Abstract
Celiac sprue is a T-cell-mediated enteropathy elicited in genetically susceptible individuals by dietary gluten proteins. To initiate and propagate inflammation, proteolytically resistant gluten peptides must be translocated across the small intestinal epithelium and presented to DQ2-restricted T cells, but the effectors enabling this translocation under normal and inflammatory conditions are not well understood. We demonstrate that a fluorescently labeled antigenic 33-mer gluten peptide is translocated intact across a T84 cultured epithelial cell monolayer and that preincubation of the monolayer with media from gluten-stimulated, celiac patient-derived intestinal T cells enhances the apical-to-basolateral flux of this peptide in a dose-dependent, saturable manner. The permeability-enhancing activity of activated T-cell media is inhibited by blocking antibodies against either interferon-γ or its receptor and is recapitulated using recombinant interferon-γ. At saturating levels of interferon-γ, activated T-cell media does not further increase transepithelial peptide flux, indicating the primacy of interferon-γ as an effector of increased epithelial permeability during inflammation. Reducing the assay temperature to 4°C reverses the effect of interferon-γ but does not reduce basal peptide flux occurring in the absence of interferon-γ, suggesting active transcellular transport of intact peptides is increased during inflammation. A panel of disease-relevant gluten peptides exhibited an inverse correlation between size and transepithelial flux but no apparent sequence constraints. Anti-interferon-γ therapy may mitigate the vicious cycle of gluten-induced interferon-γ secretion and interferon-γ-mediated enhancement of gluten peptide flux but is unlikely to prevent translocation of gluten peptides in the absence of inflammatory conditions.
Celiac sprue is a T-cell-mediated enteropathy induced in genetically susceptible individuals by dietary gluten from wheat and similar proteins in rye and barley. Gluten is uniquely resistant to gastrointestinal proteolysis in mammals. As a result, unusually long proline- and glutamine-rich peptides accumulate in the gut lumen after ingestion of gluten, some of which are recognized by inflammatory T cells that reside in the celiac small intestinal mucosa. For example, certain α- and γ-gliadin proteins from wheat release highly antigenic, metastable 33- and 26-residue peptides, respectively, when exposed to pancreatic proteases (Shan et al., 2002, 2005). Upon translocation across the intestinal epithelium into the gut-associated lymphoid tissue (GALT), peptides such as the 33-mer initiate an inflammatory immune response, the molecular basis of which is relatively well understood. Gluten peptides are deamidated at specific glutamine residues by the endogenous enzyme transglutaminase 2 (TG2) (Molberg et al., 1998). The negative charges introduced by TG2-mediated deamidation enhance the affinity of these peptides for human leukocyte antigen DQ2 (Quarsten et al., 1999), a major histocompatibility class II molecule associated with more than 90% of diagnosed celiac patients (Sollid et al., 1989). Upon recognition of these peptide-DQ2 complexes on the surface of antigen-presenting cells (APCs), CD4+ T cells in the GALT enact a T-helper 1 response dominated by interferon (IFN)-γ. Ultimately, the inflammatory response to gluten causes restructuring of the intestinal epithelial architecture, malabsorption of nutrients, and, in many patients, related clinical symptoms (Alaedini and Green, 2005). Disease progression is halted and symptoms subside upon abstention from dietary gluten.
Despite our understanding of the structural basis for the stability of gluten in the gut and for its DQ2-mediated presentation in the GALT of celiac patients, our understanding of the transepithelial uptake of gluten peptides that is required to link these two phenomena is rudimentary at best. Early work on this subject used radiolabeled EDTA to demonstrate elevated epithelial permeability in celiac patients in remission (Bjarnason et al., 1983). Subsequently, disaccharides such as lactulose were used to detect further enhancement of intestinal permeability in celiac patients with active disease (e.g., see Duerksen et al., 2005, and references therein). However, it is unknown whether transepithelial transport of small molecules such as EDTA and sugars is correlated with the uptake of antigenic gluten oligopeptides. Friis et al. (1992) reported that gliadin peptides instilled directly into the jejunum were seen in the intercellular space of celiac patient epithelia but not in healthy controls. Although that study has not been reproduced or extended in celiac patients, a more recent in vitro study using patient-derived small intestinal biopsies in Ussing chambers showed that the antigenic 33-mer peptide from α-gliadin was translocated intact from the mucosal to the serosal side (Matysiak-Budnik et al., 2005). In vivo transepithelial translocation of the intact 33-mer has also been observed in a gluten-sensitive rhesus macaque (Bethune et al., 2008). Notwithstanding their clinical relevance, neither of the latter systems is suitable for controlled, quantitative investigations into the mechanisms for transepithelial gluten peptide transport in celiac sprue. To elucidate how gluten peptides are translocated before inflammation and how this critical step in pathogenesis is modulated by disease-specific effectors, appropriate epithelial cell culture models are needed.
In the current work, we had three primary objectives: first, to identify effectors of increased epithelial permeability that are specific to the inflammatory processes in the celiac small intestine; second, to determine whether gluten peptides are translocated to a sufficient extent to initiate, as well as to propagate, inflammation; and third, to identify structural features of gluten peptides that influence their translocation. We used fluorescently labeled 33-mer, as well as other disease-relevant gluten peptides, in a T84 monolayer assay to establish that IFN-γ released by disease-specific T cells is the primary effector of increased epithelial permeability. Very recently, Schumann et al. (2008) demonstrated intact 33-mer peptide transport across a Caco-2 monolayer, albeit with partial degradation. These investigators also showed that peptide transport occurred via Rab5-mediated transcytosis and that it was modestly (1.4-fold) up-regulated by IFN-γ.In contrast to Schumann et al. (2008), we did not see an effect of IFN-γ in a subline of Caco-2 (data not shown). Instead, our findings build upon the extensive studies of Madara, Nusrat, and co-workers who have shown that IFN-γ affects the permeability of a T84 epithelial monolayer and who have studied the mechanistic underpinnings of that observation (Madara and Stafford, 1989; Bruewer et al., 2005).
Materials and Methods
Materials. Peptide synthesis reagents were purchased from Chem-Impex (Wood Dale, IL), Peptides International (Louisville, KY), and EMD Biosciences (San Diego, CA). Cy5-succinimidyl ester was purchased from GE Healthcare (Piscataway, NJ). Recombinant IFN-γ and tumor necrosis factor (TNF)-α were purchased from Peprotech (Rocky Hill, NJ). The activity of IFN-γ is 20 U/ng. Goat anti-human IFN-γ IgG and mouse monoclonal anti-IFN-γR1 IgG1 (MAB6731) were purchased from R&D Systems (Minneapolis, MN). Cell culture media, antibiotics, human serum, and fluorescently labeled dextrans were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Gluten flour was from Bob's Red Mill (Milwaukie, OR). Pepsin, trypsin, chymotrypsin, methyl β-cyclodextrin (MβCD), nystatin, cholesterol oxidase, dansylcadaverine, and wheat gliadin were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human transglutaminase 2 was prepared as described previously (Piper et al., 2002).
Peptide Synthesis. Peptides were synthesized using tert-butoxycarbonyl/O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate chemistry on solid-phase as described previously (Xia et al., 2005). For most experiments, a polyethylene glycol (PEG)-3 linker was added to the N terminus of the 33-mer by tert-butoxycarbonyl/O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate chemistry on solid phase. After cleavage from the resin, peptides were purified over a reverse-phase C18 column by high-performance liquid chromatography (RP-HPLC) using a water/acetonitrile gradient in the presence of 0.1% trifluoroacetic acid, and then they were lyophilized. Solution-phase labeling of the purified peptides with Cy5-NHS ester was carried out in dimethyl sulfoxide according to the manufacturer's instructions. Fluorescently labeled peptides were purified by RP-HPLC, lyophilized, and stored at -20°C. The correct mass for each peptide before and after labeling was confirmed by liquid chromatography-assisted mass spectrometry (LC-MS). Before use, labeled peptides were resuspended in 50 mM sodium phosphate, pH 7.0, supplemented with 0.02% NaN3, and their concentration was determined by spectrophotometric measurement of A652. Working stocks were stored at 4°C, and their integrity was confirmed periodically by RP-HPLC.
