μ M) (F-test, P < 0.001). The parameter values are ± S.D.
| Condition | K1 | A1 | K2 | A2 |
|---|---|---|---|---|
| min–1 | % | min–1 | % | |
| d-Amph (100 nM) | 0.027 ± 0.01 | 22 ± 6 | 0.19 ± 0.02 | 88 ± 5 |
| d-Amph (100 nM) + cocaine (0.175 μM) | 0.031 ± 0.015 | 36 ± 21 | 0.15 ± 0.05 | 64 ± 19 |
| d-Amph (100 nM) + cocaine (0.7 μM)† | 0.055 ± 0.002 | 100 | ||
| d-Amph (100 nM) + cocaine (2.8 μM) | 0.029 ± 0.001* | 100 | ||
| d-Amph (100 nM) + cocaine (11.2 μM | 0.016 ± 0.001* | 100 | ||
| d-Amph (100 nM) + cocaine (50 μM) | 0.011 ± 0.001* | 100 |
P < 0.05 compared with the value of the 0.75 μ M cocaine condition (ANOVA with a post hoc Bonferroni test)
This curve is statistically different from the d-Amph curve (F-test, P < 0.001)
Fig. 10.
Effect of cocaine and SoRI-9804 on selected kinetic parameter values. A, effect of cocaine on the value of K1, as reported in Table 4. B, effect of SoRI-9804 on the value of A1, as reported in Table 5.
TABLE 5.
Effect of SoRI-9804 on d-amphetamine-induced [3H]MPP+ efflux [3H]MPP+ efflux experiments were conducted as described under Materials and Methods to determine the effect of SoRI-9804 on d-amphetamine-induced (100 nM) [3H]MPP+ efflux. The data of three independent experiments were pooled and fit to one- and two-component dissociation experiments using MLAB-PC. The parameter values are ± S.D. In all cases, the two-component model fit significantly better than the one-component model (F-test, P < 0.001).
| Condition | K1 | A1 | K2 | A2 |
|---|---|---|---|---|
| min–1 | % | min–1 | % | |
| d-Amph (100 nM) | 0.027 ± 0.01 | 22 ± 6 | 0.19 ± 0.02 | 88 ± 5 |
| d-Amph (100 nM) + SoRI-9804 (0.4 μM)† | 0.028 ± 0.013 | 33 ± 18 | 0.14 ± 0.04 | 74 ± 16 |
| d-Amph (100 nM) + SoRI-9804 (2.0 μM)† | 0.035 ± 0.01 | 59 ± 12* | 0.21 ± 0.07 | 50 ± 10* |
| d-Amph (100 nM) + SoRI-9804 (10 μM)† | 0.021 ± 0.004 | 58 ± 11* | 0.15 ± 0.05 | 45 ± 10* |
| d-Amph (100 nM) + SoRI-9804 (25 μM)† | 0.023 ± 0.004 | 65 ± 10* | 0.19 ± 0.06 | 43 ± 9* |
The A values of the curve (constraint 4; see Materials and Methods) were significantly different (F-test, P < 0.01) from the 100 nM d-Amph curve. The kinetic constants of each curve did not significantly differ from those of the 100 nM d-Amph curve
The entire curve (constraint 1; see Materials and Methods) was significantly different (F-test, P < 0.01) from the 100 nM d-Amph curve
We note that the possible activity of these compounds at other central nervous system binding sites is currently underway via a screen of the receptorome (Roth et al., 2004). The results will be reported in due course.
Discussion
Pariser et al. (2008) first reported the allosteric effect of SoRI-9804 and SoRI-20040 on d-amphetamine-induced DAT-mediated DA ([3H]MPP+) release. The data we present here provide additional compelling novel evidence for allosteric modulators of the DAT. Two major findings include the identification of both “agonist” (SoRI-9804 and SoRI-20040) and “antagonist” (SoRI-20041) allosteric modulators of d-amphetamine-induced DAT-mediated DA release and that [3H]DA uptake and d-amphetamine-induced DAT-mediated efflux can be separately modulated.