Cell Culture. T84 epithelial cells from the American Type Culture Collection (Manassas, VA) were grown in T84 media [Dulbecco's modified Eagle's medium/Ham's F-12 (1:1) supplemented with antibiotics (penicillin/streptomycin) and 5% (v/v) fetal bovine serum]. Media were changed every alternate day, and the cells were split once a week. C2BBE1 epithelial cells, a clonal subline of Caco-2 selected for morphological homogeneity and exclusive apical villin localization, were from the American Type Culture Collection and grown in C2BBE1 media [Dulbecco's modified Eagle's medium supplemented with 0.01 mg/ml transferrin, antibiotics, and 10% (v/v) fetal bovine serum]. DQ2 homozygous antigen-presenting cells (Epstein-Barr virus-transformed B-cell lines 9088 and VAVY) were grown in APC media (RPMI 1640 medium/GlutaMAX (Invitrogen) supplemented with antibiotics and 10% fetal bovine serum). Every alternate day, 9088 cells were split back to 3 × 106 cells/ml, and VAVY cells were split back to 1 × 106 cells/ml. Gluten-specific, DQ2-restricted T-cell lines (P28 TCL1 and P34 TCL1) were isolated from celiac patient intestinal biopsies and expanded against chymotrypsin-digested, TG2-treated gliadin as described previously (Siegel et al., 2006). Assays using T cells were performed in T-cell media (Iscove's modified Dulbecco's medium supplemented with antibiotics, 10% fetal bovine serum, and 2% human serum). All cells were grown at 37°C with 5% atmospheric CO2. Cell-based assays and incubations were performed under these same conditions except where otherwise specified.
Preparation of Activated T-Cell Media. Whole gluten flour (30 mg/ml) was digested to proteolytically resistant fragments by treatment with pepsin (0.6 mg/ml in 0.01 N HCl; 30 min) followed by trypsin and chymotrypsin (0.375 mg/ml each, pH 6.4; 60 min). Gluten peptide fragments were deamidated by treatment with 50 μg/ml TG2 for 2 h at 37°C in the presence of 5 mM CaCl2. Deamidation reactions were quenched by boiling. Deamidated gluten fragments [or control protein, bovine serum albumin (BSA)] were incubated overnight at 1 mg/ml in T-cell media with DQ2+ APCs at 2.5 × 106 cells/ml. The next day, gluten-specific, DQ2-restricted T cells isolated from celiac patient biopsies were thawed and allowed to recover for 2 h in T-cell media. Peptide-loaded APCs were centrifuged, the supernatant was discarded, and the pellets (5 × 106 cells) were resuspended in 2.5 ml of T-cell media containing gluten-specific, DQ2-restricted T cells (5 × 106 cells). Cells were coincubated for 48 h, after which they were pelleted via centrifugation, and the supernatant was collected.
Measurement of IFN-γ in Activated T-Cell Media. Western blotting and sandwich enzyme-linked immunosorbent assays (ELISA) were used to detect and quantify IFN-γ in activated T-cell media. For Western blotting, activated T-cell media, or recombinant IFN-γ standards at 102 to 106 U/ml (1 U = 50 pg) were added to an equal volume of Laemmli buffer/β-mercaptoethanol, boiled 5 min, and analyzed by SDS-polyacrylamide gel electrophoresis. Separated proteins were transferred to a nitrocellulose membrane, probed with polyclonal anti-IFN-γ antibody, and developed chromogenically. For ELISA analysis, a commercial sandwich ELISA using matched monoclonal anti-IFN-γ antibodies was performed according to the manufacturer's instructions (BD Biosciences, San Jose, CA).
Peptide Translocation Assays. Cultured T84 cells were seeded on rat tail collagen-coated polycarbonate Transwell permeable supports (5-μm pore size, 6.5 mm in diameter; Corning Life Sciences, Acton, MA) at 5 × 104 cells/well, and the media were exchanged every other day for 2 weeks while the cells grew to confluence and formed tight junctions. After this maturation period, cell monolayers were preincubated for 48 h with media alone or with basolateral media containing either activated T-cell media at varied dilutions or purified recombinant cytokines at varied concentrations. After preincubation, the translocation assay was performed by replacing media in both the apical and basolateral chambers and by adding 2 μM each of dextran (3000 mol. wt.)-Alexa Fluor-488, dextran (70,000 mol. wt.)-Texas Red, and Cy5-labeled gluten peptide to the apical chamber. Samples of the apical and basolateral media were taken at the 0-h time point, and basolateral samples were taken every hour over a 4-h experiment. Fluorescence in collected samples was measured in 96-well format on a FlexStation II 384 (Molecular Devices, Sunnyvale, CA), monitoring three channels (excitation, 490 nm and emission, 525 nm for Alexa Fluor-488; excitation, 585 nm and emission, 620 nm for Texas Red; and excitation, 640 nm and emission 675 nm, for Cy5). The slope of basolateral fluorescence units versus time (from 1 to 4 h) was calibrated to the initial apical fluorescence and divided by the permeable support area (0.33 cm2) to yield the transepithelial flux (picomoles per square centimeter per hour). Values are reported as mean ± S.D. In experiments where the variable changed did not alter the flux of labeled dextrans (e.g., when alternative Cy5-labeled peptides were added), the transepithelial Cy5-peptide flux was normalized to the transepithelial dextran (3000 mol. wt.)-Alexa Fluor-488 flux.
In blocking antibody experiments, antibodies against IFN-γ or against its cell membrane-bound receptor were added during the 48-h preincubation. Anti-IFN-γ at varied concentrations was added to activated T-cell media 30 min before basolateral preincubation of the activated T-cell media with T84 monolayers. Anti-IFN-γR1 at varied concentrations was added to the basolateral side of T84 monolayers 30 min before basolateral addition of activated T-cell media.
In temperature drop experiments, Transwell supports were transferred to 4°C during the last hour of the 48-h preincubation and for the duration of the translocation assay. In inhibition and competition experiments, varied concentrations of inhibitors or unlabeled peptide were added with fluorescently labeled peptide to the apical chamber at the start of the translocation assay. For experiments using inhibitors of caveolae-mediated endocytosis (MβCD, nystatin, and cholesterol oxidase), wells were rinsed after cytokine preincubation with serum-free T84 media (1:1, Dulbecco's modified Eagle's medium/Ham's F-12 media supplemented with antibiotics), and serum-free T84 media were used during the translocation assay.
To determine the extent to which peptide translocation flux could be saturated, T84 monolayers were preincubated with 600 U/ml IFN-γ, and varied concentrations (up to 200 μM) of Cy5–33-mer were added to the apical chamber at the start of the translocation assay.
Chromatographic and Mass Spectrometric Analysis of Translocated Gluten Peptides. Transwell supports bearing T84 monolayers were preincubated with media alone or with 600 U/ml IFN-γ in the basolateral chamber for 48 h. Peptide translocation assays were performed as described, except serum-free T84 media were used, fluorescent dextran markers were added alone or with 20 μM Cy5-PEG3–33-mer gluten peptide to the apical chamber, and additional samples were taken from the apical and basolateral chambers at 0 and 10 h for LC-MS analysis. Samples (50 μl) were injected on a C18 RP-HPLC system (Waters, Milford, MA) coupled to a UV-visible detector and a ZQ single quadrupole mass spectrometer with an electrospray ionization source. Samples were eluted with a water/acetonitrile gradient in the presence of 0.1% formic acid. Absorbance at 640 nm and total ion current were monitored, and spectra corresponding to A640 peaks were examined. Samples were also analyzed by high-performance size exclusion chromatography coupled to a fluorescence detector (Shimadzu, Columbia, MD). Phosphate-buffered saline, pH 7.4, supplemented with 0.02% (w/v) sodium azide was used as the mobile phase, and the detector was set to monitor excitation at 647 nm and emission at 665 nm.
Analysis of Immunogenicity of Translocated Gluten Peptides. Transwell supports bearing T84 monolayers were preincubated with media alone or with 600 U/ml IFN-γ in the basolateral chamber for 48 h. Peptide translocation assays were performed as described above, except fluorescent dextran markers were added alone or with 20 μM Cy5-PEG3–33-mer gluten peptide to the apical chamber, and the basolateral media were replaced at the start of the assay with fresh T-cell media rather than T84 media. After 10 h, the basolateral media were collected and treated with vehicle or with 50 μg/ml TG2 for 2 h at 37°C in the presence of 5 mM CaCl2. Samples were boiled to quench TG2 activity, diluted 1:2 or 1:10 (v/v) into 96-well flat-bottomed plate wells containing 2.5 × 106 cells/ml DQ2+ 9088 APCs in T-cell media and incubated overnight. The next day, additional T-cell media were added to bring the 9088 cell density to 1.5 × 106 cells/ml, and 125 μl of each APC incubation was transferred to a 96-well U-bottomed plate. Gluten-specific, DQ2-restricted T cells were thawed and allowed to recover for 2 h at 1.5 × 106 cells/ml in T-cell media. Addition of 125 μl of T cells to each U-bottomed well yielded a final composition of 187,500 cells each of 9088 APCs and T cells in a 250-μl volume. Cells were incubated for 48 h, after which they were pelleted via centrifugation, and the supernatant was collected. The concentration of secreted IFN-γ in the T-cell supernatant was determined by a commercial sandwich ELISA, as described above.
Statistics. Statistical comparisons were conducted using a two-tailed Student's t test assuming unequal variances. A statistical probability of p < 0.05 was considered significant.
Results
Synthesis of Cy5-Labeled Peptides. To construct a disease-relevant probe of gluten peptide transepithelial translocation in vitro, we synthesized the 33-mer and labeled its amino terminus with Cy5 fluorescent dye (Fig. 1). The structure and purity of the Cy5-labeled product was confirmed for the 33-mer and for all other peptides used in this study by LC-MS (Supplemental Fig. 1). To prevent possible interference by the dye with 33-mer-DQ2 binding in later experiments, a PEG3 linker was inserted between Cy5 and the 33-mer amino terminus. The addition of this linker did not affect the peptide's transepithelial flux (Supplemental Fig. 2).
Fig. 1.
Panel of synthetic gluten peptides labeled with Cy5 fluorescent dye. Structures are shown for the dye and PEG linker (where present). Sequences, common literature identifiers, and references containing original identification are provided for each peptide. In peptide I (Cy5-PEG3–33-mer), the 33-mer is linked via an amide bond to a PEG3 linker, which is linked to the dye. All other peptides used are directly linked to the dye via an amide bond.
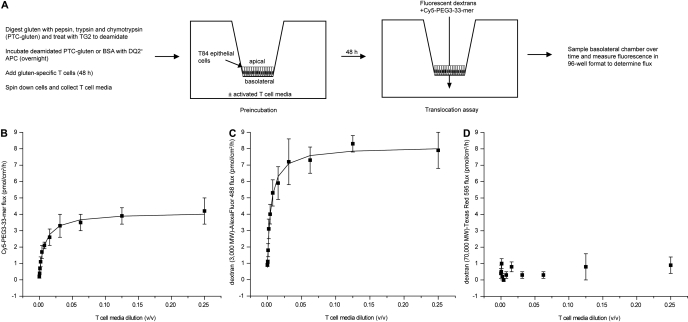
Permeabilizing Activity of Activated T-Cell Media on T84 Epithelial Monolayers. To identify effectors of increased epithelial permeability produced by the celiac patient GALT, T cells cultured from celiac patient intestinal biopsies were activated with partially digested, TG2-treated gluten, the activated T-cell media were preincubated for 48 h on the basolateral side of T84 epithelial cells cultured on Transwell supports, and the apical-to-basolateral flux of Cy5-PEG3–33-mer was measured thereafter (Fig. 2A). In agreement with observations in Caco-2 cells (Schumann et al., 2008), the 33-mer was translocated across the T84 epithelium in wells lacking activated T-cell media. Activated T-cell media increased the flux of Cy5-PEG3–33-mer across the T84 monolayer by 10- to 20-fold over this basal rate in a dose-dependent manner that could be saturated (Fig. 2B). Similar results were obtained using an alternative DQ2-homozygous APCs (VAVY) and with a second T-cell line isolated from a different celiac sprue patient (P34 TCL1; data not shown). No effect on epithelial permeability was observed at any dose with T-cell media from T cells presented with either no antigen (data not shown) or with the control protein BSA (Supplemental Fig. 2). The effect of activated T-cell media was size-selective, because the transepithelial flux of a comparably sized dextran marker (3000 mol. wt.) was similarly increased by activated T-cell media (Fig. 2C), whereas that of a much larger dextran marker (70,000 mol. wt.) was not (Fig. 2D).
Fig. 2.
Activated T-cell media enhances the apical-to-basolateral transepithelial flux of Cy5-PEG3–33-mer gluten peptide across a cultured T84 monolayer. A, experimental plan. Gluten was digested to peptide fragments and deamidated by treatment with transglutaminase 2. Deamidated gluten fragments were incubated overnight with DQ2+ antigen-presenting cells (9088), after which gluten-specific, DQ2-restricted T cells from celiac patient intestinal biopsies (P28 TCL1) were added. After 48 h, APCs and T cells were pelleted via centrifugation, and the supernatant was collected. This activated T-cell media were added at varied dilutions to the basolateral chamber of Transwell supports bearing cultured T84 epithelial cells. After 48 h of preincubation, the media in both the apical and basolateral chambers was replaced, and fluorescently labeled markers (3000 and 70,000 mol. wt. dextrans and gluten peptide, 33-mer) were added to the apical chamber at 2 μM each. Fluorescence in basolateral samples was measured over time to determine the apical-to-basolateral flux of each marker. Fluxes for Cy5-PEG3–33-mer (B), dextran (3000 mol. wt.)-Alexa Fluor-488 (C), and dextran (70,000 mol. wt.)-Texas Red 595 (D) are plotted against the dilution (v/v) of activated T-cell media used during preincubation. Mean ± S.D.s for triplicate wells are shown. Data are representative of more than three independent experiments.
Identity of the Effector of Increased Epithelial Permeability in Activated T-Cell Media. For several reasons, IFN-γ was targeted as a likely effector of epithelial permeability in activated T-cell media. First, this proinflammatory cytokine is up-regulated conspicuously in active celiac lesions (Nilsen et al., 1998) and in other inflammatory bowel diseases in which the epithelial barrier is disrupted (Clayburgh et al., 2004). Second, IFN-γ is known to increase both the paracellular and transcellular permeability of cultured epithelial monolayers in vitro (Madara and Stafford, 1989; Terpend et al., 1998), reminiscent of the dual increase of paracellular and transcellular permeability exhibited by the active celiac patient gut (Zimmer et al., 1995; Duerksen et al., 2005). Finally, activated T-cell media did not have a significant effect on Cy5-PEG3–33-mer and dextran (3000 mol. wt.) flux until 48 h of preincubation (data not shown), the same preincubation period required for IFN-γ to permeabilize T84 monolayers toward a comparably sized fluorescent dextran marker (Bruewer et al., 2003).
Consistent with previous results (Nilsen et al., 1995; Troncone et al., 1998), IFN-γ was detected in media from gluten-stimulated celiac patient-derived T cells but not from those stimulated with no antigen or with BSA (Supplemental Fig. 3). The expected molecular weight for monomeric, glycosylated IFN-γ was confirmed by Western blotting, and the concentration of IFN-γ was measured by ELISA to range from 1500 to 10,000 U/ml in various activated T-cell media preparations.
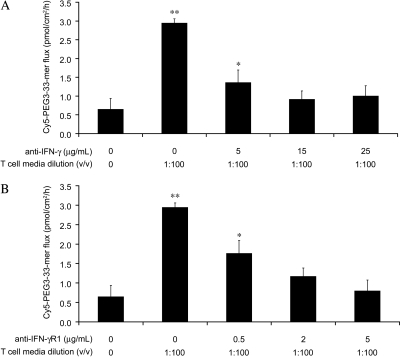
To determine whether IFN-γ contributes to the permeabilizing activity of activated T-cell media, blocking antibodies against either IFN-γ or its receptor IFN-γR1 were added during the preincubation of T84 monolayers with activated T-cell media. Blocking either IFN-γ or its receptor suppressed the permeabilizing activity of activated T-cell media in a dose-dependent manner (Fig. 3). At higher antibody concentrations (≥15 μg/ml anti-IFN-γ or ≥2 μg/ml anti-IFN-γR1), activated T-cell media had no significant effect on transepithelial Cy5-PEG3–33-mer flux.