An obvious requirement for studying these compounds on substrate-induced release is to use concentrations that do not directly induce “release.” In the DAT-mediated DA ([3H]-MPP+) release, only SoRI-9804 had no effect on release in the absence of d-amphetamine. The other compounds induced DA release (SoRI-2827 > SoRI-20041 > SoRI-20040) to varying extents. Because of the limited concentration range that did not alter control DA release, SoRI-2827 was not studied in subsequent experiments. As reported in Fig. 2, SoRI-9804 and SoRI-20040, at concentrations without effect in the absence of d-amphetamine, reversed d-amphetamine-induced DAT-mediated DA ([3H]MPP+) release in a dose-dependent manner. SoRI-20041, however, had a small barely detectable effect, raising the possibility that this compound might be able to reverse the effect of SoRI-20040. To test this hypothesis, we used [3H]DA, rather than [3H]MPP+, because SoRI-20041 had minimal effect on [3H]DA release. As reported in Fig. 5, d-amphetamine (100 nM) completely released [3H]DA (reduced retained [3H]DA to ∼ 0%). The addition of SoRI-20040 (10 μM) reversed this effect to ∼25% of control. SoRI-20041 then reduced the combined effect of SoRI-20040 plus d-amphetamine back to 0% in a dose-dependent manner (EC50 = 47 ± 5 μM). In contrast, cocaine had the opposite effect, increasing the percentage of retained [3H]DA in a dose-dependent manner. If one were to define SoRI-20040 as “agonist-like,” meaning able to produce an allosteric effect on d-amphetamine-induced DAT-mediated DA release, then SoRI-20041 could be described as an “antagonist-like” allosteric modulator. The structural difference between these two compounds is at first glance minor (Fig. 1), but such small changes in structure often produce major changes in pharmacological activity. This suggests that structural modification of these molecules may produce more potent agonist and antagonist DAT allosteric modulators.
Several control experiments revealed both the generality of the allosteric effects of the SoRI compounds and as well as their specificity. For example, SoRI-20040 (Fig. 3) and SoRI-9804 (Fig. 4) partially inhibited DAT-mediated DA ([3H]DA) release in a dose-dependent manner, similar to what was observed for DAT-mediated DA ([3H]MPP+) release, indicating that the allosteric modulation of DA release is independent of the radiotracer used in the assay. The decrease in the Emax value for d-amphetamine-induced DAT-mediated DA ([3H]DA) release produced by SoRI-20040 and SorI-9804 was less than observed with [3H]MPP+. A likely reason for this difference is that the [3H]MPP+ release assay is terminated 30 min after the addition of test compounds, whereas the [3H]DA release assay is terminated 5 min after the addition of test compounds. Also consistent with the generality of the allosteric effects of the SoRI compounds are the observations that SoRI-20040 and SoRI-9804 partially inhibit both DA-induced and MDA-induced DAT-mediated DA ([3H]MPP+) release. These data support the assertion that the allosteric modulation of DA release by SoRI-9804 and SoRI-20040 is independent of the DAT substrate used to induce release.
The experiments described in Fig. 7 show that the SoRI compounds specifically modulate the DAT, compared with the SERT and NET. It is noteworthy that SoRI-20040, SoRI-9804, and SoRI-20041 did not affect d-amphetamine-induced 5-HT release (Fig. 7B). SoRI-20041 and SoRI-9804 significantly attenuated d-amphetamine-induced NE release (Fig. 7C). The weaker effect of these same compounds in the NE release assay suggests that similar phenomena might be observed at NET. The signal-to-noise ratio of this assay (2:1), as currently implemented, precludes more detailed study of this issue. A generally similar profile of results was observed when different substrates (dopamine and MDA) were used to induce neurotransmitter release. Viewed collectively, these data support the hypothesis that the SoRI compounds specifically modulate the DAT. SoRI-2827 by itself had activity in all three release assays, suggesting a common mechanism that requires further investigation to elucidate. A possible mechanism could be drug-induced increase in the intracellular concentration of Na+, such as produced by monensin and hyperforin (Singer et al., 1999).
The [3H]MPP+ efflux experiments provided important insight into an underlying mechanism of d-amphetamine-induced DAT-mediated DA ([3H]MPP+) release and its modulation by SoRI-9804, which does not affect [3H]MPP+ release in the absence of d-amphetamine. d-Amphetamine-induced [3H]MPP+ efflux was well described by a two-component dissociation model (Table 3). d-Amphetamine increased A2 (and decreased A1) in a dose-dependent manner without significantly changing the rate constants. The estimated EC50 value for d-amphetamine increasing A2 (Fig. 8, inset) was 23 nM, a value somewhat higher than the EC50 value for d-amphetamine-induced DAT-mediated DA ([3H]MPP+) release (∼6 nM). Thus, the A1 component reflects the ability of d-amphetamine to induce a fast DAT-mediated release of [3H]MPP+. The A2 component probably reflects a slow reverse efflux of [3H]MPP+ (see below).