Fig. 3.
The ability of activated T-cell media to enhance permeability is inhibited by blocking antibodies directed against either IFN-γ or IFN-γR1. Transwell supports bearing T84 epithelial cells were preincubated with 1:100 (v/v) basolateral activated T-cell media supplemented with varied concentrations of either anti-IFN-γ blocking antibody (A) or anti-IFN-γR1 blocking antibody (B). The translocation assay was performed as described in Fig. 2 to determine the apical-to-basolateral flux of Cy5-PEG3–33-mer (2 μM initial apical concentration). Mean ± S.D.s for triplicate wells are shown. Data are representative of more than three independent experiments. Statistical comparisons were performed with respect to the control lacking activated T-cell media. *, p < 0.05; **, p < 0.01.
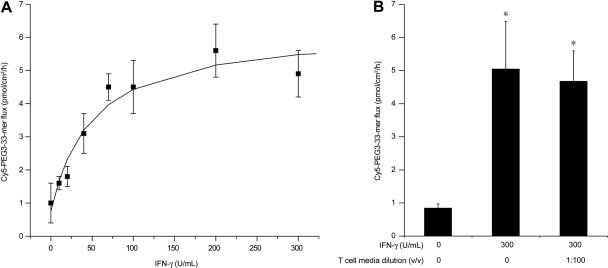
To determine whether IFN-γ is sufficient to induce increased gluten peptide transepithelial flux, recombinant IFN-γ was added during preincubation in lieu of activated T-cell media. Recombinant IFN-γ increased Cy5-PEG3–33-mer transepithelial flux in a dose-dependent, saturable manner (Fig. 4A). The EC50 value (50.0 ± 22.6 μg/ml) and peptide flux at saturation (5.5 ± 0.7 pmol/cm2/h) for recombinant IFN-γ were similar to the EC50 value (70.0 ± 9.8 μg/ml; based on measured IFN-γ content) and peptide flux at saturation (3.9 ± 0.1 pmol/cm2/h) for activated T-cell media. Saturating concentrations of recombinant IFN-γ supplemented with activated T-cell media during preincubation had no further effect on permeability relative to IFN-γ alone (Fig. 4B).
Fig. 4.
Interferon-γ is the primary effector of transepithelial gluten translocation in activated T-cell media. Transwell supports bearing T84 epithelial cells were preincubated with varied concentrations of purified recombinant IFN-γ for 48 h (A) or with a saturating concentration (300 U/ml) of purified recombinant IFN-γ supplemented with 1:100 (v/v) activated T-cell media (B). After preincubation, a translocation assay was performed as described in Fig. 2 to determine the apical-to-basolateral flux of Cy5-PEG3–33-mer (2 μM initial apical concentration). Mean ± S.D.s for triplicate wells are shown. Data are representative of more than three independent experiments. Statistical comparisons were performed with respect to the control lacking IFN-γ or activated T-cell media. *, p < 0.05.
Tumor necrosis factor-α, another proinflammatory cytokine released by celiac patient T cells upon stimulation with gluten (Nilsen et al., 1995, 1998), has been shown to increase T84 epithelial permeability in concert with IFN-γ (Bruewer et al., 2003). Recombinant TNF-α (200 U/ml) was tested alone or together with IFN-γ (100 U/ml) for an effect on gluten peptide flux in the T84 translocation assay. Consistent with previous results (Bruewer et al., 2003), TNF-α had no significant effect on its own (1.6 ± 0.6-fold change over control). The addition of TNF-α seemed to slightly potentiate the effect of IFN-γ (13.3 ± 2.4-fold change over control versus 10.6 ± 2.1-fold change over control for IFN-γ alone), but this increase was not statistically significant and was not reproducibly observed.
In contrast to our observations with T84 monolayers, no effect of IFN-γ was observed on epithelial permeability when the C2BBE1 clone of Caco-2 was tested (data not shown). A modest effect of IFN-γ on Caco-2 cell permeability has been reported by other investigators (Schumann et al., 2008). In immunofluorescence assays, we detected markedly higher levels of IFN-γR1 expression on T84 monolayers than on C2BBE1 monolayers (Supplemental Fig. 4). Because the biological activity of IFN-γ in a given cell line correlates with that cell line's level of IFN-γ receptor expression (Ucer et al., 1985), this may explain the greater sensitivity of T84 monolayers to IFN-γ in our experiments.
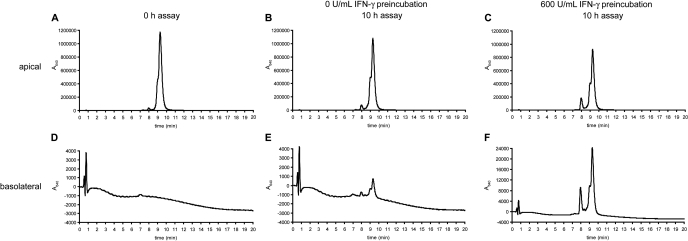
Stability and Immunogenicity of Transported Gluten Peptides. The apical and basolateral media were collected after peptide translocation assays and analyzed via LC-MS to evaluate the stability of Cy5-PEG3–33-mer. Intact Cy5-PEG3–33-mer eluted at 9 to 10 min as a single peak (Fig. 5A), whereas serum-free media contained no peaks absorbing at 640 nm (Fig. 5D). After 10 h of apical incubation with T84 cells preincubated with media alone or with saturating IFN-γ, 96 and 80% of the added Cy5-PEG3–33-mer remained intact, respectively (Fig. 5, B and C). The lower amount of intact Cy5-PEG3–33-mer remaining in the apical chamber of IFN-γ-preincubated cells was because of increased apical-to-basolateral translocation as well as increased N-terminal processing of the peptide, resulting in a larger peak for Cy5-PEG3-LQ at 8 min. No other breakdown products were observed by mass spectrometry. Intact peptide was detected in the basolateral chambers of both media-preincubated and IFN-γ-preincubated T84 cells (Fig. 5, E and F), a result confirmed by high-performance size exclusion chromatography with fluorescence detection (data not shown). Respectively, 0.15 and 2.1% of the initial 20 μM apical peptide were observed intact on the basolateral side, corresponding to 31 and 430 nM basolateral concentration of intact peptide. This area under the curve calculation agreed closely with the respective concentrations of 43 and 570 nM Cy5-PEG3–33-mer calculated by measurement of basolateral fluorescence.
Fig. 5.
Gluten peptides are translocated intact across a cultured epithelial monolayer under normal and inflammatory conditions. Transwell supports bearing T84 epithelial cells were preincubated with media alone or with 600 U/ml IFN-γ in the basolateral chamber for 48 h. Peptide translocation assays were performed as described in Fig. 2, except with serum-free media and with an initial apical concentration of 20 μM Cy5-PEG3–33-mer. Samples were taken from the apical and basolateral chambers at 0 and 10 h for LC-MS analysis. Absorbance at 640 nm was monitored, and the mass spectra corresponding to A640 peaks were examined to confirm peptide identity. The peak eluting at a retention time of 9 to 10 min is intact Cy5-PEG3–33-mer. The peak eluting at a retention time of 8 min is Cy5-PEG3-LQ, signifying limited processing of the N terminus by T84 cells. A, apical media at 0 h, containing 20 μM Cy5-PEG3–33-mer. B, apical media after 10-h incubation in wells preincubated with 0 U/ml IFN-γ. C, apical media after 10-h incubation in wells preincubated with 600 U/ml IFN-γ. D, basolateral media at 0 h (=serum-free media). E, basolateral media after 10-h incubation in wells preincubated with 0 U/ml IFN-γ. F, basolateral media after 10-h incubation in wells preincubated with 600 U/ml IFN-γ. Data are representative of more than three similar experiments.
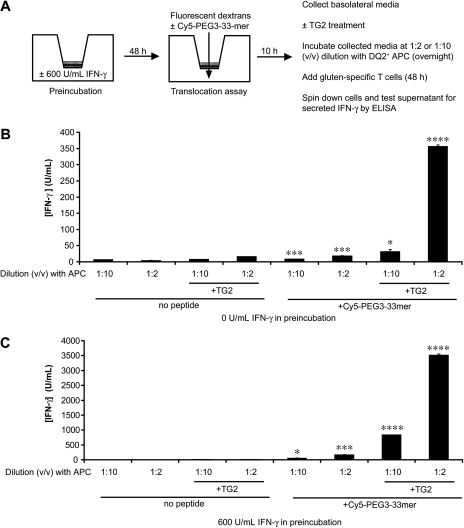
Because nanomolar concentrations of the 33-mer peptide are sufficient to stimulate proliferation of celiac patient-derived T cells in culture (Shan et al., 2002; Qiao et al., 2004), translocation assays were coupled to T-cell IFN-γ secretion assays to determine whether translocated Cy5-PEG3–33-mer retained immunogenicity. Media-preincubated and IFN-γ-preincubated T84 monolayers were incubated with apical Cy5-PEG3–33-mer for 10 h, and then the basolateral media were collected, treated with vehicle or TG2, and incubated with celiac patient-derived, gluten-specific T cells in the presence of DQ2+ APCs. After 48 h of stimulation, the concentration of IFN-γ in the media was measured by ELISA (Fig. 6A). In all cases, basolateral media from wells receiving apical Cy5-PEG3–33-mer stimulated significantly more IFN-γ release than did similarly processed basolateral media from wells receiving no peptide (Fig. 6, B and C). This stimulation was dose-dependent and was augmented up to 20.6-fold by treatment of the basolateral media with TG2. It is noteworthy that TG2-treated basolateral media from wells receiving no IFN-γ pretreatment contained sufficient intact Cy5-PEG3–33-mer to stimulate secretion of >300 U/ml IFN-γ (Fig. 6B), a concentration capable of permeabilizing epithelia toward further gluten peptide translocation (Fig. 4A). Basolateral media from wells preincubated with IFN-γ stimulated up to 26.4-fold higher levels of IFN-γ secretion (Fig. 6C), consistent with similar differences in Cy5-PEG3–33-mer flux between T84 monolayers preincubated with media alone and those preincubated with IFN-γ-containing activated T-cell media (Fig. 2B).
Fig. 6.
Gluten peptides are translocated across a cultured epithelial monolayer at sufficient levels to initiate and propagate inflammation. A, experimental plan. Transwell supports bearing T84 epithelial cells were preincubated with media alone or with 600 U/ml IFN-γ in the basolateral chamber for 48 h. Peptide translocation assays were performed as described in Fig. 2, except with 20 μM Cy5-PEG3–33-mer. Samples were taken from the basolateral chambers after 10 h and treated with vehicle or with 50 μg/ml TG2 for 2 h at 37°C in the presence of 5 mM CaCl2. Samples were boiled to quench TG2 activity, diluted 1:2 or 1:10 (v/v) into T-cell media containing DQ2+ 9088 APCs, and incubated overnight. The next day, gluten-specific, DQ2-restricted T cells were added to peptide-loaded APCs to a final composition of 187,500 cells each of 9088 APCs and T cells in a 250-μl volume. Cells were incubated for 48 h, after which they were pelleted via centrifugation, and the supernatant was collected. The concentration (units per milliliter) of IFN-γ secreted by T cells in response to basolateral media from Transwell supports preincubated with 0 U/ml IFN-γ (B) and 600 U/ml IFN-γ (C) was determined by a commercial sandwich ELISA, as described under Materials and Methods. Mean S.D.s for triplicate measurements are shown. Statistical comparisons were performed with respect to the corresponding controls in which no apical Cy5-PEG3–33mer was added. *, p < 0.05; ***, p < 0.001; ****, p < 0.0001.
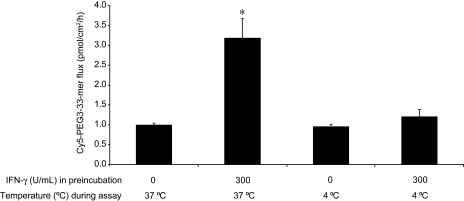
Pathway of Gluten Peptide Translocation under Basal and IFN-γ-Permeabilized Conditions. To determine whether Cy5-PEG3–33-mer translocation is energy-dependent, T84 monolayers were preincubated at 37°C with media alone or with 300 U/ml IFN-γ and then assayed for peptide transport at either 37 or 4°C. Basal peptide flux was unaffected by the temperature drop to 4°C, whereas the IFN-γ-mediated increase in peptide flux seen at 37°C was reduced to basal levels of transport at 4°C (Fig. 7). A similar temperature-dependent decrease was seen for the flux of dextran (3000 mol. wt.) (data not shown), an established marker for endocytosis (Ivanov et al., 2004).
Fig. 7.
Reducing the assay temperature to 4°C reverses the permeabilizing effect of IFN-γ but does not reduce the basal gluten peptide flux. Transwell supports bearing T84 epithelial cells were preincubated with media alone or with 300 U/ml IFN-γ in the basolateral chamber for 48 h at 37°C. Peptide translocation assays were performed as described in Fig. 2; however, some wells were assayed at 4°C rather than 37°C. Mean ± S.D.s for triplicate wells are shown. Data are representative of three similar experiments. Statistical comparisons were performed with respect to the controls lacking IFN-γ for each assay temperature. *, p < 0.05.
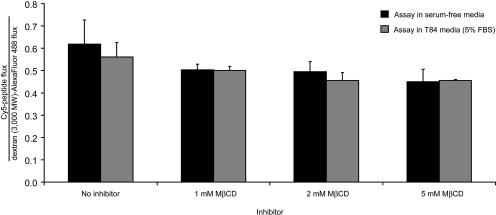
Inhibitors of caveolae-mediated endocytosis were shown previously to reduce translocation of fluorescently labeled 33-mer across Caco-2 monolayers (Schumann et al., 2008). To determine whether the translocation of Cy5-PEG3–33-mer across T84 monolayers occurs by receptor-mediated endocytosis, pharmacological inhibition, competition, and peptide titration assays were performed. After IFN-γ preincubation, the caveolae-mediated endocytosis inhibitor MβCD was added to the translocation assay at varying concentrations in serum-containing or serum-free media. The flux of Cy5-PEG3–33-mer was normalized to the flux of dextran (3000 mol. wt.), because MβCD does not affect the flux of the latter (Izquierdo-Useros et al., 2007). No reduction of Cy5-PEG3–33-mer flux was observed in wells containing MβCD in serum-free media relative to wells lacking inhibitor or to wells with inhibitor in serum-containing media (Fig. 8). Additional caveolae-mediated endocytosis inhibitors nystatin (20 μM) and cholesterol oxidase (1 U/ml), as well as the clathrin-mediated endocytosis inhibitor dansylcadaverine (200 μM) (Ivanov, 2008), also failed to inhibit Cy5-PEG3–33-mer translocation to a significant extent (data not shown). A 100-fold excess of unlabeled PEG3–33-mer had no effect on Cy5-PEG3–33-mer flux across T84 monolayers preincubated with media alone or with IFN-γ (Supplemental Fig. 5). Likewise, Cy5–33-mer flux across IFN-γ-preincubated monolayers increased linearly with initial apical Cy5–33-mer concentration (Supplemental Fig. 6), implying that the peptide translocation mechanisms operating in T84 cells cannot be saturated.
Fig. 8.
Gluten peptide translocation is not inhibited by MβCD. Transwell supports bearing T84 epithelial cells were preincubated with 300 U/ml IFN-γ in the basolateral compartment for 48 h. Peptide translocation assays were performed as described in Fig. 2, except either serum-free (black bars) or serum-containing (gray bars) media were used, and the endocytosis inhibitor MβCD was added at varied concentrations to the apical compartment with 2 μM each of fluorescent dextrans and Cy5-PEG3–33-mer. The apical-to-basolateral flux of Cy5-PEG3–33-mer in each well was normalized to the flux of dextran Alexa Fluor-488. Mean ± S.D.s for triplicate wells are shown.