The overall effect of cocaine was to slow d-amphetamine (100 nM)-induced [3H]MPP+ efflux (Fig. 9A). At concentrations of 0.7 μM cocaine or greater, the efflux curves were described by a one-component model. The simplest explanation of this observation is that, at this concentration range, cocaine was able to completely block d-amphetamine-induced DAT-mediated DA ([3H]MPP+) release. Cocaine decreased K1 in a dose-dependent manner, with an EC50 value of 3.4 μM (Fig. 10A). The cocaine sensitivity of K1 supports the idea that the slow component of efflux reflects DAT-mediated reverse transport of [3H]MPP+ (for review, see Chen and Reith, 2000).
Unlike cocaine, SoRI-9804 slowed the overall rate of d-amphetamine (100 nM)-induced [3H]MPP+ efflux in an asymptotic manner (Fig. 9B). All efflux curves were best fit by the two-component model (Table 5), and the chief effect of SoRI-9804 was to increase A1 (and decrease A2) in a dose-dependent manner (EC50 = 0.34 μM) without producing significant changes in rate constants (Fig. 10B). It is of interest to note that the potency of SoRI-9804 is 10 times lower than observed for cocaine in the same assay. Recent studies indicate that DAT can exist in a “reluctant” or “willing” state for d-amphetamine-induced DA efflux (Khoshbouei et al., 2004) depending on N-terminal phosphorylation. Viewed in this context, the effect of SoRI-9804 can be visualized as reducing the proportion of DAT that is in a willing state, thereby reducing the over all efficacy of d-amphetamine-induced DA release. This could potentially result from rendering DAT more susceptible to phosphorylation, or alternatively, shifting DAT to a conformation that is less efficient at exchanging d-amphetamine for [3H]MPP+. Several lines of evidence support the hypothesis that transporters can adopt different conformation states (Ferrer and Javitch, 1998; Reith et al., 2001; Gether et al., 2006).
Table 6 summarizes the results reported in our previous article (Pariser et al., 2008) and the current study. SoRI-9804, SoRI-20040, and SoRI-20041 share several properties. In contrast to cocaine, a classic competitive DAT inhibitor, the SoRI compounds partially inhibited DAT binding, decreased the Bmax, and slowed the dissociation of [125I]RTI-55 from the DAT, classic evidence for allosterism. One of the more striking findings of this study is that SoRI-20041 produced the same effects as SoRI-20040 and SoRI-9804 in the [3H]DA uptake assays and the DAT binding assays but did not modulate d-amphetamine-induced DA release. Some studies indicate that inward and outward transport represent distinct operational modes of DAT (Scholze et al., 2002). Our findings with SoRI-20041 support this hypothesis. To our knowledge, SoRI-20041 is the first example of a drug that separately modulates uptake versus release.
TABLE 6.
A summary of the evidence for allosteric modulation of DAT by SoRI compounds Cocaine competitively inhibits [3H]DA uptake (Zhu and Hexum, 1992) and DAT binding (Reith et al., 1992; Pariser et al., 2008).