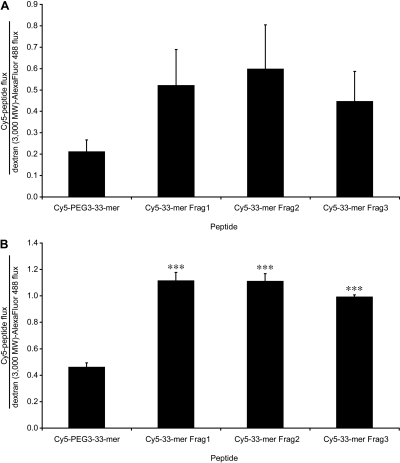
Transport of Other Disease-Relevant Gluten Peptides. We recently reported that the oral administration of the gluten-specific barley endoprotease EP-B2 prevented clinical relapse in gluten-sensitive rhesus macaques (Bethune et al., 2008). Unexpectedly, this clinical protection was accompanied by a spike in anti-gliadin antibodies. We therefore hypothesized that EP-B2 processed gluten into smaller fragments that penetrated the enterocyte barrier more efficiently than the peptides that accumulate in the absence of EP-B2. The 33-mer is ideally suited for testing this hypothesis because of its centrality to disease pathogenesis and its possession of several well defined EP-B2 cleavage sites (Bethune et al., 2006). Three fragments of the 33-mer, corresponding to the major products of EP-B2-catalyzed cleavage, were synthesized and labeled with Cy5 (Fig. 1). The transepithelial flux of these 7- to 9-mer fragments was compared with that of the full-length peptide in translocation assays, normalizing to the flux of dextran (3000 mol. wt.) in corresponding wells. The normalized transepithelial flux of each fragment across IFN-γ-preincubated cells was significantly higher than that of intact Cy5-PEG3–33-mer (Fig. 9B). All three fragments also exhibited >2-fold higher basal flux than Cy5-PEG3–33-mer (Fig. 9A), although this difference was not statistically significant. The effect of IFN-γ on the flux of the parent peptide and each fragment was comparable (∼10-fold higher than basal flux).
Fig. 9.
Synthetic proteolytic fragments of the 33-mer gluten peptide exhibit increased transepithelial flux relative to intact 33-mer. Transwell supports bearing T84 epithelial cells were preincubated with media alone (A) or with 600 U/ml IFN-γ (B) in the basolateral chamber for 48 h. Peptide translocation assays were performed as described in Fig. 2 using either intact Cy5-PEG3–33-mer or Cy5-labeled synthetic 33-mer fragments corresponding to products of digestion of 33-mer by the glutenase EP-B2. The Cy5-peptide flux in each well is normalized to the corresponding dextran (3000 mol. wt.)-Alexa Fluor-488 flux. Mean ± S.D.s for triplicate wells are shown. Data are representative of two similar experiments. Statistical comparisons were performed with respect to the flux of full-length Cy5-PEG3–33-mer. ***, p < 0.001.
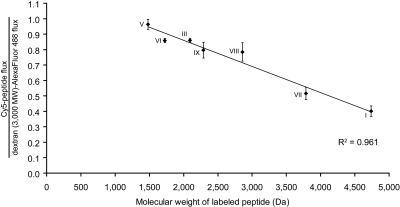
To more extensively examine the dependence of gluten peptide transepithelial flux on peptide size and sequence, we synthesized a panel of Cy5-labeled gluten peptides relevant to the pathogenesis of celiac sprue (Fig. 1) (Sturgess et al., 1994; Arentz-Hansen et al., 2000; Shan et al., 2002, 2005; Tucková et al., 2002). The transepithelial flux of each peptide was measured across T84 monolayers preincubated with media alone or with IFN-γ, normalized to the corresponding dextran (3000 mol. wt.) flux, and plotted against labeled molecular weight. Peptide flux was linearly and inversely correlated with peptide molecular weight under both basal (data not shown) and IFN-γ-preincubated conditions (Fig. 10). The slope of the flux versus peptide mol. wt. line for IFN-γ-preincubated experiments was 10-fold greater than that for the basal flux. Normalized fluxes were also plotted against the calculated log P for each peptide (spanning values of -6.8 to -4.3), but no correlation was observed (data not shown). The proline- and glutamine-rich composition of gluten peptides was not critical for transepithelial translocation, because a proteolytically resistant fragment of myoglobin (Piper et al., 2004), unrelated in sequence to gluten (Fig. 1), was also translocated intact in an IFN-γ-enhanced manner (data not shown).
Fig. 10.
A panel of disease-relevant gluten peptides exhibits a linear and inverse correlation between transepithelial peptide flux and peptide molecular weight. Transwell supports bearing T84 epithelial cells were preincubated with 600 U/ml IFN-γ in the basolateral chamber for 48 h. Peptide translocation assays were performed as described in Fig. 2 using 20 μM Cy5-peptides numbered according to Fig. 1. I, Cy5-PEG3-LQLQPF(PQPQLPY)3PQPQLPF; III, Cy5-QLQPFPQPQLPY; V, Cy5-LPYPQPQ; VI, Cy5-LPYPQPQPF; VII, Cy5-FLQPQQPFPQQPQQPYPQQ PQQPFPQ; VIII, Cy5-LGQQQPFPPQQPYPQPQPF; IX, Cy5-VSFQQPQQQYPSSQ. The Cy5-peptide flux in each well is normalized to the corresponding dextran (3000 mol. wt.)-Alexa Fluor-488 flux. Mean ± S.D.s for triplicate wells are shown.
Finally, the impact of deamidation state, and, more generally, net charge of the 33-mer on its translocation was determined by comparing the transepithelial flux of Cy5-PEG3–33-mer, representing gluten in its native state, to that of synthetically deamidated 33-mer. The fluxes of native and deamidated 33-mer were statistically indistinguishable under basal and IFN-γ-preincubated conditions (Supplemental Fig. 7).
Discussion
A better understanding of celiac sprue pathogenesis depends critically upon identifying the mechanisms by which gluten peptides are translocated across the gut epithelium and how these mechanisms are modulated in a celiac patient. Such insight could lead to the identification of new targets for celiac sprue therapy. Here, we used fluorescently labeled gluten peptides as disease-relevant probes of peptide translocation across T84 monolayers, an established model of the intestinal epithelium. In doing so, we observed that IFN-γ, secreted by gluten-activated celiac patient T cells, is the primary effector of increased peptide translocation during active disease.
The designation of IFN-γ as the primary effector of epithelial permeability in celiac sprue is based on several observations. First, IFN-γ is the dominant cytokine released from the GALT underlying epithelial lesions during active celiac sprue (Nilsen et al., 1998). Second, IFN-γ is present in gluten-stimulated T-cell media, which increases epithelial permeability, but not in BSA-stimulated T-cell media, which does not increase epithelial permeability (Fig. 2, Supplemental Figs. 2 and 3) (Nilsen et al., 1995; Troncone et al., 1998). Third, blocking IFN-γ signaling using antibodies directed against either IFN-γ or its receptor abrogates the permeabilizing activity of activated T-cell media completely (Fig. 3). Finally, purified recombinant IFN-γ recapitulates the permeabilizing activity of activated T-cell media, and activated T-cell media has no further effect in the presence of saturating levels of recombinant IFN-γ (Fig. 4). Thus, IFN-γ is temporally and spatially situated to regulate intestinal permeability in a disease state-specific manner in vivo, and it is necessary and sufficient to affect such permeability in vitro. Further studies investigating the necessity and sufficiency of IFN-γ to enhance epithelial permeability toward gluten peptides in vivo will be required to determine the relevance of these in vitro findings to disease pathogenesis.
Interferon-γ induces histological changes in celiac intestinal biopsies (Przemioslo et al., 1995). However, the mechanism by which IFN-γ increases gluten peptide translocation does not seem to be gross disruption of epithelial integrity, because T84 monolayers exposed to activated T-cell media selectively permitted translocation of dextran markers similar in size to peptides (3000 mol. wt.) while preventing passage of dextran markers the size of large proteins (70,000 mol. wt.) (Fig. 2). Moreover, a panel of gluten peptides spanning a more modest size range (1500–4700 mol. wt.) exhibited an inverse correlation between peptide size and transepithelial flux under both basal and IFN-γ-preincubated conditions (Figs. 9 and 10; data not shown). It therefore seems that IFN-γ affects a size-selective increase in gluten peptide flux across an intact epithelium via paracellular or transcellular pathways.
The flux of Cy5-PEG3–33-mer in the absence of IFN-γ preincubation was unaffected by temperature depression (Fig. 7), suggesting that basal gluten peptide translocation is attributable to the paracellular pathway or to energy-independent transcytosis. By contrast, dropping the assay temperature to 4°C reversed the effect of IFN-γ, reducing the flux to basal levels (Fig. 7). Notwithstanding the established role of IFN-γ in augmenting epithelial paracellular permeability by inducing internalization of epithelial tight junction proteins (Bruewer et al., 2005), this temperature sensitivity argues that energy-dependent transcytosis, rather than passive diffusion through permeabilized tight junctions, is the major pathway by which gluten peptides are translocated during inflammation. This is consistent with the in vivo observation of increased gluten peptide uptake in celiac patients relative to healthy controls (Friis et al., 1992), a difference that is further augmented in untreated patients (Zimmer et al., 1995). Indeed, Cy dye-labeled 33-mer uptake into epithelial cells from celiac patient intestinal biopsies is elevated ∼10-fold relative to healthy controls (Schumann et al., 2008), quantitatively similar to the ∼10-fold increase in Cy5-PEG3–33-mer flux elicited by IFN-γ in our experiments. In opposition to this interpretation, the fluxes of dextrans (3000 and 70,000 mol. wt.) and gluten peptides of varying sizes were inversely related to their molecular weights, a feature associated with the paracellular pathway rather than with fluid-phase pinocytosis. Further work will be required to parse out the relative contributions of these two pathways to gluten peptide transepithelial translocation.
If transcellular translocation is occurring after IFN-γ preincubation in the T84 epithelial cell culture model used herein, it probably occurs through an energy-dependent, non-receptor-mediated endocytic pathway. Inhibitors of clathrin-mediated and caveolae-mediated endocytosis, the two major receptor-mediated endocytic pathways, did not reduce Cy5-PEG3–33-mer flux after IFN-γ preincubation (Fig. 8; data not shown). Furthermore, the flux of labeled 33-mer could not be competed away with unlabeled 33-mer (Supplemental Fig. 5), nor could flux be saturated with increasing initial apical concentrations of labeled 33-mer (Supplemental Fig. 6), suggesting either that the receptor abundance is far in excess of those 33-mer concentrations used or that 33-mer uptake occurs without a receptor through fluid-phase endocytosis. The similar behavior of Cy-labeled peptides and the macropinocytosis marker dextran (3000 mol. wt.) in our assays, as well as the colocalization of these after uptake in Caco-2 cells (Schumann et al., 2008), supports the latter explanation.
In active celiac sprue, receptor-mediated mechanisms may additionally contribute to the transcellular translocation of gluten peptides, as proposed for major histocompatibility class II molecules and gluten-specific IgA (Zimmer et al., 1995), as well as for TG2 (Ráki et al., 2006). Of these, direct experimental evidence in support of a role in gluten peptide transcytosis has only been obtained for gluten-specific secretory IgA (Matysiak-Budnik et al., 2008). However, celiac sprue can coincide with IgA deficiency (Collin et al., 1992), and epithelial uptake of gluten peptides is elevated in patients with normal anti-gliadin IgA levels (Friis et al., 1992). Together, these data suggest other mechanisms of endocytic gluten uptake exist, some of which presumably precede inflammation and seroconversion against gluten.
Our results, as well as those of others, suggest that gluten peptides can be translocated intact across epithelial monolayers in the absence of inflammation (Fig. 5) (Schumann et al., 2008). Moreover, the translocated material is recognized and deamidated by TG2 and is immunogenic toward celiac patient biopsy-derived intestinal T cells, eliciting IFN-γ secretion at sufficient levels to increase epithelial permeability toward further gluten peptide translocation (Figs. 4 and 6). After epithelial exposure to IFN-γ, the flux of immunogenic material is increased ∼10-fold, and transported peptides correspondingly elicit 10-fold higher levels of IFN-γ secretion (Fig. 6). These experiments therefore recapitulate in a cell culture model the ability of translocated gluten peptides to both initiate and propagate permeabilization of the gut in the context of celiac sprue, a vicious cycle in which IFN-γ plays a central role.
Administration of neutralizing antibodies against IFN-γ may represent a therapeutic avenue for reducing gut permeability as well as inflammation in celiac sprue. A similar strategy, using anti-TNF-α antibodies to reduce gut inflammation, is used to treat Crohn's disease (Baert et al., 1999). Although there is no evidence for the IFN-γ gene contributing to genetic susceptibility for celiac sprue, patients in remission have 7.6-fold higher levels of IFN-γ expression than healthy controls (Wapenaar et al., 2004). This suggests that chronically up-regulated IFN-γ expression, possibly resulting from low-level exposure to gluten (Pietzak, 2005), may explain the persistence of tight junction defects (Schulzke et al., 1998), as well as the elevated epithelial uptake of gluten (Friis et al., 1992), in patients ostensibly on a gluten-free diet. Anti-IFN-γ therapy may therefore improve epithelial barrier function in treated as well as active celiac patients.
Finally, because gluten peptides are translocated even in the absence of IFN-γ, anticytokine therapy cannot prevent all ingested gluten peptides from reaching the GALT. The most promising therapeutic strategy remains digestion of ingested gluten upstream of translocation by oral glutenases capable of destroying pathogenic epitopes (Bethune and Khosla, 2008). However, we recently reported that the administration of the glutenase EP-B2 to a gluten-sensitive rhesus macaque was clinically effective but caused a spike in IgG and IgA antibodies against gluten (Bethune et al., 2008). We suggested that the incomplete proteolysis of large gluten peptides may lead to elevated seroconversion against smaller, more efficiently transported gluten peptides (Bethune et al., 2008), an important consideration given the potential for anti-gliadin IgA to facilitate further gluten translocation (Matysiak-Budnik et al., 2008). Our present observation that the flux of EP-B2-generated 33-mer fragments was greater than that for the intact peptide supports this hypothesis and suggests that a combination of glutenases with complementary specificity may be necessary to prevent seroconversion against ingested gluten.
Supplementary Material
Acknowledgments
We thank Lynne Olds and Kathy Siemers for sharing reagents and for helpful technical advice regarding cell culture. We also thank Xi Jin for generously providing recombinant transglutaminase 2 and Monica Crespo for synthesizing some of the peptides used in this study. We gratefully acknowledge Eric Sibley and W. James Nelson for provision of the T84 and C2BBE1 cell lines, respectively.
This work was supported in part by the National Institutes of Health [Grant T32-GM007276] (Cellular and Molecular Biology grant) (to M.T.B); the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK063158] (to C.K.); and Achievement Rewards for College Scientists Foundation Predoctoral Scholarship (to M.S.).
C.K. is a director and stockholder in Alvine Pharmaceuticals, a company that is developing a drug for celiac disease.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.148007.