| Drug | [3H]DA Uptake | DAT Binding ([125I]RTI-55) | Effect on [125I]RTI-55 Dissociation from DAT | Effect on d-Amph-Induced DAT-Mediated Efflux of [3H]MPP+ | Effect on d-Amph-Induced DAT-Mediated DA Release |
|---|---|---|---|---|---|
| Cocaine | “Normal” competitive inhibitor | Normal competitive inhibitor | No effecta | Slows d-Amph-induced efflux by changing apparent rate constants | Competitive inhibitor |
| SoRI-9804 | Partial inhibitor decreases Vmaxa | Partial inhibitor decreases Bmaxa | Slowed dissociation ratea | Slows d-Amph-induced efflux without changing apparent rate constants | Partial inhibitor “agonist-like” |
| SoRI-20040 | Partial inhibitor decreases Vmax | Partial inhibitor decreases Bmax | Slowed dissociation rate | Not determined; direct effects at high concentrations | Partial inhibitor agonist-like |
| SoRI-20041 | Partial inhibitor decreases Vmax | Partial inhibitor decreases Bmax | Slowed dissociation rate | Not determined; direct effects at high concentrations | No effect “antagonist-like” |
| SoRI-2827 | Partial inhibitor decreases Vmax, but less so than SoRI-20040 and SoRI-20041 | Partial inhibitor decreases Bmax | No effect on dissociation rate | Not studied; nonspecific effects | Not studied; nonspecific effects |
Unpublished data
In summary, we have identified both agonist (SoRI-9804 and SoRI-20040) and antagonist (SoRI-20041) allosteric modulators of d-amphetamine-induced DAT-mediated DA release and presented evidence that [3H]DA uptake and d-amphetamine-induced DAT-mediated efflux can be separately modulated. Such agents might have therapeutic potential for the treatment of stimulant addiction, Parkinson's disease, and other psychiatric disorders.
This research was supported by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.149088.
ABBREVIATIONS: DAT, dopamine transporter; NET, norepinephrine transporter; SERT, serotonin transporter; BAT, biogenic amine transporter; 5-HT, 5-hydoxytryptamine (serotonin); SoRI-20040, N-(2,2-diphenylethyl)-2-phenyl-4-quinazolinamine; SoRI-20041, N-(3,3-diphenylpropyl)-2-phenyl-4-quinazolinamine; SoRI-2827, [4-amino-6-[(diphenylmethyl)amino]-5-nitro-2-pyridinyl]carbamic acid ethyl ester; SoRI-9804, N-(diphenylmethyl)-2-phenyl-4-quinazolinamine; SoRI-6238, [5-amino-3-(3,4-dichlorophenyl)-1,2-dihydropyrido[3,4-b]pyrazin-7-yl]carbamic acid ethyl ester; RTI-55, 3β-(4′-iodophenyl)tropan-2β-carboxylic acid methyl ester; TB-1-099, 4-(2-[bis(4-fluorophenyl)methoxy]ethyl)-1-(2-trifluoromethyl-benzyl)-piperidine; SA, specific activity; MDA, (±)-3,4-methylenedioxyamphetamine; MPP+, 1-methyl-4-phenylpyridinium; GBR12909, 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine; ANOVA, analysis of variance; Amph, amphetamine.
References
- Akunne HC, de Costa BR, Jacobson AE, Rice KC, and Rothman RB (1992) [3H]Cocaine labels a binding site associated with the serotonin transporter in guinea pig brain: allosteric modulation by paroxetine. Neurochem Res 17 1275-1283. [DOI] [PubMed] [Google Scholar]
- Andersson A and Marcusson J (1989) Inhibition and dissociation of [3H]-paroxetine binding to human platelets. Neuropsychobiology 22 135-140. [DOI] [PubMed] [Google Scholar]
- Chang AS and Chang SM (1999) Nongenomic steroidal modulation of high-affinity serotonin transport. Biochim Biophys Acta 1417 157-166. [DOI] [PubMed] [Google Scholar]
- Chen F, Larsen MB, Neubauer HA, Sánchez C, Plenge P, and Wiborg O (2005) Characterization of an allosteric citalopram-binding site at the serotonin transporter. J Neurochem 92 21-28. [DOI] [PubMed] [Google Scholar]
- Chen N and Reith ME (2000) Structure and function of the dopamine transporter. Eur J Pharmacol 405 329-339. [DOI] [PubMed] [Google Scholar]
- Christopoulos A and Kenakin T (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54 323-374. [DOI] [PubMed] [Google Scholar]
- Ferrer JV and Javitch JA (1998) Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. Proc Natl Acad Sci U S A 95 9238-9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, and Schousboe A (2006) Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci 27 375-383. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, De Felice LJ, and Khoshbouei H (2009) Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem 284 2978-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM and Kent JM (1999) SSRIs and SMRIs: broad spectrum of efficacy beyond major depression. J Clin Psychiatry 60 (Suppl 4): 33-38. [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, and Negus SS (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29 1439-1464. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, and Javitch JA (2004) N-Terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol 2 E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Dersch CM, Kulshrestha M, Ananthan S, and Rothman RB (2004) Identification and characterization of a novel allosteric modulator (SoRI-6238) of the serotonin transporter. Synapse 53 176-183. [DOI] [PubMed] [Google Scholar]