ABBREVIATIONS: GALT, gut-associated lymphoid tissue; TG2, transglutaminase 2 (or tissue transglutaminase); APC, antigen-presenting cell; IFN, interferon; TNF, tumor necrosis factor; MβCD, methyl β-cyclodextrin; FBS, fetal bovine serum; PEG, polyethylene glycol; RP-HPLC, reverse-phase high-performance liquid chromatography; LC-MS, liquid chromatography-assisted mass spectrometry; BSA, bovine serum albumin; ELISA, enzyme-linked immunosorbent assay; EP-B2, barley endoprotease B, isoform 2.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Alaedini A and Green PH (2005) Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med 142 289-298. [DOI] [PubMed] [Google Scholar]
- Arentz-Hansen H, Körner R, Molberg O, Quarsten H, Vader W, Kooy YM, Lundin KE, Koning F, Roepstorff P, Sollid LM, et al. (2000) The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med 191 603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert FJ, D'Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, and Rutgeerts PJ (1999) Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology 116 22-28. [DOI] [PubMed] [Google Scholar]
- Bethune MT and Khosla C (2008) Parallels between pathogens and gluten peptides in celiac sprue. PLoS Pathog 4 e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethune MT, Ribka E, Khosla C, and Sestak K (2008) Transepithelial transport and enzymatic detoxification of gluten in gluten-sensitive rhesus macaques. PLoS ONE 3 e1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethune MT, Strop P, Tang Y, Sollid LM, and Khosla C (2006) Heterologous expression, purification, refolding, and structural-functional characterization of EP-B2, a self-activating barley cysteine endoprotease. Chem Biol 13 637-647. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Peters TJ, and Veall N (1983) A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet 1 323-325. [DOI] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, and Nusrat A (2003) Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171 6164-6172. [DOI] [PubMed] [Google Scholar]
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, and Nusrat A (2005) Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19 923-933. [DOI] [PubMed] [Google Scholar]
- Clayburgh DR, Shen L, and Turner JR (2004) A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 84 282-291. [DOI] [PubMed] [Google Scholar]
- Collin P, Mäki M, Keyriläinen O, Hällström O, Reunala T, and Pasternack A (1992) Selective IgA deficiency and coeliac disease. Scand J Gastroenterol 27 367-371. [DOI] [PubMed] [Google Scholar]
- Duerksen DR, Wilhelm-Boyles C, and Parry DM (2005) Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci 50 785-790. [DOI] [PubMed] [Google Scholar]
- Friis S, Dabelsteen E, Sjöström H, Norén O, and Jarnum S (1992) Gliadin uptake in human enterocytes. Differences between coeliac patients in remission and control individuals. Gut 33 1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI (2008) Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol 440 15-33. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, and Parkos CA (2004) Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell 15 176-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Blanco J, Erkizia I, Fernández-Figueras MT, Borràs FE, Naranjo-Gómez M, Bofill M, Ruiz L, Clotet B, and Martinez-Picado J (2007) Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J Virol 81 7559-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL and Stafford J (1989) Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 83 724-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matysiak-Budnik T, Candalh C, Cellier C, Dugave C, Namane A, Vidal-Martinez T, Cerf-Bensussan N, and Heyman M (2005) Limited efficiency of prolyl-endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology 129 786-796. [DOI] [PubMed] [Google Scholar]
- Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Ménard S, Candalh C, Ben-Khalifa K, Dugave C, Tamouza H, van Niel G, et al. (2008) Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med 205 143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molberg O, Mcadam SN, Körner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Norén O, Roepstorff P, et al. (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 4 713-717. [DOI] [PubMed] [Google Scholar]
- Nilsen EM, Jahnsen FL, Lundin KE, Johansen FE, Fausa O, Sollid LM, Jahnsen J, Scott H, and Brandtzaeg P (1998) Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115 551-563. [DOI] [PubMed] [Google Scholar]
- Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, and Brandtzaeg P (1995) Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut 37 766-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzak MM (2005) Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology 128 S135-S141. [DOI] [PubMed] [Google Scholar]
- Piper JL, Gray GM, and Khosla C (2002) High selectivity of human tissue transglutaminase for immunoactive gliadin peptides: implications for celiac sprue. Biochemistry 41 386-393. [DOI] [PubMed] [Google Scholar]
- Piper JL, Gray GM, and Khosla C (2004) Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo. J Pharmacol Exp Ther 311 213-219. [DOI] [PubMed] [Google Scholar]
- Przemioslo RT, Lundin KE, Sollid LM, Nelufer J, and Ciclitira PJ (1995) Histological changes in small bowel mucosa induced by gliadin sensitive T lymphocytes can be blocked by anti-interferon gamma antibody. Gut 36 874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao SW, Bergseng E, Molberg Ø, Xia J, Fleckenstein B, Khosla C, and Sollid LM (2004) Antigen presentation to celiac lesion-derived T cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion. J Immunol 173 1757-1762. [DOI] [PubMed] [Google Scholar]
- Quarsten H, Molberg O, Fugger L, McAdam SN, and Sollid LM (1999) HLA binding and T cell recognition of a tissue transglutaminase-modified gliadin epitope. Eur J Immunol 29 2506-2514. [DOI] [PubMed] [Google Scholar]
- Ráki M, Tollefsen S, Molberg Ø, Lundin KE, Sollid LM, and Jahnsen FL (2006) A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology 131 428-438. [DOI] [PubMed] [Google Scholar]
- Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, and Fromm M (1998) Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res 43 435-441. [DOI] [PubMed] [Google Scholar]
- Schumann M, Richter JF, Wedell I, Moos V, Zimmermann-Kordmann M, Schneider T, Daum S, Zeitz M, Fromm M, and Schulzke JD (2008) Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut 57 747-754. [DOI] [PubMed] [Google Scholar]
- Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, and Khosla C (2002) Structural basis for gluten intolerance in celiac sprue. Science 297 2275-2279. [DOI] [PubMed] [Google Scholar]
- Shan L, Qiao SW, Arentz-Hansen H, Molberg Ø, Gray GM, Sollid LM, and Khosla C (2005) Identification and analysis of multivalent proteolytically resistant peptides from gluten: implications for celiac sprue. J Proteome Res 4 1732-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Bethune MT, Gass J, Ehren J, Xia J, Johannsen A, Stuge TB, Gray GM, Lee PP, and Khosla C (2006) Rational design of combination enzyme therapy for celiac sprue. Chem Biol 13 649-658. [DOI] [PubMed] [Google Scholar]
- Sollid LM, Markussen G, Ek J, Gjerde H, Vartdal F, and Thorsby E (1989) Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med 169 345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess R, Day P, Ellis HJ, Lundin KE, Gjertsen HA, Kontakou M, and Ciclitira PJ (1994) Wheat peptide challenge in coeliac disease. Lancet 343 758-761. [DOI] [PubMed] [Google Scholar]
- Terpend K, Boisgerault F, Blaton MA, Desjeux JF, and Heyman M (1998) Protein transport and processing by human HT29-19A intestinal cells: effect of interferon gamma. Gut 42 538-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncone R, Gianfrani C, Mazzarella G, Greco L, Guardiola J, Auricchio S, and De Berardinis P (1998) Majority of gliadin-specific T-cell clones from celiac small intestinal mucosa produce interferon-gamma and interleukin-4. Dig Dis Sci 43 156-161. [DOI] [PubMed] [Google Scholar]
- Tucková L, Novotná J, Novák P, Flegelová Z, Kveton T, Jelínková L, Zídek Z, Man P, and Tlaskalová-Hogenová H (2002) Activation of macrophages by gliadin fragments: isolation and characterization of active peptide. J Leukoc Biol 71 625-631. [PubMed] [Google Scholar]
- Ucer U, Bartsch H, Scheurich P, and Pfizenmaier K (1985) Biological effects of gamma-interferon on human tumor cells: quantity and affinity of cell membrane receptors for gamma-IFN in relation to growth inhibition and induction of HLA-DR expression. Int J Cancer 36 103-108. [DOI] [PubMed] [Google Scholar]
- Wapenaar MC, van Belzen MJ, Fransen JH, Sarasqueta AF, Houwen RH, Meijer JW, Mulder CJ, and Wijmenga C (2004) The interferon gamma gene in celiac disease: augmented expression correlates with tissue damage but no evidence for genetic susceptibility. J Autoimmun 23 183-190. [DOI] [PubMed] [Google Scholar]
- Xia J, Sollid LM, and Khosla C (2005) Equilibrium and kinetic analysis of the unusual binding behavior of a highly immunogenic gluten peptide to HLA-DQ2. Biochemistry 44 4442-4449. [DOI] [PubMed] [Google Scholar]
- Zimmer KP, Poremba C, Weber P, Ciclitira PJ, and Harms E (1995) Translocation of gliadin into HLA-DR antigen containing lysosomes in coeliac disease enterocytes. Gut 36 703-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.