- Nightingale B, Dersch CM, Boos TL, Greiner E, Calhoun WJ, Jacobson AE, Rice KC, and Rothman RB (2005) Studies of the biogenic amine transporters. XI. Identification of a 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine (GBR12909) analog that allosterically modulates the serotonin transporter. J Pharmacol Exp Ther 314 906-915. [DOI] [PubMed] [Google Scholar]
- Pariser JJ, Partilla JS, Dersch CM, Ananthan S, and Rothman RB (2008) Studies of the biogenic amine transporters. 12. Identification of novel partial inhibitors of amphetamine-induced dopamine release. J Pharmacol Exp Ther 326 286-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, Wolf A, Rebernik P, Reither H, and Berger ML (2009) Zinc regulates the dopamine transporter in a membrane potential and chloride dependent manner. Neuropharmacology 56 531-540. [DOI] [PubMed] [Google Scholar]
- Reith ME, Berfield JL, Wang LC, Ferrer JV, and Javitch JA (2001) The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem 276 29012-29018. [DOI] [PubMed] [Google Scholar]
- Reith ME, de Costa B, Rice KC, and Jacobson AE (1992) Evidence for mutually exclusive binding of cocaine, BTCP, GBR 12935, and dopamine to the dopamine transporter. Eur J Pharmacol 227 417-425. [DOI] [PubMed] [Google Scholar]
- Roth BL, Lopez E, Beischel S, Westkaemper RB, and Evans JM (2004) Screening the receptorome to discover the molecular targets for plant-derived psychoactive compounds: a novel approach for CNS drug discovery. Pharmacol Ther 102 99-110. [DOI] [PubMed] [Google Scholar]
- Rothman RB and Baumann MH (2006) Therapeutic potential of monoamine transporter substrates. Curr Top Med Chem 6 1845-1859. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, and Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39 32-41. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Prisinzano TE, and Newman AH (2008) Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol 75 2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, and Baumann MH (2006) Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol Sci 27 612-618. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Dersch CM, Carroll FI, and Ananthan S (2002) Studies of the biogenic amine transporters. VIII: Identification of a novel partial inhibitor of dopamine uptake and dopamine transporter binding. Synapse 43 268-274. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Reid A, Mahboubi A, Kim CH, De Costa BR, Jacobson AE, and Rice KC (1991) Labeling by [3H]1,3-di(2-tolyl)guanidine of two high affinity binding sites in guinea pig brain: evidence for allosteric regulation by calcium channel antagonists and pseudoallosteric modulation by σ ligands. Mol Pharmacol 39 222-232. [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, and Glennon RA (2003) In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther 307 138-145. [DOI] [PubMed] [Google Scholar]
- Rudnick G and Clark J (1993) From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta 1144 249-263. [DOI] [PubMed] [Google Scholar]
- Sanchez C (2006) Allosteric modulation of monoamine transporters—new drug targets in depression. Drug Discov Today Ther Strategies 3 483-488. [Google Scholar]
- Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, and Sitte HH (2002) The role of zinc ions in reverse transport mediated by monoamine transporters. J Biol Chem 277 21505-21513. [DOI] [PubMed] [Google Scholar]
- Schwartz TW and Holst B (2007) Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci 28 366-373. [DOI] [PubMed] [Google Scholar]
- Singer A, Wonnemann M, and Müller WE (1999) Hyperforin, a major antidepressant constituent of St. John's wort, inhibits serotonin uptake by elevating free intracellular Na+1. J Pharmacol Exp Ther 290 1363-1368. [PubMed] [Google Scholar]
- Sur C, Betz H, and Schloss P (1998) Distinct effects of imipramine on 5-hydroxytryptamine uptake mediated by the recombinant rat serotonin transporter SERT1. J Neurochem 70 2545-2553. [DOI] [PubMed] [Google Scholar]
- Zhu J and Hexum TD (1992) Characterization of cocaine-sensitive dopamine uptake in PC12 cells. Neurochem Int 21 521-526. [DOI] [PubMed] [Google Scholar]
- Zohar J and Westenberg HG (2000) Anxiety disorders: a review of tricyclic antidepressants and selective serotonin reuptake inhibitors. Acta Psychiatr Scand Suppl 403 39-49. [DOI] [PubMed] [Google Scholar]